Abstract
Lessons from organophosphorus pesticides, which could be bioaccumulated in non-target organisms at different trophic levels and caused unexpected negative impacts, necessitate a study of the possibility of biotransfer and bioaccumulation of Bacillus thuringiensis (Bt) insecticidal toxin(s) expressed in Bt plants. Using ELISA, we evaluated the transfer of Cry1Ab toxin in a food chain of Bt rice (KMD1 and KMD2), the target insect, Cnaphalocrocis medinalis, and its predator, Pirata subpiraticus. Cry1Ab was detected in C. medinalis and P. subpiraticus. However, the concentration of Cry1Ab detected from C. medinalis and P. subpiraticus did not increase as feeding or preying time increased. A binding study of Cry1Ab to the brush border membrane vesicle of C. medinalis and P. subpiraticus indicated that P. subpiraticus does not have binding receptors in its midgut to Cry1Ab, while C. medinalis does. Survivorship and fecundity of P. subpiraticus preying on Bt rice-fed C. medinalis were not significantly different from those preying on non-Bt rice-fed C. medinalis. Developmental time of P. subpiraticus was significantly longer when it preyed on Bt rice-fed C. medinalis than on non-Bt rice-fed prey. However, a 3-year field trial indicated that Bt rice did not significantly affect the density of P. subpiraticus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the most widely consumed cereal grain and was grown on over 152 million ha worldwide in 2004 (FAO 2007). The main lepidopteran pests of rice in China are the striped stem borer, Chilo suppressalis (Walker) (Lepidoptera: Crambidae), yellow stem borer, Scirpophaga incertulas (Walker) (Lepidoptera: Pyralidae), pink stem borer, Sesamia inferens (Walker) (Lepidoptera: Noctuidae), and the leaffolder, Cnaphalocrocis medinalis (Gueneé) (Lepidoptera: Pyralidae). Collectively these insects annually cause a 3–10% yield loss equal to ca. $1 US billion, despite the intense use of insecticides (Sheng et al. 2003). Furthermore, the broad-spectrum insecticides commonly used in rice production have disrupted many biological control agents in the rice ecosystems and reduced their effectiveness for control of the Lepidoptera and other pest herbivores (Matteson 2000).
Because of the prominent pest status of stem borers and leaffolders, the limited source of resistant germplasm, and the success of genetically modified (GM) maize and cotton with Bacillus thuringiensis (Bt) insecticidal genes, there have been extensive investments in insect-resistant GM rice research and development in China (Wang and Johnston 2007). Since 1993, numerous genotypes of GM rice with insecticidal Bt gene(s) (referred as Bt rice hereafter) have been developed that confer strong resistance against rice stem borers and leaffolders (reviewed by High et al. 2004; Chen et al. 2006; Wang and Johnston 2007). Bt rice field trials were first conducted in China in 1998 and large field trials of several Bt rice lines have continued in China (Chen et al. 2006; Wang and Johnston 2007). Field trials have confirmed that Bt rice effectively controls stem borers and leaffolders (Chen et al. 2006). In a study in farmers’ fields in China, a Bt rice line resulted in yield increases of 6–9%, and 80% reduction in insecticide use, in comparison to conventional varieties (Huang et al. 2005). Because of the effectiveness of Bt rice for pest control and the extensive research on Bt rice in China, it is likely that China will be the first in the world to commercialize Bt rice (Cohen et al. 2008).
A major concern about the deployment of Bt rice is its potential impact on non-target arthropods, especially natural enemies through tritrophic interactions. Obrist et al. (2006) reported that Bt toxin could be transferred to arthropod predators (Orius spp., Chrysoperla spp., and Stethorus sp.) in Bt maize field. Chen et al. (2005) also found that Bt toxin could be taken up by the predator, Pirata subpiraticus (Bösenberg et Strand) (Araneae: Lycosidae) when it fed on Bt rice-fed Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Thus, it is possible that Bt toxins in Bt rice can appear in natural enemies through tritrophic interactions, although it is not clear what ecological consequences, if any, may arise from this phenomenon. Another question is whether Bt toxins could be bioaccumulated when consumed by natural enemies in a food chain, similar to organophosphorus pesticides being bioaccumulated in invertebrates, fish, and birds at different trophic levels and cause unexpected negative impacts (Serrano et al. 1997a, b).
