Abstract
Short-term 48 h laboratory bioassays with water from an acid mine drainage (AMD: pH 3.3, 4.4, 5.0, 5.5, 6.4, control) and water from an arsenic containing reservoir were performed with the freshwater shrimp Atyaephyra desmaresti Millet, validated in situ and compared to acidified control water (ACID). Behaviour, mortality and time to death were monitored with the Multispecies Freshwater Biomonitor (MFB). The shrimps had equal 24 h-LC50s at pH 4 in AMD and ACID. However, after 48 h AMD proved more toxic (48 h-LC50 at pH 5.2) than ACID (48 h-LC50 at pH 4.5). Stress behaviour in AMD consisted at pH ≤ 6.4 of a pH-dependent decrease in activity, with disappearance of circadian rhythmicity, and at pH 4.4 a clear increase of ventilation. At pH 5.5 bioaccumulation of metals was higher and locomotion lower than at pH 5.0. In ACID, only at pH ≤ 4.4 locomotion became abated and arythmic. Locomotion in the field was equal or higher compared to the laboratory, whereas the ventilation was higher in the laboratory. A. desmaresti is a valuable species to be used in short term behavioural bioassays of AMD in Europe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater shrimps play an increasingly important role in the fields of freshwater ecology and aquaculture: their role as large crustacean detritivores and herbivores within the food webs connected to nekton and epibenthos in mostly subtropical and tropical ecosystems is well acknowledged (e.g., Lehman et al., 1996), while increasing world demand for animal proteins have boosted freshwater shrimp aquaculture, especially in Asia (e.g., Mariappan and Balasundaram, 2003). The fact that freshwater shrimps can successfully be used in bioassays and biomonitoring has been demonstrated by, e.g., Smith et al. (1990), Hatakeyama and Yokoyama (1997), Hatakeyama and Hiroaki (1998) and Bazzanti et al. (1997). As Hatakeyama and Hiroaki (1998) pointed out, the absence of cannibalism, resulting in very high control survivals, combined with their easy culture, handling, and sensitivity to pollutants make them excellent candidates for biomonitoring programs. Several studies pointed out their potential value as biotest species in Europe (e.g., Vijayram and Geraldine, 1996; Bazzanti et al., 1997; Lombardi et al., 2000, 2001; Gerhardt et al., 2002, 2004). The small decapod Atyaephyra desmaresti Millet is one of the few freshwater shrimps in Europe, originating from the Mediterranean area (Gauthier, 1924), extending east to the Black Sea and north to The Netherlands (Fidalgo and Gerhardt, 2003) via the large European waterways. A. desmaresti is omnivorous, ingesting algae, mud and faecal pellets and is quite tolerant to temperature and salinity variations. It is also an important prey for fish (e.g., García-Berthou and Moreno-Amich, 2000). In Portugal it inhabits most watercourses, including reservoirs, rice fields, coastal lagoons and temporary streams (Fidalgo and Gerhardt, 2003). This shrimp species may represent a useful test species for pollution bioassays, along the same development taken with mysid shrimps and grass shrimps since the 1990s in the United States (e.g., USEPA, 1991; ASTM, 1993). Moreover, given the scarcity or absence of freshwater Gammarus spp. on the Iberian peninsula (Illies, 1978), which otherwise are often used as bioindicators and biotest animals in Europe (e.g., Maltby and Crane, 1994; Gerhardt, 1995, 1996; Gerhardt et al., 1998), this freshwater shrimp may offer a valuable crustacean alternative as test species for sublethal biotests in Spain and Portugal (Fidalgo and Gerhardt, 2003, Gerhardt et al., 2004). Sublethal effects of pollution were explored with a non-destructive method, a non-optical behavioural online biomonitor, based on quadropole impedance technology, able to continuously record different types of movements of individual animals (Gerhardt et al., 1994). It is anticipated that pollution stress will cause changes in behaviour, as has been demonstrated with the same technology for another freshwater shrimp in China exposed to domestic sewage and pharmaceutical waste water (Gerhardt et al., 2002) and for a marine shrimp exposed to crude oil extract (Baden and Hagerman, 1981). Behaviour is regarded as a parameter integrating a whole sequence of responses at several levels of biological organisations going from biochemical to ecological processes (Lagadic et al., 1994). Moreover, it is also true that any insights into the possible effects of certain pollution types upon this shrimp will make us better understand how tolerant it is, given its successfull expansion into Western Europe. And more generally, such study may give some answers to what kind of consequences pollution events might have upon shrimp populations, especially if such tests are field validated like in the present work.
