Abstract
An aquatic risk assessment under the U.S. Environment Protection Agency (EPA) ecological risk framework was conducted for atrazine, metolachlor, malathion, chlorpyrifos, and endosulfan in the C-111 freshwater basin (eastern boundary of the Everglades National Park), northeast Florida Bay, and south Biscayne Bay in South Florida. Based on the use of the hazard quotient approach, measured concentrations of chlorpyrifos and endosulfan in surface waters suggest potential hazards to aquatic organisms and were, therefore, considered as chemicals of potential ecological concern (COPECs). The problem formulation included an overview of the physical/chemical and environmental fate characteristics and aquatic toxicology of the COPECs. Background surface water exposure concentrations of endosulfan and toxicity data from laboratory and field studies indicate that fish and invertebrate mortality may be a concern when endosulfan is applied in agricultural areas near aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1996, the Department of the Interior (DOI) prepared a report entitled “A Comprehensive Plan for the Restoration of the Everglades,”comprising four main elements: (1) federal legislative authority for restoration activities; (2) accelerated state and federal land acquisition; (3) increased scientific research to guide restoration; and (4) federal, state, and private sector cost sharing (http://www.sfrestore.org/tf/otherres/comp.html). In 2000, Congress passed the Comprehensive Everglades Restoration Plan (CERP) as a part of the Water Resources Development Act (1996). The goal of the CERP is to restore and preserve the hydrology of the pre-drainage Everglades ecosystem, to protect the quality of the remaining habitat, to promote the return of populations of plants and animals, and to foster human development compatible with sustaining a healthy ecosystem.
Biological changes in the Everglades have been linked to levels of phosphorus and mercury and to changes in the complex hydrological patterns of the natural system resulting from water management projects to control floods and water distribution (Science Subgroup 1996). In fact, alterations in the hydrologic system are thought to be the main cause of dramatic declines of fish and wildlife populations because of habitat changes. Therefore, the basic premise behind all restoration activities identified by the Interagency Restoration Task Force for South Florida is that hydrologic restoration is a prerequisite to achieve ecosystem restoration and a sustainable South Florida Ecosystem. The restoration plan was, thus, formulated to reconstruct some key features of the natural hydrologic system in order to restore conditions that support landscape patterns, biodiversity, wildlife abundance, and clean and abundant water. In the past, little consideration, however, was given in the restoration effort to the role that organic pesticides and other contaminants play in the structure and function of ecosystems, although this was clearly recommended by the Science Subgroup (1996) in all physiographic regions that comprise South Florida. This was further supported at a workshop entitled “Linking Ecotoxicity and Risk Management to Sustainable Restoration of South Florida Ecosystems,” which recommended screening-level ecological risk assessments with retrospective and prospective diagnostic studies (LaPoint et al. 1998).
It is evident that water quantity rather than water quality issues have dominated the South Florida restoration planning (Scott et al. 2002). However, it is also evident that agriculture represents a major land use in South Florida and pesticide use presents a potential risk, especially to aquatic organisms. Based on a hazard ranking of pesticides by the National Oceanic and Atmospheric Administration (NOAA), the top three estuarine drainage areas at risk in the U.S. were in Florida-Rookery Bay, Biscayne Bay, and Tampa Bay (Pait et al. 1992). The subtropical climate, long crop-growing season, application frequency, and multitude of uses (e.g., mosquito and termite control, golf courses, and landscape management) renders pesticides particularly hazardous in South Florida ecosystems.
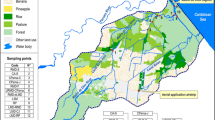
The Canal 111 (Aerojet Canal or C-111) freshwater basin (Fig. 1) is a buffer zone that separates the wetlands of the Everglades National Park (ENP) from highly productive subtropical agricultural lands and urban development to the east, and while considerable attention and resources have been allocated to altering the hydrology of this landscape, little effort has focused on understanding water quality issues that may arise from land use practices. Thus far, analytical monitoring programs have detected the presence of organic pesticides in the surface water of either the lower C-111 freshwater canal basin and/or its confluent estuaries/saltwater systems. For example, the South Florida Water Management District (SFWMD) began monitoring pesticides in the water and sediment of South Florida canals in the mid-1980s (Pfeuffer 1985, 1991). Sediment and water analyses by SFWMD indicates that atrazine, ametryn, bromacil, simazine, diuron, alpha (α)-endosulfan, beta (β)-endosulfan, endosulfan sulfate, ethion, hexazinone, and norflurazon were the most frequently detected pesticides in surface water and DDE, DDD, ametryn, atrazine, dicofol, diquat, and endosulfan sulfate were the most frequently detected pesticides in sediment samples between 1991 and 1995 (Miles and Pfeuffer 1997). Several of the sampling sites were located in the Everglades Agricultural Area (EAA) and others in the Homestead Agricultural Area (HAA) adjacent to the Everglades National Park. Detectable endosulfan residues (α and β isomers, and sulfate metabolite) in the C-111 (at S-178) were consistently present in the surface water from 1991 to 1995, and occasionally exceeded the Florida Department of Environmental Protection (FDEP) and U.S. Environment Protection Agency (EPA) water quality criteria (Miles and Pfeuffer 1997). Surface water samples collected independently by the NOAA from the southern SFWMD sampling sites confirmed these findings (Scott et al. 1994). Residues of endosulfan sulfate were also consistently found by the SFWMD in sediment samples at S-178 in the C-111, while the α and β isomers were occasionally found. All three endosulfan residues were also found in sediment samples from structures S-177 and S-18C in the C-111. The SFWMD summarized endosulfan sulfate residues in S-178 water and found that the FDEP water quality criteria were exceeded 11 times from 1996 to 2000 (two samples were from NOAA; R. Pfeuffer, personal communication). In a SFWMD monitoring program of South Florida canals from 1992 to 2001, the most common pesticides in surface water were the herbicides atrazine and ametryn, while DDE and DDD were the most frequently detected pesticides in sediment samples (Pfeuffer and Rand 2004). The U.S.EPA, in 1995, also monitored contaminants in surface water, sediment, and biota in the C-111 and creeks of northeast Florida Bay (Goodman et al. 1999). Endosulfan residues were detected in sediments of the C-111, and in sediments of Shell and Trout Creeks (in northeast Florida Bay). Organochlorine contaminants occurred at low concentrations in sediments of canals and creeks, and PCBs and PAHs were also at low concentrations but higher in the C-111 than in creeks. At most sampling sites for water and sediment, more than one pesticide was detected in each sample.
The NOAA conducted sediment toxicity tests and a contaminant monitoring study of the C-111 and Florida Bay from 1993 to 1997, but it did not evaluate cause (exposure)–effect (toxicity) relationships for contaminants (Scott et al. 2002). It did however; indicate the presence of low levels of endosulfan (total), atrazine, chlorpyrifos, and chlorothalonil in surface waters of canals adjacent to agricultural areas that drain into the C-111 and in northeast Florida Bay waters. Florida Bay waters occasionally exceeded the U.S.EPA marine water quality criterion (WQC) for endosulfan. Waters from canal sites also contained detectable concentrations of endosulfan that sporadically exceeded U.S.EPA fresh WQC. Detectable endosulfan (total) residues were also found in sediment and oysters, while chlorpyrifos was detected in fish tissue. Toxicity tests with in-place sediment and copepods and bivalves indicated potential adverse effects, but the causative agent(s) was not determined. The highest concentration of endosulfan (total) reached 477 ng/l, and 10% of the samples from canal sites exceeded the U.S.EPA chronic freshwater WQC (56 ng/l) (47% of the canal sites had detectable levels of endosulfan). The U.S.EPA chronic marine WQC (8.7 ng/l) for endosulfan was exceeded at 2% of the sites in Florida Bay, while 39% of bay sites had detectable endosulfan concentrations. The highest percentage (40%) of water quality violations for endosulfan, based on U.S.EPA standards, was detected in samples from S-178. The NOAA, in 1999–2000, also found that endosulfan concentrations were highest (mean dry season concentration ~300 ng/l) in the C-111E (Fulton et al. 2004).
Data from the NOAA’s National Status and Trends (NS&T) Program Mussel Watch Project further indicated that the mean annual concentrations of endosulfan (II, β-isomer) residue in tissues (oysters) sampled from Joe Bay (in northeast Florida Bay) were higher than the NS&T 85th percentile (i.e., it is in the highest 15% of the data set, with over 280 sites nationwide) (Cantillo et al. 1999). Recently, Harman-Fetcho et al. (2005) found that atrazine, metolachlor, chlorothalonil, chlorpyrifos, and total endosulfan (α- + β- + endosulfan sulfate) were the most frequently detected pesticides in water samples from South Florida canals based on a 2-year study from 2002 to 2004. Atrazine had the highest concentration (108 ng/l), followed by endosulfan (total; 98 ng/l), metolachlor (86 ng/l), chlorpyrifos (58 ng/l), and chlorothalonil (14 ng/l). In addition, Carriger et al. (2006) identified DDT, DDD, DDE, chlordane, and endosulfan (total) as chemicals of potential ecological concern (COPECs) in the sediment of South Florida canals based on the exceedence of sediment quality criteria in a two-tier sediment probabilistic risk assessment. Endosulfan had the highest potential risk (chronic) to arthropods at S-178 on the C-111 system.
To address the concerns about pesticides in the C-111 basin, the National Park Service (U.S. DOI) requested that an aquatic probabilistic screening level ecological risk assessment (SERA) be conducted. It focuses on the risk of adverse effects from pesticide exposures in surface water on aquatic organisms in a freshwater canal (C-111 system) and its confluent estuarine/saltwater systems (northeast Florida Bay–Joe Bay, Long Sound, Highway Creek; Card Sound in South Biscayne Bay). To date, this is the only site-specific SERA conducted as part of the Everglades restoration effort, and it is being “exposure-driven” (Suter 1993). Presently, there is little evidence that documented pesticide exposures in surface water are eliciting adverse biological effects in aquatic receptors in these systems or on their potential risk. This SERA applied the ecological risk assessment (ERA) framework under the current U.S.EPA guidelines (U.S.EPA 1998) and it addresses the likelihood and ecological significance of the potential effects of surface water exposures to the herbicides atrazine and metolachlor, and the insecticides malathion, chlorpyrifos, and endosulfan obtained from monitoring programs from the U.S. Geological Survey (USGS), the SFWMD, and the NOAA. These pesticides were detected by the various monitoring programs with the highest frequency in the C-111 system and northeast Florida Bay and south Biscayne Bay. There have been chemical-specific aquatic ecological risk assessments conducted thus far on atrazine (Solomon et al. 1996; Giddings et al. 2000), chlorpyrifos (Giesy et al. 1999; Hall and Anderson 2003), and endosulfan (U.S.EPA 2002a, 2007).
