Abstract
Giardia lamblia is one of the most recognized waterborne protozoan parasites causing gastrointestinal disease. A simple but effective DNA extraction protocol for real-time PCR detection from surface water samples was developed in this study. Eleven protocols were compared, which consisted of freeze–thaw treatments (liquid N2 and boiling water) and purification using the Qiagen DNeasy kit, together with different combinations of proteinase K, PVP360, GITC and Chelex 100 incubation. Using concentrated surface water samples spiked with G. lamblia cysts, the necessary steps for high DNA recovery were shown to be freeze–thaw, DNeasy purification and Chelex 100 incubation. Multiple rounds of freeze–thaw treatment (five cycles per round) were reported for the first time in this study to significantly increase the DNA yield from G. lamblia cysts, from ~20% after one round of freeze–thaw to 40 and 70% after two and three-rounds of freeze–thaw, respectively. More than three rounds of freeze–thaw treatment did not promote additional DNA recovery. The final protocol included three–three-rounds of freeze–thaw treatment, DNeasy purification and Chelex 100 incubation. This method was simpler, more cost-effective, and had a comparable DNA recovery to methods involving immunomagnetic separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Giardia lamblia (syn. Giardia intestinalis, Giardia duodenalis) is a waterborne protozoan parasite that infects the gastrointestinal tract of humans. It is recognized as one of the most common pathogens responsible for diarrhea in the world, with an annual infection of about 2.8 × 109 cases (Lane and Lloyd 2002). Contaminated source water and inadequate drinking water treatment are the main reasons for the reported outbreaks (Rose and Slifko 1999). In addition, Giardia cysts are more resistant than bacteria to chlorination, which is the conventional disinfection used in most water treatment plants (Betancourt and Rose 2004). To monitor and reduce the human health risk of pathogens associated with drinking water, it is important to develop a sensitive and quick method to detect G. lamblia in source and drinking water.
The immunofluorescence assay (IFA), such as USEPA method 1623 (2005), is the most widely applied diagnostic technique to detect G. lamblia from water. However, it has some shortcomings, in that the method is time-consuming, costly, and unable to differentiate between closely related species (Verweij et al. 2003; Wang et al. 2004). For this reason, molecular approaches, including real-time PCR, have been developed for Giardia detection. This quantitative PCR method has been tested by many researchers for the detection of waterborne protozoan parasites, including Cryptosporidium parvum and G. lamblia (Amar et al. 2003; Fontaine and Guillot 2002; Guy et al. 2003; Ramirez and Sreevatsan 2006; Tanriverdi et al. 2002; Verweij et al. 2003, 2004).
For G. lamblia detection, most real-time PCR testing has been done using stool samples from infected patients (Amar et al. 2003; Guy et al. 2004; Verweij et al. 2003, 2004). Because stool samples often contain a high cyst concentration, cyst purification and DNA extraction using commercial kits was not difficult. However, DNA extraction and purification from surface water can be more difficult. Usually the G. lamblia levels in surface water are very low, typically below 1 cyst/l. In addition, the composition and properties of samples are complex, so that sample concentration and DNA extraction and purification become critical steps. Various methods for DNA purification from Cryptosporidium have been developed (Fontaine and Guillot 2003; Hallier-Soulier and Guillot 2003) using immunomagnetic separation as a pretreatment from the concentrated water samples, thus preparing almost pure oocyst preparations of C. parvum before DNA extraction. Though this protocol could be adopted for G. lamblia detection, the IMS reagent is expensive, and this added cost could prevent widespread use of the method. Guy et al. (2003) and Anceno et al. (2007) developed an IMS free DNA extraction method for surface and waste water, however, the focus of this method in their papers was on wastewater, and the (oo)cysts recoveries of surface water samples were not provided.
In this study, we compared 11 protocols for direct DNA extraction of G. lamblia prior to real-time PCR detection. A protocol combining three-rounds of freeze–thaw treatment (five cycles each round) followed by DNA purification was found to be optimal for use in PCR detection.
Materials and methods
Giardia cysts
Giardia lamblia H3 isolate was purchased from Waterborne Inc. (New Orleans, LA, USA). The live cysts were stored in phosphate buffered saline (PBS) at 4°C before they were used.