In the present study we investigated survival and behavioural responses of A. desmaresti when exposed to a gradient of an acid mine drainage (AMD) in Portugal, in laboratory and in situ exposures, combined with measurements of body burdens of metals. Further, a comparison with exposure to acidified water was done, in order to discern possible effects typical for heavy metals in an acid environment from responses triggered by acid-only water, regardless of heavy metals.
Methods
Test animals
All collection of water and shrimp and all experiments on AMD were performed in June–October 2000, the ones with acidified reference water in December 2000. This period June–December is appropriate for the collection of sufficient mature animals being in a vegetative phase, not in a reproductive status (Fidalgo, 1987, 1989a, 1989b), and being all of the same size class. Animals were collected by hand net in the River Vouga, a river in Northern Portugal with very high densities of shrimps and within 50 km from the laboratory, and brought in cooling boxes to the laboratory, where they were kept in their own aerated water in a climate room at ±18 °C and 8 h night/16 h day photoperiod, 1 week (acclimation period) prior to the experiments. The animals were not cultured into new generations, but directly used for the respective experiments. For the field studies, animals were transported in cooling boxes from the laboratory until the field sites and dispatched into the respective experimental set-ups. Subsamples of live animals were taken for length measurements (tip of rostrum to tip of abdomen). These length data were compared with data of Fidalgo (1987, 1989a, 1989b), collected on animals issued from the same climatic conditions and ecoregion (River Douro, N. Portugal) in order to characterise the used animals with reference to their life cycle. All animals belonged to a size class characterised by Fidalgo (1987, 1989a, 1989b) as “mature but not reproductive” (range: 17.4±SD4.8 to 21.5±SD3.9 mm). Although the animals in the present study were not sexed, from Fidalgo (1989a) it is known that the sex ratio of natural populations in this ecoregion varies around 1.
Study site
The São Domingos mine, situated in the watershed of the River Chança (tributary of the River Guadiana), Southern Portugal, is of the cupriferrous pyrite type and is abandoned for more than 30 years (Fig. 1). Over the years, galleries and retention basins were filled with water and nowadays a continuous stream of AMD with pH=±2.3 is flowing down the main excavated valley. The AMD receives a few tributaries with pH=6–7, creating small mixing zones. It is appropriate to describe this system as a dynamic pH-gradient, dependent on pluviometry. The water chemistry is explained in detail in Gerhardt et al. (2004) and Janssens de Bisthoven et al. (2005). The confluence of the first-order Mosteirão stream with the AMD main channel was taken as a small-scale dynamic pH-gradient in order to provide water for the laboratory experiments and to validate the bioassay in the field. Near the mining village of São Domingos, a small-impounded lake (“reservoir”) provided additional reference test water with comparatively low metal concentrations (Table 1), apart for higher values of arsenic. The river Vouga in N-Portugal, from which the shrimps originated, gave control water (Table 1). It had low values for most metals, except for copper and iron. For convenience, the AMD-sites were named according to their pH-value.
Experimental design
AMD exposure
Toxicity bioassays (48 h) with water from a pH gradient in the S. Domingos mine (pH 3.3, 4.4, 5.0, 5.5, 6.4), reservoir water (pH 6.8) and Vouga water (pH 7.7) (Tables 1 and 2, Fig. 3) were performed in August in a climate room. Vouga water proved to be excellent control water, with 100% survival. The exposure duration of 48 h was chosen because (1) behavioral changes are expected to be fast responses, hence to occur within that time span (Gerhardt et al., 2004), (2) it allows for covering two days and two nights, generating a repetition of any circadian behavioral rhythmicity, and moreover, (3) LC50 differences between AMD and acidified control water (ACID) only became apparent after 48 h. The water was collected in 25-L Polyethylene tanks and stored at room temperature and in a shadowy laboratory place up to 1 week prior to the experiments. Measurement of pH and heavy metals before and after the experiments insured quality control (remaining constant) of the test water. All experiments were performed in triplicate artificial flow-through polypropylene channels of 40 cm length, 16.5 cm width and 15 cm depth and a volume of 4 L. Water from 5-L polyethylene buckets, aerated with air stones, was pumped via a multichannel pump (Watson/Marlow 2058) through each artificial stream at a maximal speed of 18 ml/min (Fig. 2). The renewal time of water in the stream was ca. 4 h, corresponding to the low flow conditions encountered in the field sites. The whole set-up was placed in a climate room at 18°C, 8 h night/16 h day cycle, illuminated per channel with two 30-W neon lights. pH, conductivity (330 WTW pH-electrode), oxygen and temperature were recorded daily. Water samples were taken at the beginning and at the end of the experiments for chemical analysis with ICP-AES and ICP-MS. Since end values of water samples did not differ from start values, only the end values are given (Tables 1 and 2). A subsample of 2 × 3 animals was taken after 48 h for analysis of metal body burdens in each pH-treatments.