This SERA was intended to assess the potential risk of these pesticides in surface water, and not to determine the actual causes of any declines in the populations of native invertebrates, fish, or plants that may be prevalent in the above ecosystems. Since each pesticide is not used in isolation to control pests and may co-occur; potential risks associated with the effects of joint actions of these pesticides were also considered. Assessment of the potential impact of the five pesticides on aquatic communities acknowledges that sea level rise, hurricanes, development, historical water management activities, and salinity changes may have altered habitats and affected populations. Furthermore, other chemical, non-chemical stressors in water and/or sediment are not being considered and may likely also contribute to adverse impacts in the study areas. Because the SERA contained a large database, the results are presented in two papers. This paper discusses the general ecological risk assessment methods and the results of Tier 1—hazard assessment and problem formulation for the pesticides in the C-111 system and northeast Florida Bay and south Biscayne Bay. The subsequent paper discusses the methods and results of Tier 2—probabilistic analyses of the chemicals (i.e., pesticides) of potential ecological concern (COPECs) in the C-111, northeast Florida Bay, and south Biscayne Bay.
Study area description
The C-111 basin (100 square miles) is located in southeastern Dade County, Florida, adjacent to the eastern boundary of the Everglades National Park (ENP) (Fig. 1). It includes lands that lie to the southeast boundary of the East Everglades and west of the coastal basins and includes the Frog Pond (i.e., agricultural area). It, thus, drains the agricultural areas of South Dade County. South of Homestead, the C-111 is joined by the C-111E and it then moves south and southeastward to cross marl marsh, which flows into Manatee Bay at the head of Barnes Sound, a semi-enclosed lagoonal estuary of south Biscayne Bay. Surface water runoff from the C-111 basin represents an important source of freshwater flow into the ENP and the estuarine ecosystems of northeast Florida Bay through Taylor Slough and south Biscayne Bay through the S-197 (in C-111) into Manatee Bay. Low tidal range and long flushing times make Manatee Bay particularly vulnerable to the effects of large freshwater inflows. Such pulses of freshwater persist for long periods of time and move within and between the shallow estuaries that make up South Biscayne Bay and its associated sounds (Fatt and Wang 1987). Unfortunately, low tidal range and long flushing times also make Manatee Bay and Barnes Sound vulnerable to hyper-saline conditions during periods of reduced freshwater flow. During the dry season, saltwater moves inland and the Western shore of south Biscayne Bay frequently experiences high salinities (Wang et al. 1978). Northeast Florida Bay includes the downstream freshwater marshes and estuarine systems that extend from the southern edge of Barnes Sound on the east to Madeira Bay on the west, and include Little Madeira Bay, Joe Bay, Highway Creek, and Long Sound.
Florida Bay is a triangular-shaped estuary composed of basins, banks, and islands that lie between the southern tip of the Florida mainland and the Florida Keys. It has a shallow depth (mean 1 m) that is perfect for light penetration and the sustainability of seagrass beds, which are a dominant habitat and a source of productivity in the Bay. The salinity of the Bay can rise to twice that of seawater as a result of the long residence time and shallow depth (McIvor et al. 1997). The sediments of the Bay are composed of carbonate mud, which sorb inorganic phosphorus from water (de Kanel and Morse 1978). The Bay was healthy until the mid-1980s, when catches of pink shrimp (Penaeus duorarum) declined (Browder et al. 1999) and the mass mortality of turtle grass (Thalassia testudinum) began (Robblee et al. 1991). By the 1990s, the Bay ecosystem appeared to shift from a clear water system dominated by benthic primary production to a turbid system dominated by algal blooms and resuspended sediment. Although there has been no dramatic decrease in total fish abundance, there has been a shift in species composition as a result of seagrass habitat loss and algal blooms (Davis and Ogden 1997). Fish that consume algae, such as the bay anchovy, are increasing.
When large volumes of freshwater are discharged from the C-111 canal, this water tends to move into coastal waters and estuaries that can create local problems due to the transfer of freshwater, contaminants, and suspended materials from urban and agricultural land. Freshwater inflow from the C-111 and surface runoff further transports nutrients and detritus from adjacent marshes and uplands into south Biscayne Bay and northeast Florida Bay. Sampling sites for water monitoring programs in the C-111, Florida Bay, and Biscayne Bay are labeled in Fig. 1. The Miami River is an additional source of contaminants into Biscayne Bay (Long et al. 2002).
In the 1960s, the C-111 area was channelized as part of the comprehensive Central and Southern Florida (C&SF) Flood Control Project. At that time, this area was envisioned as urban development, but by the 1980s, it was clear that the C-111 drainage system, which had undergone several revisions, had significantly contributed to a decline in the natural resources of the ENP. The current revision of the system, authorized in 1996, promises to restore some of the natural hydropatterns to Taylor Slough, the eastern panhandle areas of the ENP, and improve estuarine conditions in Florida Bay (project description can be found at http://www.saj.usace.army.mil/dp/mwdenp-c111/index.htm).
Methods
The SERA consisted of the first three phases of the U.S.EPA ERA framework (U.S.EPA 1998): problem formulation, analysis, and risk characterization. Problem formulation defined the problem and the plan for analyzing and characterizing the risk. Data on stressor characteristics, ecosystems at risk, ecological effects, ecosystem(s), and receptor(s) characteristics were synthesized for this phase. From this data, assessment (i.e., what we are trying to protect) and measurement (i.e., tools used to measure effects on assessment endpoints) and a conceptual model were developed to prepare the analysis plan (i.e., where risk hypotheses were evaluated). The conceptual model at the completion of problem formulation uses information on the ecosystems at risk, stressor characteristics, biological effects, and the relationship between endpoints to define exposure and effects scenarios. The objective of the conceptual model is to formulate hypotheses to determine how the pesticide stressors may affect ecosystems that are exposed.
The second phase of the SERA was risk analysis and it characterized and examined two major components of risk; exposure and effects. Risk characterization was the final phase. This provided potential risk estimates to the ecological entities listed as assessment endpoints based on the occurrence and magnitude of exposures and the severity of adverse effects resulting from such exposures. Analyses (exposure and effects characterization) and risk characterization are discussed in the follow-up paper.
A tiered ecological risk characterization approach was suggested by the ARAMDG (SETAC 1994) and endorsed by the U.S.EPA (ECOFRAM 1999) that uses a stepwise approach progressing from the simple Tier 1 hazard quotient (HQ) approach to a more complex Tier 2 probabilistic risk assessment (PRA). A two-tier approach was used in the SERA.
In Tier 1, the HQ approach was first used with a screening benchmark, followed by problem formulation. Screening benchmarks are concentrations of chemicals that are believed to constitute thresholds for the potential toxic effects of some ecological receptor exposed to a chemical in some medium (Suter and Tsao 1996). The U.S.EPA Water Quality Criteria (WQC) and Sediment Quality Criteria (SQC) are commonly used as screening benchmarks because the exceedence of one of these values constitutes cause for concern. In the SERA, actual measured environmental concentrations (AECs) of the pesticides in surface waters were compared to the U.S.EPA WQC values that were available (i.e., endosulfan, chlorpyrifos, malathion, and atrazine) to obtain an HQ. No WQC were available for metolachlor. Therefore, the AECs of metolachlor were compared to the response concentration for the most sensitive species in a toxicity test (i.e., from the lowest LC50/EC50, lowest NOEC from chronic tests) to obtain an HQ.
AECs in surface waters were obtained from monitoring programs from state (SFWMD) and federal (NOAA, USGS) agencies for 1999–2000. Site numbers and the location of sampling sites where pesticide concentrations (atrazine, chlorpyrifos, endosulfan, malathion, and metolachlor) were measured with land use characteristics are shown in Fig. 1. The SFWMD did not measure for chlorpyrifos, the NOAA did not measure for malathion, and the USGS did not measure for endosulfan. Monitoring data were available for 11 freshwater sites (S-175, S-176, S-332, Site A, S-177/site B, S-178/site C, S-18C/site E, Site W1, Site W2, Site E1, and Site E2) on or near the C-111 and three estuarine sites (Joe Bay and Highway Creek in northeast Florida Bay and Card Sound in south Biscayne Bay). Sites in W1, W2, E1, E2, Highway Creek, and Joe Bay were located in the Everglades panhandle.
When the quotient of the exposure concentration to the criteria value (or lowest acute toxicity value for metolachlor) was greater than 1, an adverse effect (i.e., high hazard) was expected to occur. For endosulfan, there are separate freshwater and saltwater criteria for α- and β-endosulfan, but no criterion exists for endosulfan sulfate, a toxic oxidation metabolite. Since the endosulfan WQC was generated from aquatic toxicity studies with technical-grade endosulfan, each criterion is applicable to the summation of the α- and β-isomers (U.S.EPA 2002b). We were, therefore, conservative in Tier 1 and compared total endosulfan concentrations (i.e., summation of concentrations of α and β isomers plus the endosulfan sulfate metabolite) to the criterion to obtain an HQ. HQ exceedences in Tier 1 were then used to focus on COPECs for problem formulation and Tier 2. Tier 2, probabilistic risk assessment, characterizes risk by comparing the probability distributions of surface water exposure concentrations with the probability distributions of species response data from laboratory toxicity studies. Results for Tier 1—hazard assessment and problem formulation are discussed below.
Results and discussion
Tier 1—hazard assessment
Sample sites, number of samples collected, and the frequency of detection for each pesticide in freshwater and estuarine sites are presented in Table 1 for the two-year period (1999–2000). Sites with exceedences of WQC for each pesticide are listed in Table 2. The herbicide atrazine was the most frequently detected pesticide. It was detected in 92% of the 185 freshwater samples in 1999 and 100% of the 106 samples taken in 2000. The highest detected concentration of atrazine in freshwater was 0.337 μg/l (at S-18C/site E). It was detected in 88% of the 24 estuarine samples in 1999 and 81% of the 26 samples taken in 2000. The highest detected concentration of atrazine in saltwater was 0.104 μg/l (at Joe Bay). Concentrations of atrazine did not exceed freshwater or marine WQC. Acute and chronic HQs were low and indicated no ecological hazard to fresh- or salt-water organisms.