Surface water sample preparation
Environmental surface water samples (10 l/sample) were taken from Laurel Creek at the University of Waterloo campus. During this study, the turbidity of the water varied from 20 to 30 NTU. Five liter of water was filtered and concentrated according to EPA method 1623 (2005). First, the water was filtered through an Envirochek capsule filter (Pall Corporation) at a speed of 2 l/min. The filter was then eluted with elution buffer by vigorously shaking at >450 rpm for 5 min. This elution step was repeated two times. In total about 250 ml of eluate was collected in two 150 ml conical tubes and centrifuged (Sorvall RC 5B PLUS, DUPONT) at 10,000 rpm for 10 min. After aspirating the supernatant, the pellet was resuspended in 0.5 ml PBS and transferred to a 2 ml screw-capped centrifuge tube. Due to the low concentration of G. lamblia in Laurel Creek, cysts were spiked into the concentrated surface water samples so that recovery could be assessed. A 40 μl aliquot containing 500 G. lamblia cysts was added to the 2 ml tube containing concentrated surface water sample, and mixed by vortexing for 10-15 s.
DNA extraction and purification protocols
Eleven DNA extraction protocols were investigated successively (Table 1). Protocol 10 resulted in the highest recovery of protozoa, and this method is described as follows. The concentrated surface water samples suspended in PBS were treated using five freeze–thaw cycles, with each cycle consisting of 2 min in liquid N2 and 2 min in boiling water. The sample was then centrifuged at 8,000 rpm for 1 min, and the supernatant transferred to a 3 ml centrifuge tube. The pellet was then resuspended in 0.5 ml PBS, and treated using two more rounds of five freeze–thaw cycles as described above. The supernatant from each round of freeze–thaw treatment were pooled, and passed through a DNeasy mini spin column (Qiagen) using the protocol described by the manufacturer. The column was washed with buffers AW1 and AW2, and eluted with two 50 μl volumes of AE buffer (Qiagen). The final pooled sample volume was 100 μl.
To further remove PCR inhibitors, the extracted DNA sample was treated using Chelex 100 (Bio-Rad). A 25 μl volume of a 1,000 mg/ml Chelex 100 solution (200 mg/ml final in H2O) was added to the eluted DNA, and the sample was shaken at 450 rpm for 30 min. The mixture of DNA and Chelex 100 was transferred into a new DNeasy column, centrifuged at 12,000 rpm for 2 min, and the final eluant volume was recorded.
The other DNA extraction methods (Table 1) are described as follows. All of the methods involved one or more rounds of freeze–thaw treatment and purification through a DNeasy spin column, as described above. For Protocol 1, a pretreatment before the freeze–thaw step was done by adding 360 μl ATL lysis buffer (Qiagen) and 40 μl proteinase K to the resuspended pellet and incubating at 55°C for 1 h. In Protocols 1 and 2, a combination of 20% Chelex 100 and 2% polyvinylpyrrolidone (PVP360) (Sigma-Aldrich) (final concentrations) were added, and the sample was incubated at 55°C for 1 h prior to DNeasy purification. Preparation of the Chelex 100 stock solution is described above. The PVP360 stock solution (8%) was made by adding PVP360 to ATL buffer (Qiagen), incubated at 55°C for 10 min and then gently mixed to dissolve.
In Protocols 3–7, various combinations of Chelex 100 and PVP360 treatments (as described in Protocols 1 and 2) were conducted before or after the freeze–thaw or DNeasy purification steps. In Protocols 7–11, the final Chelex 100 step was done as described above in method 10, by passing the DNA solution containing Chelex through a new DNeasy spin column.
In Protocol 9, preincubation of the cell pellet in a guanidine thiocyanate (GITC; Sigma-Aldrich) solution was performed prior to freeze–thaw treatment. GITC (5% in H2O final concentration) was added to the cell pellet, and the sample was incubated at 55°C for 1 h. After the first round of freeze–thaw treatment, the cell pellet was again resuspended in GITC solution and the second round of freeze–thaw treatment performed. In Protocols 8 and 9, a slurry washing step was also performed after two rounds of freeze–thaw treatment. In this step, the resuspended pellet (slurry) was directly transferred to the DNeasy column, and washed as described above.