Each artificial channel contained 10 free-living individuals to record mortalities visually. Each channel also contained 3–4 chambers connected to the Multispecies Freshwater Biomonitor (MFB; see further), containing each one individual shrimp, to continuously record online behaviour and survival during the whole exposure. Water temperature was between 17 and 18.3 °C. The measured pH-values did not vary much from the nominal pH values (SD = 0.02–0.17). Oxygen concentration remained above 7 mg/L. The conductivities of the experimental waters are mentioned in Tables 1 and 2.
Acid exposure
In a second set of experiments, performed in December 2000, adult shrimps were exposed in duplicate channels during 48 h to Vouga water which was acidified to pH = 3.3, 4.4, 5.0, 5.5 and 6.4 with HNO3, in order to discern behavioural reactions induced by stress provoked by acid-only from reactions provoked by AMD. All other experimental conditions were as in the AMD-experiment.
Field experiments
Two separate field experiments were carried out: (1) in June 2000, in the reservoir, AMD-pH 3.3 and 6.4, and (2) in October 2000, in the reservoir, AMD-pH 3.3 and 4.4. The same artificial streams as used in the laboratory were placed in the pH gradient of the mixing zone caused by the confluence of the Mosteirão stream with the AMD-channel of the S. Domingos mine, or in the reservoir near the shore, characterised by similar pollution as during the laboratory experiments (Table 2). Two replicate channels were placed in the respective sites. The channels were covered with nylon nets in order to prevent escape of free moving shrimps or introduction of local species, and were fixed between vertically planted poles in order to prevent disturbance by current. Each channel contained 10–15 free-living individuals and 3 or 4 MFB-chambers containing each one individual. At the start of the exposure (1 h acclimation time), and further two times a day, the chambers were connected to the MFB (+laptop), which was powered by a battery. Such field set-up is illustrated in Janssens de Bisthoven et al. (2004). The animals’ behaviour was then recorded in 4-min tracks every 10 min, during ca. 30 min. These data were averaged for each period of measurement and compared with behaviour recorded in the laboratory after the same duration of exposure. Eventual discrepancies due to circadian rhythmicity (e.g., 14 h of exposure in the laboratory might be in the early morning while in the field it might be in the afternoon) were not found. Mortality was visually recorded every 24 h and water chemistry measured at the end of the experiment.
Metal analysis
Metals and other elements in the water and the organisms (only the AMD-experiment) were analysed with ICP-MS for low concentrations (Cd, Co, Cu, Zn, Mn, Pb, As) and with ICP-AES for higher concentrations (Fe, Na, Mg, S, Ca, high As-values). Cl was determined with ion chromatography. Replicate analyses were not different from each other. The shrimp samples were analysed after one digestion in 500 μL 30% HNO3 at 80 °C overnight, a second digestion in 100 μL H2O2 at 80 °C and then addition of 100 μL HNO3 and 900 μL ultra-distillated water, following the same protocol as in Janssens de Bisthoven et al. (2004).
Multispecies Freshwater Biomonitor
The MFB allows for automated and quantitative recording (4-min traces) of the behaviour (locomotion, ventilation) and survival of up to 96 test chambers, each containing one animal. The plexyglass test chambers are cylindrical (length = 3 cm, diameter = 2 cm) and capped at both ends with polyamid lids, which are screwed on the cylinder, and covered with nylon gauze (mesh size = 0.5 mm) in order to allow for water exchange (Fig. 2). The animal moves within such chamber freely between four pairs of steel plates attached to the walls, which serve as electrodes. Movements of the animal provoke signals by quadropole impedance conversion (Gerhardt et al., 1994). Data analysis is based on a discrete fast Fourier transformation resulting in a histogram of the behavioural frequencies ≤8 Hz (Gerhardt et al., 1998). Different behaviours can be assigned to different frequencies. The low frequencies (0.5–2.5 Hz), generated by large body movements (locomotion), were summarised in band 1 and the higher frequencies (3.0–8), generated by small and fast movements (ventilation) in band 2.
Statistics
Normality (χ2 goodness of fit) and homogeinity of variance (Levene’s test) were tested on the data prior to parametric statistics. Mortalities (after transformation of the frequencies to arcsine (x)1\2) of free moving animals were compared between the pH-conditions by one-way analysis of variance. Mortalities in the MFB-chambers were compared with a χ2-test among each other and with the means of the mortalities of the free-living animals. Furthermore, mortalities were transformed to probits and put in linear regression with pH to determine the median pH at which 50% of the shrimps would die. The continuous MFB-data registered the exact time to death (TTD) of individual shrimps, replacing the less accurate method of visually recording the mortality every x h. Cumulative regression curves of mortalities could be constructed with linear regression as a function of time to calculate LT50, being the exposure time within each pH treatment at which 50% of the animals would die.