To date, many studies have evaluated the impact of Bt toxins through tritrophic interactions (reviewed by Romeis et al. 2006 and Chen et al. 2006), however bioaccumulation of Bt toxins at different trophic levels has not been investigated. Moreover, the ecological impact of the possible biotransfer and bioaccumulation of Bt toxins expressed in Bt rice through tritrophic interactions has not been explored. In this paper, we evaluated (1) biotransfer and bioaccumulation of Cry1Ab toxin in a food chain comprising two Bt rice lines, the herbivore C. medinalis and the predator P. subpriaticus, (2) binding activity of Cry1Ab to P. subpriaticus brush border membrane vesicles (BBMVs), (3) effect of Cry1Ab on the development and fecundity of the predator P. subpriaticus under laboratory conditions, and (4) ecological impact of Cry1Ab on P. subpriaticus in a 3-year field study.
Materials and methods
Transgenic rice
Two homogenous transgenic Bt rice lines with a synthetic cry1Ab gene, KMD1 and KMD2 (Ye et al. 2001) at the tenth generation after transformation, were used together with their non-Bt isoline japonica rice cultivar Xiushui11. The Bt rice contained a synthetic cry1Ab gene under the control of the maize ubiquitin promoter and linked in tandem with gus (encoding the β–glucuronidase), hpt (encoding the hygromycin phosphotransferase), and npt (encoding the neomycin phosphotransferase) genes (Cheng et al. 1998; Xiang et al. 1999). The Bt rice selected through four generations was homozygous for the transgenes (cry1Ab, gus and npt) (Shu et al. 1998), and could effectively control rice stem borers (Ye et al. 2001) and leaffolders (Ye et al. 2003) under field conditions. The three different rice lines were sown then transplanted into pots (dia. 15 cm) in a greenhouse on Zhejiang University’s Huajiachi Campus and kept free from insects until needed. Rice plants were used at 40 ± 2 days after transplant for the following experiments.
Arthropods tested
Eggs and early instars of C. medinalis were collected from local farmers’ rice fields in Hangzhou (120.2°E, 30.3°N) China and maintained on non-Bt rice plants (Xiushui11) in an insect rearing chamber maintained at 25 ± 1°C, RH 75–80% and a photoperiod 14:10 (L:D) h. Two nylon cages were set up in the chamber to maintain the C. medinalis colony for experimental use. Twenty pots of rice plants were moved into a rearing chamber and placed in a plastic tub in a nylon netted cage. The cage was 2.0 m long × 1.0 m wide × 1.8 m high with a zippered opening on one side. Potted rice plants were replaced as needed.
Early instars of the predator, P. subpriaticus, were also collected from local farmers’ rice fields in Hangzhou and kept in another rearing chamber under the same conditions. Because of their cannibalistic characteristic, P. subpriaticus were individually put into glass vials (length 9.5 cm, dia. 2.5 cm) lined with a water-soaked sponge at the bottom and sealed with fine mesh for ventilation. Each P. subpriaticus was supplied with two C. medinalis larvae daily and water was added to the sponge as necessary.
Biotransfer and bioaccumulation of Cry1Ab from Bt rice to C. medinalis
Cnaphalocrocis medinalis third–fourth instars were collected from the insect culture. Each larva, without starvation treatment, was placed into a Petri dish (dia. 9.5 cm) lined with a filter paper (Whatman, England). One piece of KMD1 rice leaf (cut to 6 cm length) was put into the petri dish with the two cut ends covered with moistened non-absorbent cotton (Ye et al. 2000). A C. medinalis larva was kept in the Petri dish for 1, 2, 3, 4 and 5 days, respectively. KMD1 leaves were changed daily. Meanwhile, the feeding areas were recorded with a flexible transparent grid (1 mm2 grid squares) and the excretions of the C. medinalis larvae were collected to indicate how much they fed. Five C. medinalis samples (insects and excretions) were collected at each inoculation time interval with each sample being a replicate. After the larvae and excretions were weighed, they were immediately put into different Eppendorf vials (1.5 ml) and stored at −70°C until ELISA (Enzyme-linked Immunosorbent Assay) assays were carried out using Cry1Ab/Cry1Ac QuantiPlate kit (EnviroLogix, Portland, ME) to detect the presence of Cry1Ab in C. medinalis larvae and excretions.
Experimental designs for detecting the biotransfer and bioacuumulation of Cry1Ab toxin from KMD2 rice to C. medinalis larvae were the same as described above. Five C. medinalis fed on non-Bt Xiushui11 rice were used as the controls at each time interval.