The other herbicide measured, metolachlor, was only detected in 28% of the 185 freshwater samples in 1999 and 26% of the 106 samples taken in 2000. It was not detected in 24 estuarine samples in 1999 and was only detected in 12% of the 26 samples taken in 2000. The acute and chronic HQs for metolachlor in freshwater and saltwater were close to zero when the peak exposure concentrations of the herbicide were compared with the lowest toxicity values. The pesticide with the lowest number of detections was malathion. Malathion was found about 4% of the time at freshwater sites and 0% of the time at estuarine sites, respectively, in 1999 and 2000. Atrazine, metolachlor, and malathion were not COPECs and, therefore, were not considered for Tier 2 single chemical probabilistic risk assessments. However, they were considered as potential co-joint (additive) stressors in Tier 2 when they were present at detectable concentrations.
Chlorpyrifos was detected in 48% of the 89 freshwater samples in 1999 and 85% of the 91 samples taken in 2000. It was detected in 79% of the 24 estuarine samples in 1999 and 96% of the 26 samples taken in 2000. The two highest concentrations for chlorpyrifos were found at S-177/site B at 0.0234 and 0.0232 μg/l, which were nearly four times higher than the next highest maximum concentration, which was measured at W2, where it was found 86% of the time. The maximum concentration value for chlorpyrifos was found during the dry season in February 1999. The only water quality violation for chlorpyrifos occurred in Joe Bay in 1999. The acute and chronic HQs for freshwater indicated no potential hazard, but the acute HQs for estuarine water indicated potential hazard. Although there was only one WQC violation for chlorpyrifos, it was considered as a COPEC because several AECs were just below WQC.
Endosulfan was detected in 45% of the 173 freshwater samples in 1999 and 90% of the 93 samples taken in 2000. It was detected in 96% of the 24 estuarine samples in 1999 and 96% of the 26 samples taken in 2000. Endosulfan concentrations were detected infrequently at S-176 (1 out of 32), S-332 (2 out of 32), and S-175 (0 out of 26). The highest concentration of endosulfan was found at S-178/site C in February 2000, followed by S-177/site B, where concentrations peaked in the dry season of 1999 and 2000. S-18C/site E had the third highest detected concentrations for endosulfan, which occurred in February 1999 and 2000. E1, W1, and W2, which are downstream of S-177/site B and S-178/site C, had 100% detections for endosulfan. Water quality violations for endosulfan were found in freshwater and estuarine sites.
The majority of violations occurred at S-178/site C, a site closest to the Frog Pond agricultural area. Out of 266 samples taken for analyses of endosulfan in the C-111 during 1999 and 2000, 7.5% violated freshwater WQC. Of the 20 water quality violations at S-178/site C, eight did not have detectable concentrations of α-endosulfan. However, the highest concentration (1.345 μg/l) had the highest percentage concentration of α-endosulfan and the lowest percentage concentration of endosulfan sulfate. In general, concentrations of β-endosulfan were also low. Except for one sample, the majority of total endosulfan at S-178/site C sample violations was made up of endosulfan sulfate (72–100% of each sample). The fact that endosulfan sulfate is the major metabolite of endosulfan in aquatic systems supports other recent work (Laabs et al. 2007; Shivaramaiah et al. 2005).
In estuarine sites, endosulfan water quality violations were found at all three sites sampled and, out of 50 samples taken at Highway Creek or Joe Bay, 20% violated saltwater WQC for endosulfan. In these same sites, endosulfan sulfate made up 71–94% of the total endosulfan of samples with water quality violations. The acute and chronic HQs for total endosulfan in freshwater indicated potential hazards and the acute HQ for estuarine water also indicated potential hazards. Endosulfan generally had the highest measured concentrations in freshwater and estuarine sites at the end of the dry season for each year.
Measured concentrations of chlorpyrifos and endosulfan in surface waters suggest potential hazards, therefore, the problem formulation contained an overview of their characteristics and Tier 2 focused on a PRA of these two insecticides for the C-111 and the estuarine sites.
Problem formulation
Stressor characteristics
The following section summarizes the physical and chemical characteristics and environmental fate chemistry of the two COPECs chlorpyrifos and endosulfan. This includes a summary of the various factors that influence their degradation, persistence, and transport in the aquatic environment. A review of the results of aquatic toxicity studies is also included, along with the results from previous ecological risk assessments.
For additional information on the environmental fate and aquatic toxicity of chlorpyrifos, see the reviews by Barron and Woodburn (1995) and Odenkirchen and Eisler (1988), the ecological risk assessments by Giesy et al. (1999) and Hall and Anderson (2003), and the environmental chemistry summary by Racke (1993). Additional aquatic toxicity and environmental fate information can also be obtained for endosulfan from the U.S.EPA reregistration eligibility decision (RED) (U.S.EPA 2002a) and the reviews by the NRCC (1975), U.S.EPA (1980), and Goebel et al. (1982).
Chlorpyrifos
Chlorpyrifos, [O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)-phosphorothionate], is a sulfur-bearing organophosphate (OP) insecticide and one of the most widely used in the United States because it possesses a broad spectrum of activity against a wide range of arthropod and insect pests (U.S.EPA 2000). It is commonly known as Dursban and Lorsban. Direct toxicity results from metabolic activation to form chlorpyrifos oxon with inactivation of acetylcholinesterase at the synapse (Barron and Woodburn 1995).
Of all OP insecticides, chlorpyrifos has the highest national agricultural usage (Larson et al. 1997). Florida has high chlorpyrifos usage, along with California, Washington, Georgia, Arizona, Nebraska, Iowa, Illinois, and Wisconsin (U.S.EPA 2000). In Florida, chlorpyrifos is used on tomatoes, corn, cotton, grapefruit, oranges, pecans, peaches, peanuts, sod, soybeans, sweet corn, and tobacco (FDOACS 1999). Of these crops, sweet corn is cultivated intensively in the Everglades region, with 24,400 reported acres, and 42,000 lbs a.i. chlorpyrifos are applied annually on sweet corn throughout the state (FDOACS 1999). In 1995, applications of chlorpyrifos on crops and in industrial settings were almost equivalent (Nowell et al. 1999). Recently, according to the Florida Department of Agriculture and Consumer Services (FDOACS 2003), an estimated 289,128 lbs of chlorpyrifos active ingredient was used on 18 different Florida crops. In South Florida, chlorpyrifos is also applied on or near golf courses and in residential areas (Scott et al. 2002).
Chemical/physical properties and environmental fate
Chlorpyrifos has low water solubility (~1.0 mg/l), moderate volatility (2×10−5 mm Hg at 25°C), and moderate hydrophobicity (log K oc of ~4, log K ow of ~5), and it sorbs fairly strongly to soils (U.S.EPA 2000). It, therefore, has a propensity to partition to organic matrices in aquatic systems, with little tendency to exist in dissolved form in surface waters. Abiotic and biotic degradation of chlorpyrifos in water leads to 3,5,6-trichloro-2-pyridinol (TCP), which is hydrolytically degraded in water in less than one hour (Dilling et al. 1984). Racke (1993) reports water half-lives of ≤5 days and sediment half-lives of ≤16.3 days for chlorpyrifos. In water, the half-life of chlorpyrifos is affected through sediment/particle binding, biodegradation, volatilization, hydrolysis, and photolysis (Giesy et al. 1999).
Aquatic toxicity
Chlorpyrifos is acutely toxic to freshwater and saltwater fish and invertebrates at <5.0 μg/l (Giesy et al. 1999; Mayer and Ellersieck 1986; Odenkirchen and Eisler 1988). Toxicity is higher with increased temperature and pH. Generally, aquatic crustaceans and insect larvae are the most sensitive and mollusks, annelids, and rotifers are the least sensitive aquatic species in laboratory and field studies (Giesy et al. 1999). Fish are less sensitive to chlorpyrifos than invertebrates in both acute and chronic exposures. Sublethal effects in freshwater and saltwater species include the inhibition of acetylcholinesterase (ACHE) activity in brain and blood, equilibrium loss, reduction in growth, and reproductive impairment. The latter effects were noted at exposure concentrations <1.0 μg/l (Odenkirchen and Eisler 1988). Small acute-to-chronic ratios and low persistence for chlorpyrifos indicate that short-term acute exposures rather than long-term chronic exposures are most likely to cause toxic effects in the field (Giesy et al. 1999).
Bioconcentration factors (BCFs) of chlorpyrifos for fish and invertebrates are in the range from about 50 to 6,000 and are less than that predicted from bioconcentration models because of biotransformation to polar metabolites such as TCP (Giesy et al. 1999). Chlorpyrifos will partition from the aqueous to solid organic phases in the environment, limiting exposures, indicating that laboratory bioconcentration studies may overestimate the BCF.
Freshwater pond and mesocosm studies reviewed in the North American aquatic ERA for chlorpyrifos indicated that concentrations less than 0.1 μg/l should not pose a risk to aquatic invertebrates (Giesy et al. 1999). Concentrations greater than 0.2 μg/l affect invertebrate species, but population recovery was often observed in 2–8 weeks. Concentrations greater than 0.5 μg/l affected the survival and growth of some fish species. For conservatism, 0.1 μg/l was chosen as the benchmark no observed adverse effect concentration (NOAEC) for the protection of ecological structure and function from exposures to chlorpyrifos (Giesy et al. 1999). This value was supported by field studies in outdoor mesocosms/ditches conducted by Van den Brink et al. (1996) and Van Wijngaarden et al. (1996).
Based on the available acute and chronic core and supplementary studies in the Reregistration Eligibility Decision (RED), the U.S.EPA Environmental Fate and Effects Division (EFED) found chlorpyrifos to be toxic to aquatic invertebrates and fish (U.S.EPA 2000). The acute and chronic hazard quotients utilized by the U.S.EPA indicated that chlorpyrifos is a concern for freshwater and estuarine fish. This was based on many typical usage scenarios in exposure modeling. Acute risks for amphibian tadpoles were also of concern to EFED based on peak EECs (estimated environmental concentrations) from modeling data. For all possible uses of chlorpyrifos, chronic reproductive effect values were exceeded by 21-day EECs. For freshwater and estuarine aquatic invertebrates, hazard quotients surpassed acute and chronic concern levels for all outdoor uses.