Protocols 10 and 11 tested the effects of three to five rounds of freeze–thaw on DNA recovery rates. In Protocols 8–11, the amount of DNA recovered from the cell pellet was measured after each round of freeze–thaw treatment or slurry washing treatment.
PCR amplification
PCR primers and the fluorescently labeled probe (Table 2) were described by Verweij et al. (2003). The primer pair could amplify a 63 bp fragment of the G. lamblia small subunit ribosomal RNA gene (Genbank accession number M54878).
Real-time PCR was carried out using an iCycler Multicolor Real-time PCR Detection System (Bio-Rad). Bovine serum albumin (BSA) was added to the mixture to reduce the effect of amplification inhibitors. Each reaction (25 μl) contained: 300 nM of each primer; 200 nM of probe; 10 mM dNTP; 4 mM MgCl2; 1× PCR buffer; 1 U Jumpstart Taq polymerase (Sigma-Aldrich); 20 ng/μl BSA; 10 μl DNA template. The amplification conditions were 1 cycle at 95°C for 5 min, 50 cycles at 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. The fluorescent signal was detected at the end of each amplification cycle.
Each sample was tested in triplicate. A standard DNA series equivalent to 10,000, 1,000, 100, 10 and 1 cyst(s) from the pure culture and blank (H2O) controls were included in every assay. Standard curves and data analysis were completed using MS Excel 2003.
We evaluated the specificity of the oligonucleotides using genomic DNA from negative control strains including C. hominis (Synthesized DNA fragament, IDT inc.), C. parvum (Waterborne P102), C. muris (Waterborne P104), G. muris (Waterborne P105) Acanthamoeba castellanii (ATCC 30010D) and six bacterial strains including Aeromonas hydrophila (ATCC 7966), Campylobacter jejuni (ATCC 33291), Legionella pneumophila (ATCC 33152), Escherichia coli 0157:H7 (ATCC 43895), Salmonella typhimurium (ATCC 13311), and Yersinia enterocolitica (ATCC 9610).
Statistical analyses
All statistical analyses were performed by using Excel 2003 (Microsoft, US). When different DNA extraction protocols were compared, at least three replicates were used for statistical analyses. The protocols were compared in a pairwise pattern, where t tests were applied to determine if one protocol could yield significant higher DNA extraction than another.
Results
The efficiency of various DNA extraction and purification protocols for G. lambia was tested. This was done by comparing the results from two to three protocols in parallel, followed by modification and retesting of the various methods. This process was repeated until the optimal protocol was found.
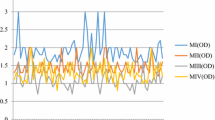
The purified DNA was then quantified using a real-time PCR assay that targets the small subunit ribosomal RNA gene of G. lamblia (Verweij et al. 2003). In this assay, the standard DNA series showed a good linear relationship, usually with a correlation coefficient more than 0.95 (Fig. 1). The specificity of the primer and probes were tested beforehand and they could only amplify the target gene specifically.
Effects of BSA, proteinase K, PVP360 and Chelex 100
The DNA extraction efficiency of various protocols was initially tested by adding 500 G. lamblia cysts to a concentrated surface water sample. Initially, BSA was not added to the PCR amplification mixture. It was unexpected that in this first assay using Protocol 1 (without BSA) there was no amplification signal (Fig. 1a). After the addition of 20 ng/μl BSA to the PCR amplification mixture, a weak amplification signal was obtained (Table 3) which suggests that BSA was effective at suppressing the effects of environmental inhibitors in the DNA samples. For this reason, BSA was used in all the following PCR assays. However, even with the addition of BSA, the Ct values were still greater than the lowest value on the standard curve, resulting in a calculated cyst recovery efficiency of 0%. For this reason, we tested a variety of other protocols to improve cyst recovery from concentrated surface water samples.