Biotransfer and bioaccumulation of Cry1Ab from Bt rice-fed C. medinalis to P. subpiraticus
Pirata subpiraticus adults, without starvation treatment, were individually put into glass vials. Each P. subpiraticus was daily supplied with two C. medinalis third–fourth instars that had fed on KMD1 rice plants for 1 day. C. medinalis (either dead or live) were taken with forceps when new ones were supplied to P. subpiraticus each day. After continuously feeding on KMD1 rice-fed C. medinalis larvae for 1, 2, 3, 4 and 5 days, respectively, five P. subpiraticus adults (as five replicates) were sampled at each inoculation time interval, immediately weighed and stored at −70°C until ELISA assays were conducted to detect the presence of Cry1Ab. In order to detect whether P. subpiraticus adults excrete Cry1Ab toxin, the white excretions of P. subpiraticus adults were washed from the glass vial’s inner wall with 1 ml Extraction/Dilution Buffer (Supplied in Cry1Ab/Cry1Ac QuantiPlate kit) after P. subpiraticus had fed on KMD1 rice plant-fed C. medinalis larvae for 5 days. Samples were stored at −70°C before analyzing the presence of Cry1Ab.
Experimental designs were the same as above for detecting the biotransfer and bioaccumulation of Cry1Ab from KMD2 rice-fed C. medinalis larvae to P. subpiraticus. Five P. subpiraticus preying on Xiushui11 rice-fed C. medinalis were used as controls at each time interval.
Binding of Cry1Ab to C. medinalis and P. subpiraticus midgut BBMVs
Preparation of BBMVs
Cnaphalocrocismedinalis and P. subpiraticus midguts were prepared as described by Wolfersberger et al. (1987). Their BBMVs were extracted by differential magnesium precipitation in a procedure modified from Wolfersberger et al. (1987). The final vesicles were resuspended in binding buffer (8 mM Na2HPO4, 2 mM KH2PO4, 150 mM NaCl, pH 7.4, containing 0.1% bovine serum albumin) to a final protein concentration of 1 mg/ml and stored at −70°C for blotting analysis.
Ligand blotting of binding proteins
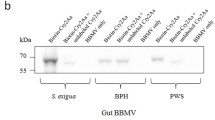
A total of 30 μg BBMV proteins of C. medinalis and P. subpiraticus were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membrane by Bio-Rad Trans Blot apparatus. The membrane was incubated with activated Cry1Ab (1 μg/ml) for 2 h (Liao et al. 2005). The bound Cry1Ab was detected using Cry1Ab monocolonal antibody (1:2000 dilution, v/v) followed by a second antibody (HRP-conjugated goat anti-mouse antibody, Sigma, USA). Color development was induced by incubation with TMB solution (Promega, USA). A negative control of each treatment was processed the same as above except without incubation with Cry1Ab.
Effect of Bt rice on survival, developmental time and fecundity of P. subpiraticus
Pirata subpiraticus has eight instars before reaching adulthood under the rearing chamber conditions. Pirata subpiraticus fifth instars were selected for survival and development tests, because the earlier instars have high natural mortalities (data not shown). Fifth instars were placed into glass vials individually and divided into three groups (group I, II and III). Each group had 60 P. subpiraticus fifth instars. Group I individuals were supplied with C. medinalis larvae that had fed on KMD1 rice for 1 day. Group II and III individuals were supplied C. medinalis larvae that had fed for 1 day on KMD2 and Xiushui11, respectively. Each P. subpiraticus nymph was supplied with two C. medinalis larva daily in each group until adult stage. The number of dead P. subpiraticus in each group was recorded daily. The total developmental time of each P. subpiraticus nymph from fifth instar to adult stage was also recorded. After P. subpiraticus reached the adult stage, females and males were paired within each group. After mating, females were put into glass vials individually and supplied with KMD1-fed C. medinalis larvae for group I, KMD2-fed C. medinalis larvae for group II, and Xiushui11-fed C. medinalis larvae for group III until the first egg bag of each P. subpiraticus was laid and hatched. The number of eggs per egg bag and egg hatching rate were recorded.