The North American ERA showed that, in high usage cornbelt areas (i.e., Midwest, Lake Erie, California, and various other agricultural and urban watersheds), the acute 10th centile (effects benchmark) or the NOAEC from field/mesocosm studies (0.1 μg/l) was exceeded in less than 10% of any of the Lake Erie areas for chlorpyrifos with sufficient monitoring data. Freshwater fish acute toxicity 10th centiles from species’ sensitivity distributions were not exceeded by exposure concentrations in Lake Erie for any year monitored. However, when maximum exposure concentrations were compared to acute 10th centiles for freshwater arthropods “they suggested that, in some rivers in this drainage basin, and in some years, significant effects on invertebrates could occur.” In addition, in small tributaries and agricultural basins in California, there was a likelihood that threshold concentrations for invertebrates would be exceeded, but not for fish.
Hall and Anderson (2003) recently conducted a probabilistic analyses of chlorpyrifos surface water data in the San Joaquin River (California) from 1991 to 2001 and showed that the California Department of Fish and Game acute and chronic criteria for all sites (n=49) was exceeded in 13.7% and 19.1%, respectively. The probability of exceeding acute and chronic criteria was greater at pooled tributary than main stem sites.
Endosulfan
Endosulfan, commercially known as Thiodan®, is a sulfur-bearing chlorinated hydrocarbon of the cyclodiene subgroup. Technical endosulfan is a mixture of two stereoisomers; α (70%) and β (30%) endosulfan (NRCC 1975). It is used as an insecticide and acaricide on a wide variety of row crops, fruits, nuts, vegetables, and cotton. Cyclodiene pesticides disrupt nervous system function by preventing chloride ions from entering neurons through the inhibition of GABA receptors (Ware 1991). The main transformation product identified in the environment is endosulfan sulfate, but endosulfan diol, endosulfan lactone, and endosulfan hydroxy carboxylic acid also appears in lesser quantities.
Endosulfan has been detected in surface water, groundwater, and sediment (U.S.EPA 2002a). It is one of the most ubiquitous organochlorine insecticides in the atmosphere (Shen et al. 2005). There is evidence for the long-range transport of endosulfan and endosulfan sulfate because there are reported concentrations in environmental matrices from the Arctic regions (http://www.amap.no/). Results from the global monitoring network for persistent organic pollutants (POPs) published in 2006 also revealed that endosulfan is abundant and its use has increased (Pozo et al. 2006; Harner et al. 2006). The U.S.EPA STORET database (http://www.epa.gov/storet/) indicates surface water detections of one or more endosulfan residues in 38 states in the U.S., with the highest number of endosulfan detections in California, Florida, Louisiana, Washington, Mississippi, and Ohio. In the surface water database (SURF) from the California Department of Pesticide Regulation Environmental Hazard Assessment Program (EHAP), endosulfan sulfate had the highest detection frequency compared to parent endosulfan and β-endosulfan (CDPR 2000). The presence of endosulfan is well documented in the National Sediment Quality Survey, which reports endosulfan residues in stream sediments in 30 out of 76 watersheds from 12 states in the U.S. (U.S.EPA 1997). The U.S.EPA evaluation of the National Sediment Contaminant Point Source Inventory (NSI) from 1980 to 1999 in the U.S. also indicates that the number of detections of β-endosulfan was about three times more than that for α-endosulfan and endosulfan sulfate in sediment, with also a significantly higher concentration range (U.S.EPA 2001).
Endosulfan usage within the South Florida Water Management District, which contains 16 counties in South Florida, was approximately 36 tons annually (Miles and Pfeuffer 1997). In the vicinity of the Biscayne Bay watershed alone, approximately 36,562 lbs of endosulfan were applied per year (Pait et al. 1992). Endosulfan is utilized on tomatoes and squash in the Biscayne Bay region (Pait et al. 1992). Recently, the total amount of active ingredient endosulfan used in Florida was estimated by the Florida Department of Agriculture and Consumer Services to be 98,302 lbs (FDOACS 2003).
Chemical/physical properties and environmental fate
Endosulfan is not soluble in water (60–100 μg/l @ 25°C) and it has a moderate volatility (1×10−5 mmHg @ 25°C) (ATSDR 2000). α-Endosulfan has been found to be more volatile and less persistent than β-endosulfan, which has a higher volatility than the oxidation product endosulfan sulfate (NRCC 1975; Goebel et al. 1982). Volatilization may be a major source of removal for endosulfan from the aquatic environment. The log K ow (>3–4.8) for α- and β-endosulfan and endosulfan sulfate indicates a potential for bioconcentration in aquatic organisms (Ney 1998; German Federal Environment Agency 2007).
The hydrolysis of both isomers in alkaline solutions (pH > 7) is likely to be an important degradation route from aqueous systems (NRCC 1975; U.S.EPA 2002a). Endosulfan diol is the major decomposition product from alkaline hydrolysis (Goebel et al. 1982). Endosulfan sulfate is the major oxidation product expected in slightly acidic waters under aerobic conditions (NRCC 1975). At a pH of 7, both isomers hydrolyze with half-lives less than three weeks, but at a pH of 9, both isomers have half-lives less than six hours. Under acidic conditions, both isomers are stable to hydrolysis. For example, the reported half-lives in water with pH < 7 were over a month (Callahan et al. 1979). Persistence typically increases at pH < 7 and under anaerobic conditions (NRCC 1975; U.S.EPA 2002a). Furthermore, α- and β-endosulfan isomers are resistant to photolysis in water (U.S.EPA 2002a; Callahan et al. 1979; Goebel et al. 1982), while the sulfate and diol metabolites are susceptible to photolysis (http://www.inchem.org/documents/hsg/hsg/hsg017.htm).
In the soil environment, both α- and β-endosulfan and endosulfan sulfate have an affinity to sorb to soil and are, thus, not expected to be mobile (U.S.EPA 2002a). The distribution coefficient, K d, between soil and water of the β-isomer is higher than that of the α-isomer (Peterson and Batley 1993; U.S.EPA 2002a). Zhou et al. (2003) also found that α-endosulfan had a lower distribution coefficient than β-endosulfan. The endosulfan K d in soil and sediment increases as organic carbon content increases (Peterson and Batley 1993; Parkpian et al. 1998). The organic carbon normalized sorption coefficient (K oc) is about 10,600, 13,500, and 12,400 for the α-, β-isomers, and endosulfan (technical), respectively, with variation between soils (U.S.EPA 2002a; Wauchope et al. 1992). All K ocs indicate that parent endosulfan and both isomers are hydrophobic and strongly sorbed to soils and sediments. Endosulfan and its isomers will, thus, have preference for sediment (Peterson and Batley 1993) or particulates in the aquatic environment (Greve and Wit 1971).
Degradation by a variety of fungi and bacteria in soils is the major route of endosulfan degradation, and endosulfan sulfate is the primary oxidation transformation product (NRCC 1975). The half-lives in acidic to neutral soils under aerobic conditions of α-endosulfan range from one to two months and for β-endosulfan from three to nine months (U.S.EPA 2002a). Endosulfan sulfate appears to be as persistent as the parent compound under aerobic conditions. Actual field dissipation studies also indicate that endosulfan will persist in surface soils for months after application (U.S.EPA 2002a). For example, in field dissipation studies, the DT50 for endosulfan sulfate from soils in Spain (Hardy 2001) and Greece (Balluff 2001) were 75 days and 47–161 days, respectively.
The residual activity of α- and β-isomers and endosulfan sulfate in soils indicate that repeated applications of endosulfan will produce accumulation, with potential carry-over from year-to-year (U.S.EPA 2002a). This suggests that surface water runoff and surficial soils may serve as a source for receiving waters long after application. Recently, it was shown that the loss of α-endosulfan dissolved in runoff water and through leaching was higher than that of β-endosulfan, while the loss of α-endosulfan from runoff sediments was lower than that of β-endosulfan (Zhou et al. 2003).
Although several metabolites of endosulfan, i.e., sulfate, diol, ether, hydroxy ether, and lactone, have been shown to occur (Callahan et al. 1979; Goebel et al. 1982), endosulfan sulfate is typically the major metabolite in aquatic systems (Shivaramaiah et al. 2005) and in tissues (Lehotay et al. 1998).
Bioconcentration
The reported log K ow for α- and β-isomers and endosulfan sulfate (~3–4.8) indicate potential for bioconcentration in aquatic organisms. Bioconcentration studies were available for seven fish species: sheepshead minnow (Cyprinodon variegatus), zebra fish (Brachydanio rerio), yellow tetra (Hyphessobrycon bifasciatus), striped mullet (Mugil cephalus), pinfish (Lagodon rhomboids), long whiskers catfish (Mystus gulio), and spot (Leiostomus xanthurus) (U.S.EPA 2007). According to the U.S.EPA, the methodology in these studies did not meet all of the standard criteria (i.e., achieved steady-state, measurement and stability of exposure concentrations, analytical confirmation of parent and metabolites) for a bioconcentration study under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA). The two highest quality studies, based on meeting some of these three criteria, indicate that the BCF range for fish is 1,000 (striped mullet; Schimmel et al. 1977) to 3,000 (sheepshead minnow; Hansen and Cripe 1991). Depuration half-lives in fish for α- and β-endosulfan and endosulfan sulfate were 2–6 days.
Bioconcentration studies were available for five species of invertebrates: blue mussel (Mytilus edulis), grass shrimp (Palaemonetes pugio), oyster (Crassostrea madrasensis), clam (Katelysia opima), and red swamp crayfish (Procambarus clarkii). Bioconcentration studies with invertebrates and endosulfan indicate a BCF range of 12–600. More recently, an average BCF of 2,682 and 3,278 was determined for freshwater green algae (Pseudokirchneriella subcapitatum) and the cladoceran (Daphnia magna), respectively (DeLorenzo et al. 2002). D. magna neonates accumulated little endosulfan when exposed via the ingestion of contaminated phytoplankton. Therefore, it appeared that uptake from water is the dominant route for endosulfan bioconcentration in zooplankton. Information on depuration in invertebrates was limited.