Circadian rhythmicity was fitted by a sinus function (Gerhardt et al., in press), B = α + β[sin((t − θ)/γ)], where B = % time spent on behaviour, t = time units of 20 min expressed in decimals, α = correction constant to adjust the sinus curve according to the position of the behavioural data on the Y-axis (= mean of B), β = correction factor to adjust the amplitude of the sinus to the observed minima and maxima of the behavioural data B, θ = correction constant to adjust the sinus curve according to the position of the behavioural data on the X-axis (correspondence of data oscillations with predicted oscillations), γ = correction factor to adjust the periodicity of the sinus curve according to the observed circadian rhythmicity. For every time-block of 6 h, bands 1 and 2 data were compared between the different treatments (pH, day-night, laboratory-field) by one-way ANCOVA (covariate time) after arsine (x)1\2 transformation. Degrees of freedom were provided by the number of 4 min recordings x number of shrimps. Bioaccumulation data were compared after ln(x + 1) transformation by one-way MANOVA between the different AMD-conditions and correlated by Spearman Rank with each other and with the pH-values of the respective sites.
Results
Metal concentrations
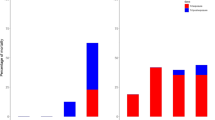
The chemical characteristics of the water used in the laboratory experiment are given in Tables 1 and 2 and Fig. 3. Regarding the metals, the River Vouga showed drinking water quality, except for high values of Fe and Cu. The reservoir water had for all elements relatively low concentrations, except for values of As. The metals Cd, Co, Cu, Fe, Pb, Mn and Zn, as well as S increased with decreasing pH (Sp. Rank, r 2 > 0.73, p < 0.04). The aqueous metals and salts in the first field experiment in June (Table 2) were generally similar to those in the laboratory experiment in August. However, the values measured during the second field experiment in October were 1.5–10× higher (p < 0.05), especially at pH 3.3, which was typically related to evaporation effects at the end of the summer dry season as stated in Gerhardt et al. (2004) and Janssens de Bisthoven et al. (2005). In the reservoir most elements remained constant, except for arsenic, which was 10× higher in the second field experiment. Contrary to aqueous concentrations, body burdens of metals were not linearly dependent on pH. This is because most metals bioaccumulated more at pH 5.5 than at other pHs (Fig. 3). When comparing metal body burdens in shrimps exposed in the laboratory with the corresponding field treatments (Fig. 3), there is a good agreement at pH 3.3 between laboratory and field (1), but less so with field (2), where the shrimp bioaccumulated less.
Mortality
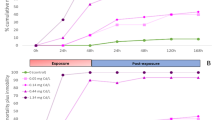
The LC50s were similar for the animals moving freely in groups of 10–15 within the artificial channels, and the animals confined individually in the MFB-chambers (Table 3). Mortalities increased with decreasing pH. After 24 h of exposure, 50% of the shrimps died at a pH = ~4, both in AMD and in ACID. However, after 48 h the LC50 for ACID only increased by ~0.6 pH-units, while the LC50 for AMD increased until over pH = 5.1, showing more sensitivity towards AMD (paired t-test on predicted mortalities: t = 3.53, p = 0.017). Analysis of LT50s on the basis of mortality-times to death regressions was only significant for AMD (r² > 0.80, p < 0.03), showing a median TTD of 20 min. at AMD-pH 3.3, of ~32 h (95% CL = 31–38) at AMD-pH 4.4 and of ~40 h (95% CL = 36–47) at AMD-pH 5.0.
The mortalities after 24 and 48 h registered during the two field experiments (in the MFB chambers and free-living) concord well with the results obtained in the laboratory for the shrimps exposed to reservoir water (survival >98%) and the ones exposed to pH 3.3 (most died within 1 h). The mortality after 48 h in the first field experiment (AMD-pH 6.4) reached 10–15%, which was comparable to the mortality obtained in AMD-pH 6.4 in the laboratory (±10%). The mortality after 48 h in the second field experiment (AMD-pH 4.4) reached 77–88%, also comparable to the mortality obtained in AMD-pH 4.4 in the laboratory (±88%).
Normal behaviour
The normal behaviour could be observed in animals exposed to their original water, from the River Vouga (Fig. 4). The shrimps showed a clear circadian activity, characterised by swimming during the night and ventilation during the day. The shrimps exposed to Vouga water showed in both AMD and ACID experiments locomotion behaviour for 25–35% of the time (SE = 0.8–4), while ventilation behaviour took 3–10% of the time (SE = 2.8–9).