Effect of the Bt rice on P. subpiraticus population in fields
Bt rice, KMD1 and KMD2, were used for field studies at the Experimental Farm of Zhejiang University in Hangzhou City (120.12°E, 30.13°N), China in 2002, 2003 and 2004, together with their non-Bt isoline rice cultivar Xiushui11. Each year, Bt and non-Bt rice were transplanted 1 month after sowing. In 2002 and 2003, rice seeds were sown on May 27, and the seedlings transplanted on June 27 at the Experimental Farm. In 2004, seeds were sown on April 25, and the seedlings were transplanted on May 25. Each year, the field was divided into nine experimental plots in a three (treatments, KMD1, KMD2, and non-Bt) × 3 (replications) completely randomized design. Each experimental plot was 20 m × 25 m. Each plot was bordered on all sides by an unplanted 50 cm-wide earthen walkway. Seedlings were hand transplanted at one seedling per plant or hill spaced 16.5 cm × 16.5 cm apart, and the entire experimental field was surrounded by five border rows of the untransformed control plants. Normal cultural practices for growing rice, such as fertilization and irrigation, were followed during the course of the experiment except that no insecticides were applied after sowing and transplanting.
Population density of P. subpiraticus, a predominant predator, in different rice plots was evaluated using a vacuum-suction machine. The machine was based on a description by Carino et al. (1979), supplemented with a square sampling frame (50 cm × 50 cm × 90 cm high with a planar area of 2,500 cm2) made of Mylar sheets to enclose nine rice hills. Each year, samples were taken in all plots on a 15 ± 2 days schedule starting ca. 1.5 month after transplanting. On each sampling date, a square sampling frame was placed at random along a diagonal line of each tested plot, with five samples per plot. Arthropods inside the frame enclosure were removed using a vacuum-suction machine, and then transferred into a coded glass vial containing 75% ethanol. All samples were returned to the laboratory for counting the number of P. subpiraticus.
There were six sampling dates (from August to October) in 2002 and five sampling dates (from July to September) in 2003 and 2004.
Statistical analysis
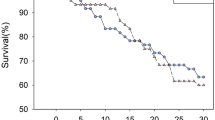
Data on the accumulative feeding areas and excretions of C. medinalis larvae on different rice plants and concentration of Cry1Ab in C. medinalis and P. subpiraticus were analyzed using GLM PROC in SPSS and Fisher’s protected LSD means separation test. Developmental time, the number of the eggs, egg hatching rate of P. subpiraticus supplied with Bt rice-fed C. medinalis larvae were also analyzed using GLM PROC. Survival analysis of P. subpiraticus on Bt rice-fed C. medinalis larvae was conducted using the Kaplan–Meier procedure and Logrank Test (Norusis 2005). Survival data were recorded until all P. subpiraticus nymphs either died or reached adulthood stages. All live P. subpiraticus adults were recorded as “surviving until the last recorded day”.
Population density of P. subpiraticus (means by sampling date) in different rice fields within each year was analyzed using GLM PROC. The rice type (Bt treatments) and sampling date variables were entered as fixed factors. Since Bt treatment may have a possible chronic effect on P. subpiraticus population, rice type × sampling date interaction was considered for population dynamics of P. subpiraticus, and was analyzed using two-way ANOVA (rice type verses sampling date). All count data were square root (X + 1) or log 10 (X + 1) transformed, as necessary, before univariate analysis, but untransformed means are presented. All statistical analyses were conducted using SPSS for Windows version 11.5 (SPSS Inc., Chicago, USA).
Results
Biotransfer and bioaccumulation of Cry1Ab from Bt rice to C. medinalis
Bt rice lines KMD1 and KMD2 significantly reduced C. medinalis larval food consumption, compared to non-Bt rice Xiushui 11 (F = 321.46, df = 2,135; P < 0.001). (Fig. 1). At day one, feeding areas of C. medinalis larvae on Xiushui11 rice plants were 276 mm2, and 11.38 and 7.24 mm2 on KMD1 and KMD2 rice plants, respectively (Fig. 1). The feeding areas of C. medinalis larvae on each type of rice did not significantly vary as the feeding times increased from 1 to 5 days (F = 1.706, df = 4,135; P = 0.152). Similarly, average excretion weight of C. medinalis larvae after feeding on different rice plants was significantly affected by rice type (F = 28.135, df = 4,135; P < 0.001) and feeding time (F = 10.022, df = 2,135; P < 0.001) (Fig. 2).
Cry1Ab was detected using ELISA in both KMD1 rice-fed and KMD2 rice-fed C. medinalis larvae (Fig. 3a). However, no Cry1Ab was detected from Xiushui11 rice-fed C. medinalis larvae. Concentrations of Cry1Ab in different Bt rice-fed C. medinalis larvae were not significantly affected by Bt rice type (KMD1 and KMD2) (F = 1.188, df = 1,20; P = 0.289), feeding time (F = 0.769, df = 4,20; P = 0.558) and the interactions of Bt rice and feeding time (F = 0.680, df = 4,20; P = 0.614). No Cry1Ab accumulation was found in C. medinalis as feeding time increased from 1 to 5 days. Cry1Ab was also detected from the excretions of KMD1 rice-fed and KMD2 rice-fed C. medinalis larvae. Rice type (F = 0.136, df = 1,20; P = 0.716), feeding times (F = 0.819, df = 4,20; P = 0.528) and interactions of rice type and feeding time (F = 0.167, df = 4,20; P = 0.953) did not significantly affect the presence of Cry1Ab in excretions (Fig. 4).