Protocols 2–7 all used one round of freeze–thaw (consisting of five freeze–thaw cycles) and DNeasy purification, and different combinations of Chelex 100 and PVP360 treatment (Table 1). Protocols 2–5 were first compared (Fig. 2a). Protocol 2 and 3, which included PVP360, had the lowest DNA recovery of about 2%. Protocol 4, which included Chelex 100 but not PVP360 treatment, resulted in an increased recovery of 9.7 ± 0.5%. However, the highest recovery occurred in Protocol 5 (35.6 ± 5.4%) in which both the Chelex 100 and PVP360 treatment steps were removed. Protocol 5 was the simplest, only freeze–thaw and DNeasy purification was included. The results suggest that when PVP360 and Chelex100 were mixed with the concentrated sample directly, they actually had no promotion on DNA recovery efficiency in our study.
Based on the above results, we next tested the addition of Chelex100 after the freeze–thaw step (Protocol 6), or after DNeasy purification (Protocol 7). A comparison of Protocol 5, 6 and 7 resulted in recoveries of 23.4 ± 5.2, 19.5 ± 4.1 and 29.6 ± 7.8%, respectively (Fig. 2b), which suggested Protocol 7 was the most effective among them. When Protocol 7 was compared with Protocol 5 and 6 again, protocol 7 again showed the highest DNA recovery from G. lamblia (Fig. 2c, d).
Effect of increased freeze–thaw cycles
To improve cyst lysis, we tested an increased number of freeze–thaw cycles. Protocols 1–7 used five freeze–thaw cycles, and we tested DNA recovery following up to eight freeze–thaw cycles, but this showed no promotion in DNA recovery (data not shown). However, when we tested multiple rounds (five cycles each round) of freeze–thaw treatment (Protocol 8–11), we did obtain an increase in DNA recovery. In these protocols, two to five rounds of freeze–thaw were applied, and the DNA recovery for each round was assessed separately, and then added to get the total recovery. In Protocols 8, 9 and 10 (Figs. 3, 4), the first round freeze–thaw could obtain an average DNA recovery of 17.5 ± 8.8%, and the second round obtained an additional 24.6 ± 7.9%. Therefore, the sum of the two rounds reached 42.0 ± 16.6%. These results suggest that after one round of five cycles of liquid N2—boiling water freeze–thaw, there were still many cysts that remained intact in the concentrated surface water pellet. More rounds of freeze–thaw were an effective approach to obtain more DNA from the mixture.
Since Protocols 8 and 9 proved that two rounds of freeze–thaw could effectively increase DNA extraction efficiency, we then tested the effect of additional rounds to explore the potential of this method. Figure 4 shows the results of four different trials of the three round freeze–thaw method (Protocol 10), and results show that the third round could still increase the overall recovery by an average of 23.8 ± 12.4%, resulting in a total average recovery of 72.5 ± 30.6%. However, when more freeze–thaw rounds were tested, an increase in DNA recovery was not detected (Fig. 5). These results show that three rounds of freeze–thaw treatment were sufficient for optimal DNA recovery from G. lamblia cysts in this study.
Effects of direct slurry washing and GITC
In both Protocol 8 and 9, after the second round freeze–thaw, the concentrated surface water sample (slurry) left in the centrifuge tube was resuspended and directly applied and processed through a DNeasy column for purification without freeze–thaw. This step was taken in order to investigate if any DNA attached to the particulates and would be detached through the purification process. However, this step was not effective at improving DNA recoveries.
Protocol 9 also used a GITC treatment, which is often applied to lyse cells in RNA or DNA extraction, to resuspend the concentrated pellet before freeze–thaw treatment. The average DNA recovery of this protocol was 15.4 ± 19.0% from each round of freeze–thaw, which was some lower and fluctuated more than that of Protocol 8 (19.6 ± 17.0%). This result suggested that additional GITC treatment had no promotion in the DNA extraction of Giardia.
Comparison of the DNA extraction efficiencies of the protocols
DNA extraction efficiencies were compared in a pairwise pattern using t test. From the results in Fig. 2a, P2, 3, 4 could be compared with P5, respectively. While, P5, 6 were compared with P7, respectively, based on Fig. 2b, c, d. P7 and P8, P8 and P9, P8 and P10, P10 and P11 could also be compared with the results from Figs. 3, 4 and 5, respectively. All of the comparison showed statistically significant difference (P < 0.01), except for P10 and P11. This step-by-step development and modification of the protocols through comparison showed that P10 and P11 had the highest DNA extraction efficiency. However, five rounds of free-thaw treatment made P11 more laborious and time-consuming. So P10 with three rounds were the optimal one and should be used in the future.