Mean locomotion and ventilation of A. desmaresti measured in the laboratory by the MFB as a function of exposure time in different pH treatments of (a) ACID and (b) AMD water. Data are fitted to least squares. For Vouga and Reservoir, a sinus function is represented when a clear circadian rhythmicity of the data is noted. Black bars = night periods.
Behaviour in AMD- and ACID-laboratory experiments
Locomotion was in all pH treatments higher in animals exposed to ACID than to AMD (range between F(1, 2164) = 25.5, p < 0.0001 and F(1, 5140) = 5977, p < 0.0001). This was also the case for ventilation in the higher pH treatments (Fig. 4). However, at pH = 4.4 ventilation became more pronounced in AMD than in ACID (F(1, 2164) = 13.7, p < 0.0002). Animals exposed to ACID at pH > 5.5 showed equal or more locomotion than in Vouga water, and maintained their circadian rhythmicity. However, at pH < 5.5 the rhythmicity was weakened or lost (F(21, 6188) = 326.0, p < 0.0001). With decreasing pH in the AMD the animals exhibited a dose-dependent decreasing locomotion (F(5, 2869) = 431, p < 0.0001), combined with a disappearance of circadian rhythmicity. However, during the second day and night they performed better at pH = 5.0 than at pH = 5.5 (F(5, 3050) = 500.9, p < 0.001) (Fig. 4), which might be due to higher bioaccumulation at pH 5.5 (all metals except Cu and Pb, Fig. 3). For ventilation this pH-dependent decrease was counteracted by a pronounced increase at pH = 4.4, here interpreted as a clear stress reaction to AMD.
Behaviour in AMD, in situ
A good agreement was found for locomotion between laboratory- and field-exposed shrimps for reservoir, and a satisfactory one for pH 4.4 and 3.3 (Table 4). However, the field data for locomotion in pH 6.4 were systematically higher than the ones obtained in the laboratory, although the water chemistry was similar (Table 1, Fig. 3). When looking at the body burden data (Fig. 3), the field shrimps accumulated more Co, Fe, Mn and Pb than the laboratory shrimps (p < 0.05). On the other hand, the laboratory shrimps accumulated more As, Cd and Zn.
Discussion
Short-term exposure to acidified water showed the pH-tolerance (≥pH 5.0) of these freshwater shrimps in terms of survival and behaviour (see also Gerhardt and Fidalgo, 2003). This tolerance could contribute to the invasive character of the species in the inland waters of Western Europe. Differences in 48 h-LC50s and levels of activity between ACID and AMD indicate the important toxic role of the metals in the AMD. As observed by Kotyak et al. (1972) and Gerhardt et al. (2004), only with progressive neutralisation of AMD, Crustacea have a chance of survival. In a separate paper, we described an experiment where we tested the behaviour of A. desmaresti when exposed to water from the same AMD-gradient (only pH 3.3, 4.4 and 5.0) taken in a different season (spring) and tapwater as control, and we combined the results in a multimetric index, integrating several macroinvertebrate community metrics (Gerhardt et al., 2004). The then obtained 48 h-LC50 was at pH = 5.8, a pH value only slightly higher (±0.6 pH units) than the 48 h-LC50 obtained here. Factors which might have contributed to the slightly higher sensitivity in the study of Gerhardt et al. (2004) could be a higher toxicity of the AMD water in spring or a younger (more sensitive) age of the cohort of shrimps. The same circadian activity could be observed in the controls in both studies, which disappeared in AMD-water. Both studies show that the behaviour of A. desmaresti is highly predictable in terms of behaviour (e.g., locomotion between 20 and 35% of the time), circadian rhythmicity, and pH-dependent decrease of locomotion within increasingly acid AMD. These are robust criteria in favour of the species as model organism in Europe for biomonitoring studies with pollution related to AMD. An interesting aspect is that the locomotion in the AMD at pH 5.5 was lower than at pH 5.0 and that this could be related to higher bioaccumulation of metals at pH 5.5 than at pH 5.0. It is known that within the range pf pH 5.0–5.5 many metals start becoming bioavailable due to changes in speciation (Stumm and Morgan, 1981), generating subsequent sublethal toxic stress, reflected in the behaviour. Ökland and Ökland (1986) reported that pH 5.0–5.5 were the lowest possible viable acidity for a majority of aquatic invertebrates. This was also confirmed by Engblom and Lingdell (1984), who showed a clear decrease of number of taxa, including Crustacea, around pH 5.5.