Presence of Cry1Ab toxin in C. medinalis (a) after feeding on Bt (KMD1 and KMD2) and non-Bt (Xiushui11) rice and P. subpriaticus (b) preying on Bt (KMD1 and KMD2) and non-Bt (Xiushui11) rice-fed C. medinalis for different time periods. No Cry1Ab was detected in Xiushui11-fed C. medinalis and P. subpriaticus preying on Xiushui11-fed C. medinalis
Biotransfer and bioaccumulation of Cry1Ab from Bt rice-fed C. medinalis to P. subpiraticus
Cry1Ab was detected from P. subpiraticus after preying on Bt rice (KMD1 and KMD2)-fed C. medinalis for 1 day (Fig. 3b). No Cry1Ab was detected from P. subpiraticus that fed on Xiushui11 rice-fed C. medinalis. Cry1Ab did not accumulate in P. subpiraticus as feeding time increased from 1 to 5 days. Rice type (F = 0.1.134, df = 1,20; P = 0.283), preying time (F = 0.768, df = 4,20; P = 0.579) and interactions of rice type and preying time (F = 0.432, df = 4,20; P = 0.712) did not significantly affect the presence of Cry1Ab in P. subpiraticus. A trace amount of Cry1Ab was also detected using ELISA from the excretions of P. subpiraticus that had fed on KMD1and KMD2 rice-fed C. medinalis larvae (data not shown).
Binding of Cry1Ab to C. medinalis and P. subpiraticus midgut BBMVs
BBMV proteins (30 μg) of C. medinalis and P. subpiraticus were prepared and separated by 10% SDS–PAGE (Fig. 5a), then transferred to a polyvinylidene difluoride membrane. In vitro binding of activated Cry1Ab (1 μg/ml) to C. medinalis and P. subpiraticus BBMV proteins is shown in Fig. 5. The results indicate that Cry1Ab binds to several BBMV proteins in the midgut of C. medinalis; however, Cry1Ab does not have binding receptors in BBMVs of P. subpiraticus (Fig. 5b).
Survival, developmental time and fecundity of P. subpiraticus
Survival probability of P. subpiraticus from the fifth instar to adult stage was not significantly affected by rice type (χ 2 = 2.240, df = 2, P = 0.326) (Table 1). Total developmental time of P. subpiraticus preying on KMD1-fed and KMD2-fed C. medinalis larvae was significantly longer than that on Xiushui11 rice-fed C. medinalis larvae (F = 69.588, df = 2,82; P < 0.001) (Table 1). In total 12, 14 and 16 pairs of P. subpiraticus adults were collected from group I (KMD1), II (KMD2) and III (Xiushui11), respectively. After P. subpiraticus adults were mated within each group, females were reared until eggs were laid. The number of eggs per egg bag (F = 0.133, df = 2,40; P = 0.876) and egg hatching rate (F = 2.244, df = 2,40; P = 0.112) of P. subpiraticus between Bt rice treatments and the control were not statistically different (Table 1).
Effect of the Bt rice on P. subpiraticus population in fields
Results of the 3-year field study indicated that KMD1 and KMD2 did no significantly affect P. subpiraticus populations in 2002 (F = 1.950, df = 2,72; P = 0.15), 2003 (F = 0.098, df = 2,60; P = 0.907), and 2004 (F = 0.246, df = 2,60; P = 0.783), compared to the non-Bt control Xiushui11 rice (Table 2). However, P. subpiraticus populations in different rice fields were significantly impacted by sampling date in each tested year, i.e., 2002 (F = 23.329, df = 5,72; P < 0.001), 2003 (F = 7.344, df = 4,60; P < 0.001), and 2004 (F = 3.301, df = 4,60; P = 0.016). In order to investigate whether Bt treatment had a possible chronic effect on P. subpiraticus population, rice type × sampling date interactions were analyzed. No significant impact was found for rice type × sampling date interaction on P. subpiraticus populations in 2002 (F = 1.360, df = 10,72; P = 0.216), 2003 (F = 1.403, df = 8,60; P = 0.214), and 2004 (F = 0.641, df = 8,60; P = 0.740).