There were no data available on the bioconcentration of endosulfan sulfate in aquatic organisms.
Aquatic toxicity—laboratory
Endosulfan (technical) is acutely toxic to aquatic organisms with a range of 0.1 for striped bass (Morone saxatilis) to 166 μg/l for Daphnia magna (U.S.EPA 2002a). The latter acute toxicity range does not include tests used in the U.S.EPA (1980) water quality criteria document, which were conducted mostly under static conditions. Acute toxicity values for saltwater organisms are generally lower than those for freshwater organisms. For example, the 96-h LC50s for two freshwater invertebrates, scuds (Gammarus lacustris) and stoneflies (Pteronarcys sp.), were 5.8 and 3.3 μg/l, respectively (Johnson and Finley 1980). However, the 96-h LC50s for saltwater species such as grass shrimp (Palaemonetes pugio) and the amphipod (Gammarus palustris) were 0.62 μg/l (Wirth et al. 2001) and 0.43 μg/l (Leight and Van Dolah 1999). There is limited data for aquatic organisms on the effects of chronic exposure to endosulfan. The chronic data available indicate that the no observed effect concentrations for fathead minnows (Pimephales promelas) and the cladoceran Daphnia magna were 0.2 and 2.0 μg/l, respectively. The most sensitive endpoints were reduced growth and survival. The literature shows that α-endosulfan may be more acutely toxic than β-endosulfan and endosulfan sulfate to fish and invertebrates in static water tests, but a combination of (α + β)-endosulfan plus endosulfan sulfate appears to be more toxic than any single isomer, especially to H. azteca, where the combination decreased growth and reduced survival at 0.37–4.84 mg endosulfan/Kg sediment (Wan et al. 2005). The estimated 28-d LC50s for the (α + β)-endosulfan combination and endosulfan sulfate to H. azteca were 0.83 and 1.73 mg/kg, respectively, and 0.36 mg/kg for the (α + β)-endosulfan plus endosulfan sulfate combination.
Recently, laboratory acute toxicity data for endosulfan sulfate generated by the registrants in support of the RED on endosulfan indicate that, for freshwater and estuarine fish, the metabolite is equally toxic to the parent compound (U.S.EPA 2007).
The 10-d sediment LC50 values for the freshwater midge (Chironomus tentans) were 0.96, 3.24, and 5.22 μg/goc (normalized to TOC), while the LC50 values for the amphipod (Hyalella azteca) were 51.7, >1,000, and 873 μg/goc for α-endosulfan, β-endosulfan, and endosulfan sulfate, respectively (You et al. 2004). It appears that, in sediment, α-endosulfan may also be more toxic than β-endosulfan and endosulfan sulfate to benthic organisms. Differences in acute toxicity of the two isomers may be accounted for by the greater binding affinity of the β-isomer to sediment than the α-isomer, which is more bioavailable. The U.S.EPA (2007) also presented recent acute sediment toxicity data for 10-day tests with endosulfan sulfate and showed that, in C. tentans and Leptocheirus plumulosus, the NOAEC was 2.7 and 27 μg/l (pore water), based on growth and survival, respectively. The 28-day chronic NOAEC for L. plumulosus was 1.58 μg/l, based on growth.
Aquatic toxicity—field studies and risk assessments
Case studies on the detection of endosulfan in surface waters indicate a range of values. The results of a monitoring program on insecticide loss to stream water from agricultural areas in Ontario, Canada, showed the presence of endosulfan at a low range of 0.01–0.17 μg/l from agricultural watersheds (Frank et al. 1982) to a high range of 0.11–2.0 μg/l from rural ponds (Frank et al. 1990). In addition, investigators found that, in farm ditches in British Columbia, Canada, endosulfan concentrations ranged from a low of 0.01–13.4 μg/l (Wan et al. 1995) to 1,530 μg/l (Wan 1989). Endosulfan concentrations in the Lourens River, South Africa, ranged from a low range of 0.03–0.16 μg/l (Schulz et al. 2001a) to a high of 8.5–12.3 μg/l (Schulz et al. 2001b). Fulton et al. (1999) also found that endosulfan ranged from <0.1 to >0.8 μg/l at estuarine sites in South Carolina. Lehotay et al. (1998) found that surface water samples of two tributaries of the Chesapeake Bay, Maryland, contained up to 0.035 μg/l (Patuxent River) and up to 0.225 μg/l (Choptank River) of endosulfan (β). The latter concentration exceeded the U.S.EPA freshwater water quality criteria (0.056 μg/l) for endosulfan. Approximately 54% of the total residue detected was endosulfan sulfate. Muir et al. (2004) showed the presence (pg/l) of α- and β-endosulfan from four temperate lakes in south-central Canada with no agriculture areas within 30 miles, suggesting the atmospheric transport and deposition of endosulfan.
Schulz (2004) showed that, when concentrations of 23 insecticides from field studies (~70) were compared to their water quality guideline for the protection of aquatic life, the water quality guideline for endosulfan was exceeded more frequently in field studies than all other insecticides.
Table 3 lists the available field studies on the effects of endosulfan in surface waters. In most of the studies, there appeared to be no clear relationship between endosulfan exposure and the effects observed. In some studies, the results are based on experimental exposure (i.e., direct spraying, endosulfan injection, or actual application). Alternatively, in some studies, the results are based on non-point-source pollution events monitored during and/or after normal farming practice. There is also a distinction between effects on organisms exposed in situ, which reflects an artificial, experimental exposure, and effects on abundance, drift, and community dynamics in the field under actual field exposures.
What is clear from the acute toxicity studies conducted in the field and the U.S.EPA STORET 90th centile exposure concentration for endosulfan (0.31 μg/l) and the California Department of Pesticide Regulation 95th centile exposure concentration range for the sulfate, parent, and β-endosulfan (0.07–0.14 μg/l) is that fish and invertebrate mortality in the field may be a real concern when endosulfan is applied in agricultural areas near aquatic systems. The latter gains in importance because a review of the Ecological Incident Information System (EIIS; U.S.EPA 1994) shows that, since 1971 with 91 reported aquatic incidents, endosulfan accounted for the majority (62%) of the cyclodiene incidents. The majority (96%) of the incidents were in the aquatic environment; 82% were fish-related, and 7% were related to macroinvertebrates. The highest percentage of reported incidents were from California, Louisiana, North Carolina, and South Carolina. Since the U.S.EPA (1994) incident data, an additional 18 incidents (15 involving aquatic and three involving terrestrial organisms) have been reported associated with endosulfan use and mostly in California (U.S.EPA 2007).
For the endosulfan RED, the U.S.EPA conducted two primary risk assessments for effects on nontarget aquatic organisms (U.S.EPA 2002a). The first utilized hazard quotients (HQs) from standard toxicity tests. For freshwater fish, acute and chronic HQs were above levels of concern (LOCs), with ranges from 1.2 to 23 for acute effects and from 0.5 to 29 for chronic effects. Acute HQs for freshwater aquatic invertebrates were above LOCs, with ranges from 0.17 to 3.3. Freshwater aquatic invertebrate chronic HQs were also above LOCs, with ranges from 1.1 to 61. HQs for estuarine and marine fish and invertebrates were higher than HQs for freshwater species. Acute HQs for estuarine and marine fish ranged from 9.8 to 191. Chronic HQs for estuarine and marine fish ranged from 5 to 316. For estuarine and marine invertebrates, acute and chronic HQs were in the ranges of 2.2–42 and 1.6–85, respectively. Unlike chlorpyrifos, ranges for HQs in the assessment for endosulfan were higher for freshwater and saltwater species of fish than for freshwater and saltwater species of invertebrates from standard toxicity tests. From the distribution of acute freshwater fish HQs and Monte Carlo simulations to predict exceedences from typical usages in crop scenarios, acute LOCs set by the U.S.EPA would be exceeded 99% of the time by acute HQs for seven out of eight crops modeled.
In the second phase of the endosulfan RED, the U.S.EPA conducted a probabilistic assessment of aquatic risk. Modeling data used application rates for the compound with a 300-ft spray drift buffer. The resulting joint probability curves predicted that, for a scenario involving tomato sprayings in Florida, there is a 50% probability that at least 75% of aquatic species would experience mortality. For a spraying scenario involving apples, there was a 50% probability that at least 5% of aquatic species would experience mortality.
Ecosystems potentially at risk
The ecosystems addressed in the SERA were the C-111 freshwater basin, northeast Florida Bay, and south Biscayne Bay. To determine the areas that were potentially at risk, we considered: (1) where, when, and at what quantities the pesticides were found; and (2) stressor characteristics. Most of the analytical data from surface water exposure monitoring programs were for the C-111 system and secondarily for northeast Florida Bay.
Assessment endpoints
The U.S.EPA (1998) provides three criteria for the selection of assessment endpoints: (1) ecological relevance, (2) susceptibility to the known or potential stressors, and (3) relevance to management goals. This leads to the following general assessment endpoints for freshwater and estuarine sites in Tier 2:
-
1.
Survival and production of algae, periphyton, and seagrasses provide habitat, food, and energy for consumers and control the diversity of consumers within the different ecosystems. One of Florida Bay’s major ecological attributes that is both an indicator of its health and is important to society is the seagrass community. They are a productive base for the food web, a habitat for higher trophic levels, and a regulator of the Bay’s water quality (i.e., through nutrient uptake, binding of sediments by their roots, and trapping of particles through their leaf canopy).
-
2.
Survival and function of microbial decomposers essential to the recycling of nutrients in sediments and surface waters of the ecosystems.
-
3.
Survival and production of arthropods (e.g., pink shrimp) which provide food in and on sediments. Florida Bay is a nursery ground for pink shrimp in that it supports the shrimp fishery of the Tortugas. They are a component of the diet of gamefish and wading birds, and are also an indicator of the Bay’s productivity.
-
4.
Survival and production of invertebrate herbivores that exert functional control over the primary producers.
-
5.
Survival and production of fish that exert functional control over the primary producers and primary consumers (herbivores). Biscayne Bay and Florida Bay sport-fishing is of economic importance to the region.