In the present study, exposure of the shrimps in the first field experiment validated the laboratory results concerning the locomotion in reservoir water. Concerning pH 6.4 in the field, some complex patterns of bioaccumulation and eventually other uncontrolled factors (e.g., organic matter, temperature variations) provoked less sublethal toxicity than in the laboratory.
Observed differences in behaviour between the second field experiment and the laboratory results might be attributed to the higher sublethal toxicity of the water (mortalities remained the same) due to typical estival evaporation effects (Gerhardt et al., 2004, Janssens de Bisthoven et al., 2005). The generally higher ventilation observed in the laboratory could be due to artefactal laboratory conditions such as a lower amount of particulate matter, triggering a higher ventilation effort for feeding or a lower flow rate provoking lower oxygen content. Given the short-term character of the observed sublethal and lethal responses, such results can only be extrapolated to real world scenario’s within the specific time frame of maximum two days. The animals exposed to their original Vouga water showed the highest time spent on locomotion, although copper concentrations were relatively high. However, Crustacea are known to be quite tolerant to copper, an essential element used in their hemolymph (Maund et al., 1992; Gerhardt, 1995). Also, the freshwater prawn Macrobrachium sp. proved to be rather unsensitive to particulate copper pollution in the Fly River of Papua New Guinea, affected by a copper mine (Smith et al., 1990). Even though in the reservoir water survival was optimal and the animals retained their circadian rhythmicity, locomotion was lower than in Vouga water, while ventilation was higher. It suggests that arsenic, the main metal differentiating both waters, might possibly exert some slight sublethal stress upon the organisms.
Two studies (Gerhardt et al., 2002, 2005a), involving the comparison of a crustacean and a small fish, reported crustaceans (respectively the freshwater shrimp Macrobrachium nipponense and Daphnia magna) to be equally or more sensitive to pollution (AMD, domestic sewage and pharmaceutical sewage) than fish (respectively the medaka Oryzias latipes and the moskitofish Gambusia holbrooki). For these sensitivity reasons, combined with (1) the higher ethical acceptance of using invertebrates rather than fish in ecotoxicology, (2) the rising economic importance of shrimp in aquaculture and (3) their ecological importance, especially in subtropical and tropical ecosystems, freshwater shrimp offer a valuable alternative as biotest organisms.
Circadian rhythmicity of behaviour could be recorded with the MFB in other invertebrates such as, e.g., D. magna (Gerhardt et al., 2005a, Gerhardt et al., in press), Choroterpes picteti (Ephemeroptera; Gerhardt et al., 2005b), the caddis fly Hydropsyche angustipennis (Gerhardt, 1996) and the stone fly Dinocras cephalotes (Gerhardt, 2000), but not in the dipteran benthic insect Chironomus (Janssens de Bisthoven et al., 2004). Aguzzi et al. (2004) could relate circadian night activity of the decapod Norway lobster to endogenous heart beat periodicities. As external stimulus for increased swimming activity water current has been mentioned, creating positive rheotaxis in the common Australian freshwater shrimp Paratya australiensis (Hancock and Bunn, 1999). Enhanced swimming behaviour at night was observed by net sampling and underwater video transect methods in the tropical freshwater shrimp Caridina nilotica of Lake Victoria, and was related to visual planktivory (Lehman et al., 1996). The occurrence of circadian behavioral rhythmicities stresses the importance of a continuous monitoring of behaviour with non-optical methods such as the MFB, especially at night, where optic techniques are impeded. Changes in circadian rhythmicity could become interesting parameters for pollution detection.
Increased ventilation, as observed in the AMD at pH 4.4 is a clear sign of stress. A gradual increase in arhythmic scaphognathite activity (ventilation) was observed with impedance techniques in the marine shrimp Palaemon adspersus when exposed to crude oil for several weeks (Baden and Hagerman, 1981). They attributed it to damage to gill membranes or nerve tissues, especially to the neurons in the suboesophageal ganglion controlling scaphognathite activity.
In conclusion, A. desmaresti proved to be a sensitive and appropriate test species in ecotoxicological tests and online biomonitoring, and its use should be further encouraged in the Iberian peninsula and in the rest of Europe.
References
Aguzzi J., Abelló P., Depledge M.H. (2004). Endogenous cardiac activity rhythms of continental slope Nephrops norvegicus (Decapoda: Nephropidae) Mar. Freshw. Behav. Physiol. 37, 55–64
ASTM. (1993). Standard guide for conducting static and flow-through acute toxicity tests with mysids from the west coast of the United States. ASTM 1996 Annual Book of Standards Vol. 11.05, E1463–92, pp. 881–902. West Conshohocken, PA: American Society of Testing and Materials.