Discussion
DNA extraction and inhibitor removal can have a great impact on successful target gene amplification, and are critical steps in PCR. This is particularly important in detecting protozoan pathogens in surface water, due to the low concentration of target organisms and the large volumes of water that must be concentrated for detection. Various protocols have been developed to achieve a high DNA recovery from Giardia cysts. Based in a review of the literature, we selected several steps to test in this study. The freeze–thaw method was chosen because results in the literature showed that it was effective for obtaining efficient cyst lysis (Guy et al. 2004; Lane and Lloyd 2002; Ramirez and Sreevatsan 2006; Tanriverdi et al. 2002;). The violent temperature change from liquid N2 to boiling water has proven to be the most effective method to break the hard (oo)cyst wall. In addition, we used DNeasy purification in all the procedures tested. Our previous work (unpublished data) has shown that it is useful in purifying DNA from Giardia before real-time PCR protocols, resulting in a lower Ct value and stronger amplification signal. Additional treatments used in other studies (Alberti and Fornaro 1990; Kotlowski et al. 2004) have included proteinase K as a cell lysis agent, Chelex 100 and PVP360 to reduce the PCR inhibitors, and GITC to enhance cell lysis and stabilize extracted DNA.
Our results differed from those of others (Amar et al. 2003; Anceno et al. 2007; Chung et al. 1998; Guy et al. 2003) in that proteinase K, PVP360 and Chelex 100 exhibited no promotion in DNA extraction and detection efficiency (Protocols 1–6) when added before DNA purification using resin columns. This may be attributed to the complex nature of materials present in environmental samples such as surface waters. When water samples are concentrated, not only microbial cells but also inorganic particulates, colloidal materials including humic substances, and other inorganic and organic material were retained by the filters. The physical and chemical properties of these materials can vary greatly. For example, the largest particulates could have a diameter in the scale of mm, and the smallest organic matter might have a molecular weight of <100, the colloidal materials may contain different electric charges, the humic substances (which are an extremely heterogeneous supramolecular system, possessing both aromatic and aliphatic characteristics) can act as chelator and detergent. Therefore, the complicated electrochemical and colloidal chemical characteristics and the possible physical processes such as interception may prevent PVP360 and Chelex 100 from effectively removing PCR inhibitors from different types of environmental samples. Furthermore, the concentrated slurry from the surface water samples might contain too many inhibitors to be effectively removed by Chelex and PVP360 if they are used before the resin column purification step. We also found that proteinase K and GITC did not show improved DNA recovery from either pure cultures or environmental samples.
When added to the DNA solution after DNeasy column purification, Chelex 100 could increase DNA recovery (Fig. 2b, c). The addition of BSA to the PCR reaction mixture could also remove PCR inhibitors, and this has been shown in numerous other studies (Anceno et al. 2007; Guy et al. 2004; Ghosh et al. 2000). Both of these results are evidence of the strong inhibition caused by compounds that remain present in the DNA extracted from concentrated surface water samples. In our final procedure, Chelex 100 chelation was the last step, and the mixture including the resin particles were transferred to the column for spinning together, thus preventing a loss of DNA.