The specific assessment endpoint for Tier 2 is the protection of at least 90% of the species 90% of the time (10th centile from species sensitivity distributions) from acute and chronic stressor exposures (Solomon et al. 1996). A total of five general assessment endpoints are described above. Only assessment endpoints 1, 3, and 5 are being considered in light of both single-chemical and multiple-chemical exposures. These endpoints are in keeping with the management goals set forth by the Science Subgroup (1996). Microbial decomposers, invertebrate herbivores, and seagrasses cannot be addressed because of the limited toxicity databases for these groups. Not presently included within the assessment endpoints are any specific endangered or threatened species.
Conceptual model
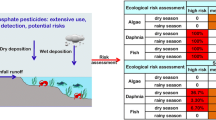
The major uses of the pesticides in the C-111 basin (e.g., Frog Pond) are for agriculture. Spraying is the major form of application that may lead to the transport of the pesticides to surface waters through surface runoff and drift. The highest concentration of pesticides would be expected to be in the C-111 canal system and, to a lesser extent, in the shallow coastal estuarine sites directly following drift. Chlorpyrifos, and especially endosulfan (the sulfate form), may be expected at estuarine sites because they are more hydrophobic and, thus, may be transported sorbed to sediment from freshwater to saltwater. Because pesticide input would occur mainly during spraying and secondarily as a result of surface runoff events (e.g., especially endosulfan and chlorpyrifos), and because they dissipate rapidly in surface water, the pesticides would be expected to be present in intermittent pulses, rather than continuously. Defining the spatial and temporal exposure distribution and risk of the five pesticides in the C-111 system and estuarine sites was a major objective of the SERA. The site conceptual exposure model is illustrated in Fig. 2.
The pesticides being considered here have specific receptor-mediated modes of toxicity. Therefore, aquatic organisms vary in their sensitivity to the five pesticides based on possessing the target site for the pesticide. Therefore, aquatic plants (e.g., algae, periphyton, seagrass) are more likely to be affected by the herbicides (i.e., atrazine, metolachlor) and invertebrates and fish are more likely to be affected by the insecticides (i.e., malathion, chlorpyrifos, endosulfan). There are limited toxicity data for amphibians, periphyton, invertebrate herbivores, microbial decomposers, and seagrass. Chlorpyrifos, malathion, and endosulfan exposure may reduce or eliminate some invertebrate populations, and possibly cause indirect effects on fish. Indirect effects may also occur as a result of predators consuming contaminated prey. Only the risk associated with the direct effects of pesticides was assessed in the SERA.
The SERA focused on phytoplankton/plants, arthropods, and fish. The seasonal timing of the pesticide usage, especially the insecticides, coincide with the spawning periods (January, February, March) of fish species (i.e., sensitive life stages) in freshwater areas. Important invertebrate prey as a food resource may also be affected. This is especially relevant when these prey are critical to the growth and survival of fish early life stages. The site conceptual effects model is illustrated in Fig. 3.
Measurement endpoints are measurable ecological characteristics related to the valued characteristic chosen as the assessment endpoint (U.S.EPA 1998). These are the focus in the SERA and they link the assessment endpoint and attributes measured to the characterization of risk potentials.
Based on the conceptual model of potential exposure and effects, the following questions (as risk hypothesis), including measurement endpoints, were addressed in Tier 2:
-
1.
What is the likelihood that the pesticide concentrations in the C-111 system and related estuarine sites will be high enough to cause acute effects? (Measurement of effect: survival of test organisms in acute laboratory tests.)
-
2.
What is the likelihood that the pesticide concentrations are high enough and stable enough in water to cause chronic effects on the survival, growth, and/or reproduction of organisms? (Measurement of effect: survival and NOECs of growth, and reproduction of test organisms in chronic laboratory tests.)
The first and second questions are related to assessment endpoints 1, 3, 4, and 5. Assessment endpoint 2 was not addressed because of insufficient microbial toxicity information. All questions were addressed in light of single- and multiple-chemical pesticide exposures in water. If the pesticides do produce acute and/or chronic toxic effects on aquatic organisms, the following questions will be examined:
-
1.
At what sites and when are the effects likely to be the greatest?
-
2.
Which trophic groups are at the greatest risk?
-
3.
If some species are likely to be affected, are these species critical food organisms, such that, for example, reductions in invertebrate populations will affect fish growth and survival?
Questions 1 and 2 will be addressed, but question 3 will not be examined. Questions 1 and 2 were addressed because, in the C-111 system, northeast Florida Bay, and south Biscayne Bay, hazard to aquatic receptors was predicted for fresh- and salt-water organisms as separate groups, and sampling sites in these systems were also analyzed separately during the dry (February) and wet (June) seasons to determine where the risks were greatest.
Conclusions
Tier 1—hazard assessment and problem formulation of an aquatic risk assessment under the U.S.EPA ecological risk framework were summarized for pesticides in the C-111 freshwater basin (eastern boundary of the Everglades National Park), northeast Florida Bay, and south Biscayne Bay. For hazard assessment, the hazard quotient approach was used, in which the surface water exposure concentrations of malathion, atrazine, endosulfan, and chlorpyrifos from monitoring studies were compared to the U.S.EPA water quality criteria (WQC). No WQC were available for metolachlor, therefore, the surface water concentrations were compared to the response for the most sensitive species in a toxicity test. Based on the results of hazard assessment, the measured surface water concentrations of chlorpyrifos and endosulfan suggest potential hazards. Both insecticides were classified as chemicals of potential ecological concern (COPECs) and were then considered in the problem formulation.
Based on measured concentrations of atrazine, metolachlor, and malathion, there was no indication of hazard to aquatic organisms in surface waters. The problem formulation phase of the risk assessment contained an overview of their physical/chemical and environmental fate characteristics and aquatic toxicity. The problem formulation also addressed the ecosystems at risk, endpoints (measurement and assessment), and a conceptual model for risk assessment. What is clear from the literature review on endosulfan surface water concentrations in the field and the results of aquatic laboratory and field toxicity studies is that, when endosulfan is applied for agricultural purposes near aquatic ecosystems, adverse effects on fish and invertebrates is a concern, especially following acute exposures. The subsequent paper presents the probabilistic aquatic risk assessment of endosulfan and chlorpyrifos, individually and jointly with atrazine, malathion, and metolachlor, for the C-111 freshwater basin, northeast Florida Bay, and south Biscayne Bay.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (2000) Toxicological profile for endosulfan. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA
Balluff M (2001) Field soil dissipation of AE F002671 (endosulfan) following a single application to bare (preemergence) cotton plots at 1 location in Greece. Aventis Crop Science Study 20003033/GR1-FS
Barron MG, Woodburn KB (1995) Ecotoxicology of chlorpyrifos. Rev Environ Contam Toxicol 144:1–93
Barry MJ, Davies W (2004) Effects of invertebrate predators and a pesticide on temporary pond microcosms used for aquatic toxicity testing. Environ Pollut 131:25–34
Barry MJ, Logan DC (1998) The use of temporary pond microcosms for aquatic toxicity testing: direct and indirect effects of endosulfan on community structure. Aquat Toxicol 41:101–124
Browder JA, Restrepo VR, Rice JK, Robblee MB, Zein-Eldin Z (1999) Environmental influences on potential recruitment of pink shrimp, Farfantepenaeus duorarum, from Florida Bay nursery grounds. Estuaries 22:484–499
Buchmann MF (1999) NOAA screening quick reference tables, NOAA HAZMAT report 99-1. National Oceanic and Atmospheric Administration, Coastal Protection and Restoration Division, Seattle, WA, 12 pp
California Department of Pesticides (CDPR) (2000) Memorandum. Recommendation for priority surface water monitoring studies on selected pesticides. Available online at: http:///www.cdpr.ca.gov/docs/emon/pubs/ehapreps/m080200.pdf
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR, Jennings P, Durfree RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, Gould C (1979) Water-related environmental fate of 129 priority pollutants: I. Introduction and technical background, metals and inorganics, pesticides and PCBs. EPA-440/4-79-029a, U.S.EPA, Office of Water, Washington, DC
Cantillo AY, Lauenstein CG, O’Connor TP, Johnson WE (1999) Status and trends of contaminant levels in biota and sediments of South Florida. National Oceanic and Atmospheric Administration, National Ocean Service, National Centers for Coastal Ocean Science, Center for Coastal Monitoring and Assessment, Silver Spring, MD
Carriger JF, Rand GM, Gardinali PR, Perry WB, Tompkins MS, Fernandez AM (2006) Pesticides of potential ecological concern in sediment from south Florida canals: an ecological risk prioritization for aquatic arthropods. Soil Sed Contam 15:21–45
Davis SM, Ogden JC (1997) Everglades, the ecosystem and its restoration. St. Lucie Press, Boca Raton, FL