Discussion
Development and commercialization of Bt plants have revolutionized insect pest management (Shelton et al. 2002). Since commercialization in 1996, the rate of adoption has been unprecedented in agriculture and in 2007 Bt crops were grown on 42.1 million ha worldwide (James 2007). However, their potential impact on nontarget organisms, especially natural enemies, continues to be the focus of considerable debate (Romeis et al. 2006; Marvier et al. 2007). Although many field studies to date have shown negligible impact on non-target organisms (Romeis et al. 2006; Marvier et al. 2007), some laboratory studies have shown negative effects (Ferry et al. 2003). So far, there are no clear universal guidelines for assessing the effects of Bt plants on selected non-target arthropods. A tiered system that has been adapted from the ecotoxicological discipline of plant protection products is currently suggested for such evaluation, which includes a first tier “worst-case” study under laboratory conditions, with additional studies if needed (Romeis et al. 2008).
Our “worst-case” study indicated that Cry1Ab toxin expressed in KMD1 and KMD2 rice plants could be transferred from Bt rice to the herbivore, C. medinalis, and then to the predator, P. subpiraticus (Figs. 3, 4). However, as the feeding time increased from 1 to 5 days, the presence of Cry1Ab did not show a tendency to bioaccumulate in different consumers (primary and secondary) belonging to the food chain comprising Bt rice, C. medinalis, and P. subpriaticus. In a biomass pyramid, it is possible that any toxin/pollutant in producers (plants) would move into primary consumers (herbivores) by feeding, then into secondary consumers (predators) by preying; thus bioaccumulation and biomagnification would take place (Serrano et al. 1997a, b). However, our results indicate that, instead of being bioaccumlated in the predator, the concentration of Cry1Ab in P. subpriaticus was ca. 10-fold lower than that in C. medinalis. First this might be due, in part, to the exudation of Cry1Ab by C. medinalis and P. subpriaticus (Fig. 3). Similarly, Bernal et al. (2002) found Cry1Ab in the honeydew of N. lugens after it fed on Bt rice. These results may suggest that water-soluble toxins (pollutants) usually cannot be bioaccumulated and biomagnified in a food chain because they can dissolve in the bodily fluids of the consumers and are excreted (McShaffrey 1995). Secondly, effective degradation of Cry1Ab toxin by crude protease extracts of P. subpriaticus midgut considerably reduced the amount of Cry1Ab in P. subpriaticus (Chen et al. 2005). In one previous study, D’Adamo et al. (1997) demonstrated that the differences of the pollutant bioaccumulation in the food chain comprising Dunaliella tertiolecta (microalga), Mytilus galloprovincialis (mussel) and Dicentrarchus labrax (fish) were caused by the efficient detoxification enzymatic system located in the liver of the fish. Similarly, Forcada et al. (1999) found that midgut enzymes of a Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae) strain resistant to Bt were able to process and degrade the Bt toxin in vitro, and to minimize the amount of the toxin present in the midgut lumen. Those results are in agreement with our findings that P. subpriaticus has an enzyme system in its midgut to prevent itself from being harmed by a Bt toxin.
Movement patterns and impacts on non-target organisms of Bt toxins through tritrophic interactions have been reported in some previous studies (reviewed by Romeis et al. 2006 and Chen et al. 2006). However, the ecological impact of Bt toxin movement has not always been clearly evaluated. Our laboratory feeding experiments demonstrated that Bt rice did not cause higher mortality in P. subpriaticus compared to non-Bt rice (Table 1). However, developmental time of P. subpriaticus preying on Bt rice-fed C. medinalis was significantly longer in comparison with non-Bt rice-fed C. medinalis. Since no binding receptors of Cry1Ab were found in the BBMVs of P. subpriaticus midgut (Fig. 5), it may be inferred that longer developmental time of P. subpriaticus was due to an indirect effect (i.e., host quality) instead of direct toxicity of Cry1Ab. It is unclear whether a slower developmental time could cause a chronic effect on P. subpriaticus population in fields. Our 3-year field trial indicates that P. subpriaticus population density was very similar between Bt and non-Bt rice fields. In addition, the interaction of rice type and sampling date did not significantly affect the overall P. subpriaticus population (Table 2).