Baden S., L. Hagerman (1981). Ventilatory responses of the shrimp Palaemon adspersus to sublethal concentrations of crude oil extract Mar. Biol. 63, 129–133
Bazzanti M., Chiavarini C., Cremisini C., Soldati P. (1997). Distribution of PCB congeners in aquatic ecosystems: a case study Environ. Int. 23, 799–813
Engblom E., Lingdell P.-E. (1984). The mapping of short-term acidification with the help of biological pH indicators Rep. Inst. Freshw. Res. Drottningholm 61, 28–33
Fidalgo M.L. (1987). About the individual productivity of the freshwater shrimp Atyaephyra desmaresti Millet Limnética 3, 197–203
Fidalgo M.L. (1989a). Biology of the freshwater shrimp Atyaephyra desmaresti Millet (Decapoda: Natantia) in the River Douro, Portugal. I. Life cycle and individual growth Arch. Hydrobiol. 116, 97–106
Fidalgo M.L. (1989b). Some additional observations on the population dynamics of the freshwater shrimp Atyaephyra desmaresti Millet (Decapoda: Natantia) in Crestuma/Lever Reservoir (River Douro, Portugal), Vol. 214, pp. 3–14. Publicações do Instituto de Zoologia “Dr. Augusto Nobre”, Faculdade de Ciências do Porto
Fidalgo M.L., A. Gerhardt (2003). Distribution of the freshwater shrimp, Atyaephyra desmaresti (Millet, 1831) in Portugal (Decapoda, Natantia) Crustaceana 75, 1375–1385
García-Berthou E., Moreno-Amich R. (2000). Food of introduced pumpkinseed sunfish: ontogenic diet shift and seasonal variation J. Fish Biol. 57, 29–40
Gauthier H. (1924). Recherches sur le développement larvaire d’Atyaephyra desmaresti (Millet, 1831) (Décapodes, Natantia, Caridea, Atyidés) Bull. Soc. Hist. Nat. Afr. Nord 15, 337–376
Gerhardt A. (1995). Monitoring behavioural responses to and effects of metals in Gammarus pulex (Crustacea) with impedance conversion Environ. Sci. Pollut. Res. 2(1), 15–23
Gerhardt A. (1996). Behavioural early warning responses to polluted surface water: Performance of G. pulex L. (Crustacea) and H. angustipennis Curtis (Insecta) to a complex industrial effluent Environ. Sci. Pollut. Res.3(2), 63–70
Gerhardt, A. (2000). A new Multispecies Freshwater Biomonitor for ecologically relevant surveillance of surface waters. In: Butterworth, F. et al. (eds) Biomonitors and Biomarkers as Indicators of Environmental Change, Vol. II, pp. 301–317. Dordrecht: Kluwer-Plenum Press
Gerhardt A., Janssens de Bisthoven L., AMVM Soares (2004). Macroinvertebrate response to Acid Mine Drainage: community metrics and on-line behavioural toxicity bioassay Environ. Pollut. 130, 263–274
Gerhardt A., Janssens de Bisthoven L., AMVM Soares (2005a). Evidence for the Stepwise Stress Model: Gambusia holbrooki and Daphnia magna under AMD and ACID stress Environ. Sci. Technol. 39/11, 4150–4158
Gerhardt A., Janssens de Bisthoven L., AMVM Soares (2005b). Effects of Acid Mine Drainage and acidity on the activity of Choroterpes picteti (Ephemeroptera) measured with the Multispecies Freshwater Biomonitor Arch. Environ. Contamin. Toxicol. 48(4), 450–458
Gerhardt, A., Janssens de Bisthoven, L., Schmidt, S. (2005). Automated recording of vertical negative phototactic behaviour in Daphnia magna Straus (Crustacea). Hydrobiologia, in press
Gerhardt A., Clostermann M., Fridlund B., Svensson E. (1994). Monitoring of behavioral patterns of aquatic organisms with an impedance conversion technique Environ. Int. 20(2), 209–219
Gerhardt A., Carlsson A., Resseman C., Stich K.P. (1998). New online biomonitoring system for Gammarus pulex (L.) (Crustacea): in situ test below a copper effluent in South Sweden Environ. Sci. Technol. 32, 150–156
Gerhardt A., Janssens de Bisthoven Mo L., Wang C., Wang Z. (2002). Short-term behavioral responses of Orizias latipes (Pisces) and Macrobrachium nipponense (Crustacea) to municipal and pharmaceutical waste water in Beijing, China. Chemosphere 47, 35–47
Hancock M.A., Bunn S.E. (1999). Swimming response to water current in Paratya australiensis Kemp, 1917 (Decapoda, Atyidae) under laboratory conditions Crustaceana 72, 313–323