The use of multiple rounds of freeze–thaw treatment was shown to be a critical factor in recovering high amounts of DNA from G. lamblia added to concentrated surface water samples. In other studies, only one round of three to five cycles of freeze–thaw was applied to obtain acceptable DNA recoveries (Anceno et al. 2007; Guy et al. 2003). However, in this study we found that a second and third round of freeze–thaw treatment substantially increased DNA recoveries. This suggests the large amount of particulate material in the slurry pellet might hinder cyst lysis and protect intact cysts from the effects of the freeze–thaw treatment. These protected cysts may then be more susceptible to cell lysis following removal of the supernatant after each round of treatment. Based on a general understanding of reaction kinetics, the release of DNA during freeze–thaw would approach a steady and saturated state in the first several cycles, so increasing the cycle number was not practical. However, by removing the released DNA and breaking the reaction balance, the addition of new solvent, through a new round of freeze–thaw, would re-establish the DNA mass transfer drive. In addition, the removal of humic substances after each freeze–thaw round would have reduced the amount of inhibitors remaining in the pellet, which was clearly observed by the reduction in color of the supernatant after each round of freeze–thaw. Removal of this humic material may have resulted in decreased protection from the effects of freeze–thaw lysis in subsequent rounds of treatment. This was the probable reason that the additional DNA recovery from the second and third freeze–thaw rounds were similar to those from the first round.
Immunomagnetic separation could be applied as a first step in the purification of G. lamblia cysts, to remove PCR inhibitors prior to cell lysis. Hu et al. (2004) have reported a cyst recovery of 89.0 ± 4.7% using IMS and fluorescent assay (FA). Our tests also showed a DNA recovery of around 100% using IMS and real-time PCR. However, the commercial IMS reagent kit is very expensive, and would add a significant cost per sample. In contrast, the treatment reagents used in our method, including liquid N2 and the DNA column purification kit, have a much lower cost. Because of the efficient DNA recovery and PCR detection that could be obtained using our method, the cost savings is a significant reason for the application of the method developed in this study.
Conclusions
A new protocol using the combination of a multiple rounds of freeze–thaw, DNeasy column and Chelex 100 purification was shown to be effective for direct DNA extraction and preparation of G. lamblia for real-time PCR detection from surface water. To our knowledge, this is the first description for the application of a multiple-round freeze–thaw treatment for DNA extraction from protozoa. Though very simple, its effectiveness for efficient detection of G. lamblia from surface water is evident. The low reagent costs are of benefit when compared with protocols that require IMS as part of the DNA extraction procedure. Since G. lamblia is one of the most common threats for human health resulting from water, the future application of this protocol would contribute to the sensitive detection of this pathogen. In addition, it may also prove useful in the molecular detection of C. parvum and other protozoan pathogens.
References
Alberti S, Fornaro M (1990) Higher transaction efficiency of genomic DNA purified with a guanidinium thiocyanate-based procedure. Nucleic Acids Res 18(2):351–353. doi:10.1093/nar/18.2.351
Amar CFL, Dear PH, McLauchlin J (2003) Detection and genotyping by real-time PCR/RFLP analyses of Giardia duodenalis from human faeces. J Med Microbiol 52(8):681–683. doi:10.1099/jmm.0.05193-0
Anceno AJ, Katayama H, Houpt ER, Chavalitshewinkoon-Petmitr P, Chuluun Band Shipin OV (2007) IMS-free DNA extraction for the PCR-based quantification of Cryptosporidium parvum and Giardia lamblia in surface and waste water. Int J Environ Health Res 17(4):297–310. doi:10.1080/09603120701372573
Betancourt WQ, Rose JB (2004) Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet Parasitol 126:219–234