Progress on the research and development of GM rice, especially Bt rice, in China has been rapid in recent years. More than 100 GM rice varieties have been in field testing in China by 2005 (Wang and Johnston 2007). However, to date, none of them has been approved for commercialization not only because of ecological risk concerns from domestic and international bodies, but because of political, economic, and trade concerns. From a standpoint of scientifically sound ecological risk assessments (ERA), the data we presented here used a tier-based method to rigorously evaluate the potential impact of Bt rice on an important non-target predator and did not find a negative direct effect. As the first study to investigate the possibility of bioaccumulation of Cry toxin used in Bt rice in a food chain and its ecological impact in the fields, our results present valuable information that can be used by regulatory agencies to develop scientifically based ERA policies and have broad implications for ecological safety of Bt plants.
References
Bernal JS, Griset JG, Gillogly PO (2002) Impacts of developing on Bt maize-intoxicated hosts on fitness parameters of a stem borer parasitoid. J Entomol Sci 37:27–40
Carino FO, Kenmore PE, Dyck VA (1979) The Farmcop suction sampler for hoppers and predators in flooded rice fields. Int Rice Res Newsl 4:21–22
Chen M, Ye GY, Lu XM, Hu C, Peng YF, Shu QY, Illimar A (2005) Biotransfer and bioaccumulation of Cry1Ab insecticidal protein in rice plant-brown planthopper-wolf spider food chain. Acta Entomol Sin 48:208–213
Chen M, Zhao JZ, Ye GY, Fu Q, Shelton AM (2006) Impact of insect-resistant transgenic rice on target insect pests and non-target arthropods in China. Insect Sci 13:409–420. doi:10.1111/j.1744-7917.2006.00110.x
Cheng X, Sardana R, Kaplan H, Altosaar I (1998) Agrobacterium-transformed rice plants expressing synthetic cry1A(b) and cry1A(c) genes are highly toxic to yellow stem borer and striped stem borer. Proc Natl Acad Sci USA 95:2767–2772. doi:10.1073/pnas.95.6.2767
Cohen M, Chen M, Bentur JS, Heong KL, Ye GY (2008) Bt rice in Asia: potential benefits, impact, and sustainability. In: Romeis J, Shelton AM, Kennedy G (eds) Integration of insect-resistant GM crops within IPM programs. Springer, Dordrecht, pp 223–248
D’Adamo R, Pelosi S, Trotta P, Sansone G (1997) Bioaccumulation and biomagnification of polycyclic aromatic hydrocarbons in aquatic organisms. Mar Chem 56:45–49. doi:10.1016/S0304-4203(96)00042-4
FAO (2007) FAOSTAT <http://faostat.fao.org/site/567/default.aspx> accessed September 1, 2007
Ferry N, Edwards MG, Mulligan EA, Emami K, Petrova A, Frantescu M, Davison GM, Gatehouse AMR (2003) Engineering resistance to insect pests. In: Christou P, Klee H (eds) Handbook of plant biotechnology. Wiley, Chichester, pp 373–394
Forcada C, Akcácer EM, Garcerá NDM, Tato A, Martínez R (1999) Resistance to Bacillus thruingiensis cry1Ac toxin in three strains of Heliothis virescens: proteolytic and SEM study of the larval midgut. Arch Insect Biochem Physiol 42:51–63. doi:10.1002/(SICI)1520-6327(199909)42:1<51::AID-ARCH6>3.0.CO;2-6
High SM, Cohen MB, Shu QY, Altosaar I (2004) Achieving successful deployment of Bt rice. Trends Plant Sci 9:286–292. doi:10.1016/j.tplants.2004.04.002
Huang JK, Hu RF, Rozelle S, Pray C (2005) Insect-resistant GM rice in farmers’ fields: assessing productivity and health effects in China. Science 308:688–690. doi:10.1126/science.1108972
James C (2007) Global status of commercialized biotech/GM crops: 2007. ISAAA briefs, no. 37, ISAAA: Ithaca, NY
Liao CY, Trowell SC, Akhurst R (2005) Purification and characterization of Cry1Ac toxin binding proteins from the brush border membrane of Helicoverpa armigera midgut. Curr Microbiol 51:367–371. doi:10.1007/s00284-005-0051-9
Marvier M, McCreedy C, Regetz J, Kareiva P (2007) A meta-analysis of effects of Bt cotton and maize on non-target invertebrates. Science 316:1475–1477. doi:10.1126/science.1139208
Matteson PC (2000) Insect pest management in tropical Asian irrigated rice. Annu Rev Entomol 45:549–574. doi:10.1146/annurev.ento.45.1.549