Hatakeyama S., Yokoyama N. (1997). Correlation between overall pesticide effects monitored by shrimp mortality test and change in macrobenthic fauna in a river Ecotoxicol. Environ. Safe. 36, 148–161
Hatakeyama S., Hiroaki S. (1998). Biomonitoring with shrimp to detect seasonal change in river water toxicity Environ. Toxicol. Chem. 17, 687–694
Illies, J. (1978). Limnofauna Europaea: A Checklist of the Animals Inhabiting European Inland Waters, with Accounts of Their Distribution and Ecology, 2nd edn, 532 pp. Fisher
Janssens de Bisthoven L., Gerhardt A., AMVM Soares (2004). Effects of Acid Mine Drainage on larval Chironomus (Diptera, Chironomidae) measured with the Multispecies Freshwater Biomonitor Environ. Toxicol. Chem. 23, 1123–1128
Janssens de Bisthoven L., Gerhardt A., AMVM Soares (2005). Chironomidae larvae as bioindicators of an Acid Mine Drainage in Portugal Hydrobiologia 532, 181–191
Kotyak M., Shapiro M.A., Sykora J.L. (1972). Riffle benthos in streams receiving Acid Mine Drainage Water Res. 6, 1239–1242
Lagadic L., Caquet T., Ramade P.M. (1994). The role of biomarkers in environmental assessment (5). Invertebrate populations and communities Ecotoxicology 3, 193–208
Lehman J.T., Mbahinzireki G.B., Mwebaza-Ndawula L. (1996). Caridina nilotica in Lake Victoria: abundance, biomass, and diel vertical migration Hydrobiologia 317, 177–182
Lombardi J.V., Machado-Neto J.G., Brossi-Garcia A.L., Marques H.L.A., E. Kubo (2000). Acute toxicity of the fungicide copper oxychloride to the freshwater prawn Macrobrachium rosenbergii De Man Bull. Environ. Contamin. Toxicol. 65, 383–390
Lombardi J.V., Machado-Neto J.G., Brossi-Garcia A.L., Marques H.L.A., E. Kubo (2001). Acute toxicity of the pesticides endosulfan and ametryne to the freshwater prawn Macrobrachium rosenbergii De Man Bull. Environ. Contamin. Toxicol. 67, 665–671
Maltby L., M. Crane (1994). Responses of Gammarus pulex (Amphipoda, Crustacea) to metalliferous effluents: identification of toxic components and the importance of interpopulation variation Environ. Pollut. 84, 45–52
Mariappan P., Balasundaram C. (2003). Sheltering behaviour of Macrobrachium nobilii (Henderson and Matthai, 1910) Acta Ethol. 5, 89–94
Maund S.J., Taylor E.J., Pascoe D. (1992). Population responses of the freshwater amphipod crustacean Gammarus pulex (L.) to copper Freshw. Biol. 28, 29–36
Ökland J., Ökland K.A. (1986). The effects of acid deposition on benthic animals in lakes and streams. Experientia 42, 471
Smith R.E.W., Ahsanullah M., G.E. Batley (1990). Investigations of the impact of effluent from the Ok Tedi copper mine on the fisheries resource in the Fly River, Papua New Guinea Environ. Monit. Assess. 14, 315–331
Stumm W., Morgan J.J. (1981). Aquatic Chemistry. An Introduction Emphasizing Chemical Equilibria in Natural Waters John Wiley &Sons New York 780 pp
USEPA. (1991). Mysid, Mysidopsis bahia. In Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 4th edn. U.S. Environmental Protection Agency, Environmental Monitoring Systems Laboratory, Office of Research and Development, Cincinnati, OH, EPA/600 4–90/027
Vijayram K., Geraldine P. (1996). Regulation of essential heavy metals (Cu, Cr and Zn) by the freshwater prawn Macrobrachium malcolmsonii (Milne Edwards) Bull. Environ. Contamin. Toxicol. 56, 335–342
Acknowledgments
This study was financed by CETERA, IAV/82/00, PRAXIS/C/MGS/10200/1998 and by FCT Fundação para a Ciência e a Tecnologia Ministério da Ciência e do Ensino Superior, Portugal (SFRH/BPD/8891/2002 and SFRH/BPD/8345/2002). We are grateful to T. Olsson (Lund University) who performed the metal analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janssens de Bisthoven, L., Gerhardt, A., Guhr, K. et al. Behavioral Changes and Acute Toxicity to the Freshwater Shrimp Atyaephyra desmaresti Millet (Decapoda: Natantia) from Exposure to Acid Mine Drainage. Ecotoxicology 15, 215–227 (2006). https://doi.org/10.1007/s10646-005-0052-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-005-0052-2