de Kanel J, Morse JW (1978) The chemistry of orthophosphate uptake from seawater on to calcite and aragonite. Geochim Cosmochim Acta 42:1335–1340
DeLorenzo ME, Scott GI, Ross PE (1999) Effects of the agricultural pesticides atrazine, deethylatrazine, endosulfan, and chlorpyrifos on an estuarine microbial food web. Environ Toxicol Chem 18:2824–2835
DeLorenzo ME, Taylor LA, Lund SA, Pennington PL, Strozier ED, Fulton MH (2002) Toxicity and bioconcentration potential of the agricultural pesticide endosulfan in phytoplankton and zooplankton. Arch Environ Contam Toxicol 42:173–181
Dilling WL, Lickly LC, Lickly TD, Murphy PG, McKellar RL (1984) Organic photochemistry. 19. Quantum yields for O,O-diethyl O-(3,5,6-trichloro-2-pyridinyl) phosphorothioate(chlorpyrifos) and 3,5,6-trichloro-2-pyridinol in dilute aqueous solutions and their environmental phototransformation rates. Environ Sci Technol 18:540–543
ECOFRAM (1999) Ecological committee on FIFRA risk assessment methods: report of the aquatic workgroup. U.S. Environmental Protection Agency, Office of Pesticide Programs, Washington, DC
Ernst WR, Jonah P, Doe K, Julien G, Hennigar P (1991) Toxicity to aquatic organisms of off-target deposition of endosulfan applied by aircraft. Environ Toxicol Chem 10:103–114
Faria MS, Nogueira AJA, Soares AMVM (2007) The use of Chironomus riparius larvae to assess effects of pesticides from rice fields in adjacent freshwater ecosystems. Ecotoxicol Environ Saf 67:218–226
Fatt JC, Wang JD (1987) Canal discharge impacts on Biscayne Bay salinities. Research/Resources Management Report SER-89, United States Department of the Interior, National Park Service, Southeast Region, Atlanta, GA
Fischer R (1994) Simulated or actual field testing: a comparison. In: Graney RL, Kennedy JH, Rodgers JH Jr (eds) Aquatic mesocosm studies in ecological risk assessment. Lewis Publishers, Boca Raton, FL
Florida Department of Agriculture, Consumer Services (FDOACS) (1999) Summary of agricultural pesticide usage in Florida: 1995–1998. FDOACS, Division of Agricultural Environmental Services, Bureau of Pesticides, Tallahassee, FL
Florida Department of Agriculture, Consumer Services (FDOACS) (2003) Summary of agricultural pesticide usage in Florida: 1999–2002. FDOACS, Division of Agricultural Environmental Services, Bureau of Pesticides, Tallahassee, FL
Fox PJ, Matthiessen P (1982) Acute toxicity to fish of low-dose aerosol applications of endosulfan to control tsetse fly in the Okavango Delta, Botswana. Environ Pollut Ecol Biol 27:129–142
Frank R, Braun HE, Holdrinet MVH, Sirons GJ, Ripley BD (1982) Agriculture and water quality in the Canadian Great Lakes basin. V. Pesticide use in 11 agricultural watersheds and presence in stream water, 1975–1977. J Environ Qual 11:497–505
Frank R, Braun HE, Ripley BD, Clegg BS (1990) Contamination of rural ponds with pesticide, 1971–85, Ontario, Canada. Bull Environ Contam Toxicol 44:401–409
Fulton MH, Moore DW, Wirth EF, Chandler GT, Key PB, Daugomah JW, Strozier ED, Devane J, Clark JR, Lewis MA, Finley DB, Ellenberg W, Karnaky KJ Jr, Scott GI (1999) Assessment of risk reduction strategies for the management of agricultural nonpoint source pesticide runoff in estuarine ecosystems. Toxicol Ind Health 15:201–214
Fulton MH, Scott GI, DeLorenzo ME, Key PB, Bearden DW, Strozier ED, Madden CJ (2004) Surface water pesticide movement from the Dade County agricultural area to the Everglades and Florida Bay via the C-111 canal. Bull Environ Contam Toxicol 73:527–534
German Federal Environment Agency (2007) Endosulfan: draft dossier prepared in support of a proposal of endosulfan to be considered as a candidate for inclusion in the Annexes to the Stockholm Convention. Umweltbundesamt, Germany. February 2007
Giddings JM, Anderson TA, Hall LW, Hosmer AJ, Kendall RJ, Richards RP, Solomon KR, Williams WM (2000) Aquatic ecological risk assessment of atrazine: a tiered probabilistic approach. A report of an expert panel. Report no. 709–00. Novartis Crop Protection, Inc., Greensboro, NC
Giesy JP, Solomon KR, Coats JR, Dixon KR, Giddings JM, Kenaga EE (1999) Chlorpyrifos: ecological risk assessment in North American aquatic environments. Rev Environ Contam Toxicol 160:1–129
Goebel H, Gorbach S, Knuaf W, Rimpau RH, Hüttenbach H (1982) Properties, effects, residues, and analytics of the insecticide endosulfan. Residue Rev 83:1–174
Goodman LR, Lewis MA, Macauley JM, Smith R Jr, Moore JC (1999) Preliminary survey of chemical contaminants in water, sediment, and aquatic biota at selected sites in Northeastern Florida Bay and canal C-111. Gulf Mex Sci 17:1–16
Greve PA, Wit SL (1971) Endosulfan in the Rhine river. J Water Pollut Control Fed 43:2338–2348
Hall LW, Anderson RD (2003) Parametric and probabilistic analysis of historical chlorpyrifos surface water monitoring data from the San Joaquin River watershed: 1991–2001. Water Air Soil Pollut 150:275–298
Hansen DJ, Cripe GM (1991) Interlaboratory comparison of the early life-stage toxicity test using sheepshead minnows (Cyprinodon variegatus). In: Mayes MA, Barron MG (eds) Aquatic toxicology and risk assessment, vol 14, ASTM STP 1124, Philadelphia, PA, pp 354–375
Hardy IAJ (2001) Endosulfan: field soil dissipation study in Spain. Aventis Crop Science Study 26644
Harman-Fetcho JA, Hapeman CJ, McConnell LL, Potter TL, Rice CP, Sadeghi AM, Smith RD, Bialek K, Sefton KA, Schaffer BA, Curry R (2005) Pesticide occurrence in selected South Florida canals and Biscayne Bay during high agricultural activity. J Agric Food Chem 53:6040–6048
Harner T, Pozo K, Gouin T, MacDonald AM, Hung H, Cainey J, Peters A (2006) Global pilot study for persistent organic pollutants (POPs) using PUF disk passive air samplers. Environ Pollut 144:445–452
Hose GC, Van den Brink PJ (2004) Confirming the species-sensitivity distribution concept for endosulfan using laboratory, mesocosm, and field data. Arch Environ Contam Toxicol 47:511–520
Hose GC, Wilson SP (2005) Toxicity of endosulfan to Paratya australiensis Kemp (Decapoda: Atyidae) and Jappa kutera Harker (Ephemeroptera: Leptophlebiidae) in field-based tests. Bull Environ Contam Toxicol 75:882–889
Hose GC, Lim RP, Hyne RV, Pablo F (2002) A pulse of endosulfan-contaminated sediment affects macroinvertebrates in artificial streams. Ecotoxicol Environ Saf 51:44–52
Hose GC, Lim RP, Hyne RV, Pablo F (2003) Short-term exposure to aqueous endosulfan affects macroinvertebrate assemblages. Ecotoxicol Environ Saf 56:282–294
Jergentz S, Mugni H, Bonetto C, Schulz R (2004) Runoff-related endosulfan contamination and aquatic macroinvertebrate response in rural basins near Buenos Aires, Argentina. Arch Environ Contam Toxicol 46:345–352
Johnson WW, Finley MT (1980) Handbook of acute toxicity of chemicals to fish and aquatic invertebrates. Resource publication 137, U.S. Fish and Wildlife Service, Washington, DC, pp 1–98
Laabs V, Wehrhan A, Pinto A, Dores E, Amelung W (2007) Pesticide fate in tropical wetlands of Brazil: an aquatic microcosm study under semi-field conditions. Chemosphere 67:975–989
LaPoint T, Rodgers J Jr, Delphino J, Atkeson T, McCutcheon S (1998) Advisory panel report on the Workshop on Ecological Risk of Toxic Substances in South Florida Ecosystems, Roz and Cal Kovens Conference Center; Florida International University, Florida, 20–21 October 1998, p 20
Larson SJ, Capel PD, Majewski MS (1997) Pesticides in surface waters: distribution, trends, and governing factors. Ann Arbor Press, Chelsea, MI
Lehotay SJ, Harman-Fetcho JA, McConnell LL (1998) Agricultural pesticide residues in oysters and water from two Chesapeake Bay tributaries. Mar Pollut Bull 37:32–44
Leight AK, Van Dolah RF (1999) Acute toxicity of the insecticides endosulfan, chlorpyrifos, and malathion to the epibenthic estuarine amphipod Gammarus palustris (Bousfield). Environ Toxicol Chem 18:958–964
Leight AK, Scott GI, Fulton MH, Daugomah JW (2005) Long term monitoring of grass shrimp Palaemonetes spp. population metrics at sites with agricultural runoff influences. Integr Comp Biol 45:143–150
Leonard AW, Hyne RV, Lim RP, Chapman JC (1999) Effect of endosulfan runoff from cotton fields on macroinvertebrates in the Namoi river. Ecotoxicol Environ Saf 42:125–134
Leonard AW, Hyne RV, Lim RP, Pablo F, Van den Brink PJ (2000) Riverine endosulfan concentrations in the Namoi River, Australia: link to cotton field runoff and macroinvertebrate population densities. Environ Toxicol Chem 19:1540–1551
Long ER, Hameedi MJ, Sloane GM, Read LB (2002) Chemical contamination, toxicity, and benthic community indices in sediments of the lower Miami river and adjoining portions of Biscayne Bay, Florida. Estuaries 25:622–637
Mayer FL Jr, Ellersieck MR (1986) Manual of acute toxicity: Interpretation and database for 410 chemicals and 66 species of freshwater animals. Resource Publication 160, United States Department of the Interior, Fish and Wildlife Service, Washington, DC
McIvor CC, Ley JA, Bjork RD (1997) Changes in freshwater inflow from the Everglades to Florida Bay including effects on biota and biotic processes: a review. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Boca Raton, FL, pp 117–146
Miles CJ, Pfeuffer RJ (1997) Pesticides in canals of south Florida. Arch Environ Contam Toxicol 32:337–345
Muir DC, Teixeira C, Wania F (2004) Empirical and modeling evidence of regional atmospheric transport of current-use pesticides. Environ Toxicol Chem 23:2421–2432
National Registration Authority for Agricultural and Veterinary Chemicals (NRA) (1998) NRA review of endosulfan. NRA, Australia
National Research Council of Canada (NRCC) (1975) Endosulfan: its effects on environmental quality. NRCC publication no. 14098, NRC Associate Committee on Scientific Criteria for Environmental Quality, Subcommittee on Pesticides and Related Compounds, Ottawa, Canada
Ney RE Jr (1998) Fate and transport of organic chemicals in the environment: a practical guide, 3rd edn. Government Institutes, Rockville, MD
Nowell LH, Capel PD, Dileanis PD (1999) Pesticides in stream sediment and aquatic biota: distribution, trends and governing factors. Lewis Publishers, Boca Raton, FL