Chung E, Aldom JE, Chagla AH, Kostrzynska M, Lee H, Palmateer G, Trevors JT, Ungerd S, De Grandis S (1998) Detection of Cryptosporidium parvum oocysts in municipal water samples by the polymerase chain reaction. J Microbiol Methods 33:171–180. doi:10.1016/S0167-7012(98)00050-5
Fontaine M, Guillot E (2002) Development of a taqMan quantitative PCR assay specific for Cryptosporidium parvum. FEMS Microbiol Lett 214:13–17. doi:10.1111/j.1574-6968.2002.tb11318.x
Fontaine M, Guillot E (2003) An immunomagnetic separation-real-time PCR method for quantification of Cryptosporidium parvum in water samples. J Microbiol Methods 54:29–36. doi:10.1016/S0167-7012(03)00005-8
Ghosh S, Debnath A, Sil A, Chattopadhyay DJ, Das P (2000) PCR detection of Giardia lamblia in stool: targeting intergenic spacer region of multicopy rRNA gene. Mol Cell Probes 14(3):181–189. doi:10.1006/mcpr.2000.0302
Guy RA, Payment P, Krull UJ, Horgen PA (2003) Real-time PCR for quantification of Giardia and Cryptosporidium in environmental water samples and sewage. Appl Environ Microbiol 69:5178–5185. doi:10.1128/AEM.69.9.5178-5185.2003
Guy RA, Xiao C, Horgrn PA (2004) Real-time PCR assay for detection and genotype differentiation of Giardia lamblia in stool specimens. J Clin Microbiol 42(7):3317–3320. doi:10.1128/JCM.42.7.3317-3320.2004
Hallier-Soulier S, Guillot E (2003) An immunomagnetic separation-reverse transcription polymerase chain reaction (IMS-RT-PCR) test for sensitive and rapid detection of viable waterborne Cryptosporidium parvum. Environ Microbiol 5(7):592–598. doi:10.1046/j.1462-2920.2003.00442.x
Hu J, Feng Y, Ong SL, Ng WJ, Song L (2004) Improvement of recoveries for the determination of protozoa Cryptosporidium and Giardia in water using method 1623. J Microbiol Methods 58:321–325. doi:10.1016/j.mimet.2004.04.013
Kotlowski R, Martin A, Ablordey A, Chemlal K, Fonteyne P, Portaels F (2004) One-tube cell lysis and DNA extraction procedure for PCR-based detection of Mycobacterium ulcerans in aquatic insects, molluscs and fish. J Med Microbiol 53:927–933. doi:10.1099/jmm.0.45593-0
Lane S, Lloyd D (2002) Current trends in research into the waterborne parasite Giardia. Crit Rev Microbiol 28(2):123–147. doi:10.1080/1040-840291046713
Ramirez NE, Sreevatsan S (2006) Development of a sensitive detection system for Cryptosporidium in environmental samples. Vet Parasitol 136:201–213. doi:10.1016/j.vetpar.2005.11.023
Rose JB, Slifko TRG (1999) Cryptosporidium, and Cyclospora and their impact on foods: a review. J Food Prot 64:1793–1798
Tanrıverdi S, Tanyeli A, Baslamish F, Koksal F, Kılınc Y, Feng X, Batzer G, Tzipori S, Widmer G (2002) Detection and genotyping of oocysts of Cryptosporidium parvum by real-time PCR and melting curve analysis. J Clin Microbiol 40(9):3237–3324. doi:10.1128/JCM.40.9.3237-3244.2002
US Environmental Protection Agency (2005) Method 1623: Cryptosporidium and Giardia in water by filtration, immunomagnetic separation, and fluorescent antibody. Office of Water, Washington, DC. Publication EPA-821-R-99-006
Verweij JJ, Schinkel J, Laeijendecker D, van Rooyen MAA, van Lieshout L, Polderman AM (2003) Real-time PCR for the detection of Giardia lamblia. Mol Cell Probes 17:223–225. doi:10.1016/S0890-8508(03)00057-4
Verweij JJ, Blangé RA, Templeton K, Schinkel J, Brienen EAT, van Rooyen MAA, van Lieshout L, Polderman AM (2004) Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J Clin Microbiol 42(3):1220–1223. doi:10.1128/JCM.42.3.1220-1223.2004
Wang Z, Vora GJ, Stenger DA (2004) Detection and genotyping of Entamoeba histolytica, Entamoeba dispar, Giardia lamblia, and Cryptosporidium parvum by oligonucleotide microarray. J Clin Microbiol 42(7):3262–3271. doi:10.1128/JCM.42.7.3262-3271.2004
Acknowledgments
This research was completed while the first author was a Postdoctoral Fellow with the Natural Sciences and Engineering Research Council of Canada (NSERC) Chair in Water Treatment at the University of Waterloo. It was supported by the Ontario (Canada) Ministry of the Environment, Best in Science Program. The Partners of the NSERC Chair may be found at www.civil.uwaterloo.ca/watertreatment/.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, X., Van Dyke, M.I., Portt, A. et al. Development of a direct DNA extraction protocol for real-time PCR detection of Giardia lamblia from surface water. Ecotoxicology 18, 661–668 (2009). https://doi.org/10.1007/s10646-009-0347-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0347-9