McShaffrey D (1995) Leaders in environmental activism-trophic levels. http://www.marietta.edu/~mcshaffd/lead/trophic.pdf. pp 1–6. Accessed January 20, 2008
Norusis M (2005) SPSS 13.0 advanced statistical procedure companion. Prentice Hall, Upper Saddle River, pp 103–122
Obrist LB, Dutton A, Albajes R, Bigler F (2006) Exposure of arthropod predators to Cry1Ab toxin in Bt maize fields. Ecol Entomol 31:143–154. doi:10.1111/j.0307-6946.2006.00762.x
Romeis J, Meissle M, Bigler F (2006) Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat Biotechnol 24:63–71. doi:10.1038/nbt1180
Romeis J, Bartsch D, Bigler F, Candolfi M, Gielkens MMC, Hartley SE et al (2008) Non-target arthropod risk assessment of insect-resistant GM crops. Nat Biotechnol 26:203–208. doi:10.1038/nbt1381
Serrano R, Hernández F, López FJ, Peña JB (1997a) Bioconcentration, depuration and chronic toxicity of the organophosphorus pesticide chlorpyrifos in the marine molluse Mytilus edulis. Arch Environ Contam Toxicol 33:47–52. doi:10.1007/s002449900222
Serrano R, Hernández F, López FJ, Peña JB (1997b) Study on bioconcentration of chlorpyrifos, chlorfenvinfos and methidathion in Mytilus galloprovincialis. Relationships with physicochemical properties and biotransformation. Bull Environ Contam Toxicol 59:968–975. doi:10.1007/s001289900577
Shelton AM, Zhao JZ, Roush RT (2002) Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu Rev Entomol 47:845–881. doi:10.1146/annurev.ento.47.091201.145309
Sheng CF, Wang HT, Gao LD, Xuan JW (2003) The occurrence status, damage cost estimate and control strategies of stem borers in China. Plant Prot 29:37–39
Shu QY, Ye GY, Cui HR, Xiang YB, Gao MW (1998) Development of transgenic Bacillus thuringiensis rice resistant to rice stem borers and leaf folders. J Zhejiang Agric Uni 24:579–580
Wang YQ, Johnston S (2007) The status of GM rice R&D in China. Nat Biotechnol 25:717–718. doi:10.1038/nbt0707-717
Wolfersberger MG, Luethy P, Maurer A, Parenti P, Sacchi VF, Giordana B, Hanozet GM (1987) Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp Biochem Physiol 86A:301–308. doi:10.1016/0300-9629(87)90334-3
Xiang YB, Cheng X, Liang Z, Shu QY, Ye GY, Gao MW, Altosaar I (1999) Agrobacterium-mediated transformation of insecticidal Bacillus thuringiensis cry1Ab and cry1Ac genes and their expression in rice. Chin J Biotechnol 15:494–500
Ye GY, Shu QY, Cui HR, Gao MW, Xia YW, Cheng XY, Altosaar I (2000) A leaf-section bioassay for evaluating rice stem borer resistance in transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner. Bull Entomol Res 90:179–182. doi:10.1017/S0007485300000298
Ye GY, Shu QY, Yao HW, Cui HR, Cheng XY, Hu C, Xia YW, Gao MW, Altosaar I (2001) Field evaluation of resistance of transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner to two stem borers. J Econ Entomol 94:270–276
Ye GY, Yao HW, Shu QY, Cheng XY, Hu C, Xia YW, Gao MW, Altosaar I (2003) High levels of stable resistance in transgenic rice with a synthetic cry1Ab gene from Bacillus thuringiensis Berliner to rice leaffolder, Cnaphalocrocis medinalis (Guenée) under field conditions. Crop Prot 22:171–178. doi:10.1016/S0261-2194(02)00142-4
Acknowledgments
We thank Dr. I. Altosaar (University of Ottawa) for providing activated Cry1Ab toxin and Dr. D. B. Zhang (College of Agriculture and Biology, Shanghai Jiaotong University, China) for providing the monoclonal antibody of Cry1Ab. Financial supports from the National Program on Key Basic Research Projects (973 Program, 2007CB109202), the Ministry of Science and Technology of China, the National Natural Science Foundation of China (39970507) and the Special Foundation for the Winner of National Excellent Doctoral Dissertation, the Ministry of Education of China (199944) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, M., Ye, Gy., Liu, Zc. et al. Analysis of Cry1Ab toxin bioaccumulation in a food chain of Bt rice, an herbivore and a predator. Ecotoxicology 18, 230–238 (2009). https://doi.org/10.1007/s10646-008-0276-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0276-z