Odenkirchen EW, Eisler R (1988) Chlorpyrifos hazards to fish, wildlife, and invertebrates: a synoptic review. Biological Report 85, US Department of the Interior, Fish and Wildlife Service, Patuxent Wildlife Research Center, Laurel, MD, 34 pp
Pait AS, DeSouza AE, Farrow DRG (1992) Agricultural pesticide use in coastal areas: a national summary. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Rockville, MD
Parkpian P, Anurakpongsatorn P, Pakkong P, Patrick WH Jr (1998) Adsorption, desorption and degradation of alpha-endosulfan in tropical soils of Thailand. J Environ Sci Health B 33:211–233
Peterson SM, Batley GE (1993) The fate of endosulfan in aquatic ecosystems. Environ Pollut 82:143–152
Pfeuffer RJ (1985) Pesticide residue monitoring in sediment and surface water within the South Florida water management district. Technical publication 85-2, South Florida Water Management District (SFWMD), West Palm Beach, FL
Pfeuffer RJ (1991) Pesticide residue monitoring in sediment and surface water within the South Florida water management district, volume 2. Technical publication 91-01, South Florida Water Management District (SFWMD), West Palm Beach, FL
Pfeuffer RJ, Rand GM (2004) South Florida ambient pesticide monitoring program. Ecotoxicology 13(3):195–205
Pozo K, Harner T, Wania F, Muir DC, Jones KC, Barrie LA (2006) Toward a global network for persistent organic pollutants in air: results from the GAPS study. Environ Sci Technol 40:4867–4873
Racke KD (1993) Environmental fate of chlorpyrifos. Rev Environ Contam Toxicol 131:1–150
Robblee MB, Barber TR, Carlson PR Jr, Durako MJ, Fourqurean JW, Muehlstein LK, Porter D, Yarbro LA, Zieman RT, Zieman JC (1991) Mass mortality of the tropical seagrass Thalassia testudinum in Florida Bay (USA). Mar Ecol Progr Ser 71:297–299
Rohr JR, Crumrine PW (2005) Effects of an herbicide and an insecticide on pond community structure and processes. Ecol Appl 15:1135–1147
Ross P, Scott GI, Fulton MH, Strozier ED (1996) Immunoassays for rapid, inexpensive monitoring of agricultural chemicals. In: Richardson M (ed) Environmental xenobiotics. Taylor and Francis, London, pp 161–178
Schimmel SC, Patrick AM Jr, Wilson AJ Jr (1977) Acute toxicity to and bioconcentration of endosulfan by estuarine animals. In: Mayer FL, Hamelink JL (eds) Aquatic toxicology and hazard evaluation, 1st symposium, AST STP 634, Philadelphia, PA, pp 241–252
Schulz R (2003) Using a freshwater amphipod in situ bioassay as a sensitive tool to detect pesticide effects in the field. Environ Toxicol Chem 22:1172–1176
Schulz R (2004) Field studies on exposure, effects, and risk mitigation of aquatic nonpoint-source insecticide pollution: a review. J Environ Qual 33:419–448
Schulz R, Peall SKC (2001) Effectiveness of a constructed wetland for retention of nonpoint-source pesticide pollution in the Lourens River catchment, South Africa. Environ Sci Technol 35:422–426
Schulz R, Peall SKC, Dabrowski JM, Reinecke AJ (2001a) Current-use insecticides, phosphates and suspended solids in the Lourens River, Western Cape, during the first rainfall event of the wet season. Water SA 27:65–70
Schulz R, Peall SKC, Dabrowski JM, Reinecke AJ (2001b) Spray deposition of two insecticides into surface waters in a South African orchard area. J Environ Qual 30:814–822
Science Subgroup (1996) South Florida ecosystem restoration: scientific information needs. A Science Subgroup report to the Working Group of the South Florida Ecosystem Restoration Task Force, Miami, FL
Scott GI, Fulton MH, Daugomah J, Strozier ED, Key PB, Pennington PL, Thompson BC, Wirth EF, Thayer G (1994) Monitoring of pesticides in surface waters of Florida Bay and adjacent agricultural watersheds: implications for future management of freshwater inputs to Florida Bay. U.S. National Marine Fisheries Service, Charleston, SC
Scott GI, Fulton MH, Wirth EF, Chandler GT, Key PB, Daugomah JW, Bearden D, Chung KW, Strozier ED, DeLorenzo M, Sivertsen S, Dias A, Sanders M, Macauley JM, Goodman LR, LaCroix MW, Thayer GW, Kucklick J (2002) Toxicological studies in tropical ecosystems: an ecotoxicological risk assessment of pesticide runoff in South Florida estuarine ecosystems. J Agric Food Chem 50:4400–4408
Shen L, Wania F, Lei YD, Teixeixeira C, Muir DCG, Bidleman TF (2005) Atmospheric distribution and long-range transport behavior of organochlorine pesticides in North America. Environ Sci Technol 39:409–420
Shivaramaiah HM, Sanchez-Bayo F, Al-Rifai J, Kennedy IR (2005) The fate of endosulfan in water. J Environ Sci Health B 40:711–720
Society of Environmental Toxicology and Chemistry (SETAC) (1994) Pesticide risk and mitigation: final report of the Aquatic Risk Assessment and Mitigation Dialogue Group. SETAC, Foundation for Environmental Education, Pensacola, FL
Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, La Point TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW Jr, Williams WM (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–76
Suter GW II (1993) Ecological risk assessment. Lewis Publishers, Boca Raton, FL
Suter GW II, Tsao CL (1996) Toxicological benchmarks for screening of potential contaminants of concern for effects on aquatic biota on Oak Ridge Reservation: 1996 revision. ES/ER/TM-96/R2, Oak Ridge National Laboratory, Oak Ridge, TN, 104 pp
U.S.EPA (1980) Ambient water quality criteria for endosulfan. EPA 440/5-080-046, United States Environmental Protection Agency, Office of Water, Washington, DC
U.S.EPA (1985) Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. United States Environmental Protection Agency, Office of Research and Development, Environmental Research Laboratories, Duluth, MN
U.S.EPA (1994) Ecological incident information system. EPA 734-K-94-001
U.S.EPA (1997) The incidence and severity of sediment contamination in surface waters of the United States, 3 volumes. EPA 823-R-97-006, -007, -008. September 1997
U.S.EPA (1998) Guidelines for ecological risk assessment. EPA/630/R-95/002F. United States Environmental Protection Agency, Office of Water, Washington, DC
U.S.EPA (2000) Reregistration eligibility science for chlorpyrifos: fate and environmental risk assessment chapter. United States Environmental Protection Agency, Office of Prevention Pesticides and Toxic Substances, Washington, DC
U.S.EPA (2001) National sediment quality survey. Database 1980 to 1999. MS Access 97 database files. Prepared by the EPA’s Office of Science and Technology, Standards and Health Protection Division (EPA-823-C-01-001)
U.S.EPA (2002a) Environmental fate and ecological risk assessment for the reregistration eligibility decision on endosulfan (Thiodan®). DP Barcode D238673. United States Environmental Protection Agency, Office of Pesticide Programs, Environmental Fate and Effects Division, Washington, DC
U.S.EPA (2002b) National recommended water quality criteria (WQC): 2002. EPA/822/R-02/047. Office of Water, Washington, DC
U.S.EPA (2007) Appendix 1 to 2007 addendum: environmental fate and ecological risk assessment of endosulfan. United States Environmental Protection Agency, Office of Pesticide Programs, Environmental Fate and Effects Division, Environmental Risk Branch V, Washington, DC
Van den Brink PJ, van Wijngaarden RPA, Lucassen WGH, Brock TCM, Leeuwangh P (1996) Effects of the insecticide Dursban 4E (active ingredient chlorpyrifos) in outdoor experimental ditches: II. Invertebrate community responses and recovery. Environ Toxicol Chem 15:1143–1153
Van Wijngaarden RPA, van den Brink PJ, Crum SJH, Voshaar JHO, Brock TCM, Leeuwangh P (1996) Effects of the insecticide Dursban 4E (active ingredient chlorpyrifos) in outdoor experimental ditches: I. Comparison of short-term toxicity between the laboratory and the field. Environ Toxicol Chem 15:1133–1142
Wan MT (1989) Levels of selected pesticides in farm ditches leading to rivers in the lower mainland of British Columbia, Canada. J Environ Sci Health B 24:183–203
Wan MT, Szeto S, Price P (1995) Distribution of endosulfan residues in the drainage waterways of the Lower Fraser Valley of British Columbia. J Environ Sci Health B 30:401–433
Wan MT, Kuo JN, Buday C, Schroeder G, Van Aggelen G, Pasternak J (2005) Toxicity of alpha-, beta-, (alpha plus beta)-endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchus kisutch, and biological implications in streams. Environ Toxicol Chem 24:1146–1154
Wang JD, Daddio E, Horwitz MD (1978) Canal discharges into south Biscayne Bay. Report to the Department of Environmental Resources Management, Metropolitan Dade County, Rosenstiel School of Marine and Atmospheric Science (RSMAS), University of Miami, Miami, FL
Ware G (1991) Fundamentals of pesticides: a self-instructional guide. Thompson Publications, Fresno, California
Wauchope RD, Buttler TM, Hornsby AG, Augustijn-Beckers PWM, Burt JP (1992) The SCS/ARS/CES pesticide properties database for environmental decision-making. In: Georage W (ed) Rev Environ Contam Toxicol 123:1–164
Wirth EF, Lund SA, Fulton MH, Scott GI (2001) Determination of acute mortality in adults and sublethal embryo responses of Palaemonetes pugio to endosulfan and methoprene exposure. Aquat Toxicol 53:9–18
You J, Schuler LJ, Lydy MJ (2004) Acute toxicity of sediment-sorbed endrin, methoxychlor, and endosulfan to Hyalella azteca and Chironomus tentans. Bull Environ Contam Toxicol 73:457–464
Zhou M, Li YC, Nkedi-Kizza P, O’Hair SK (2003) Endosulfan losses through runoff and leaching from calcareous gravelly or marl soils. Vadose Zone J 2:231–238
Acknowledgments
This studied was funded by the Critical Ecosystems Studies Initiative, Everglades National Park, U.S. Department of the Interior, Cooperative Agreement no. H5284-02-0094. This is Southeast Environmental Research Center (SERC) contribution no. 386.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carriger, J.F., Rand, G.M. Aquatic risk assessment of pesticides in surface waters in and adjacent to the Everglades and Biscayne National Parks: I. Hazard assessment and problem formulation. Ecotoxicology 17, 660–679 (2008). https://doi.org/10.1007/s10646-008-0230-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0230-0