Abstract
To examine regional specific diversity in development and growth of Ayu (Plecoglossus altivelis) larvae, we collected and compared collections from the Kalong estuary in Vietnam, and the Shimanto and Muko estuary, and the Niigata coast in Japan. Among the four areas, most of the morphometrics through ontogeny were similar except that the snout tended to be shorter and the anus hardly migrated in Kalong larvae. The snout length increased gradually with growth in the Vietnamese larvae, while this value increased significantly until ca. 10 mm BL, subsequently being constant up to 30 mm body length (BL) in the Japanese larvae. The water temperature when the larvae were collected was higher in the Kalong than in most of the Japan sites. Growth-rates estimated from otolith increments were from highest to lowest, Niigata (mean = 0.54 mm/day), Kalong (0.47), Shimanto (0.38) and Muko (0.34). The higher growth-rates were obtained not in Niigata of highest latitudinal region, but in Kalong of lowest latitudinal region. This indicates that Ayu could experience their early developmental stages from the cool temperate to tropical regions, implying the potential biodiversity of this fish species in the world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Ayu (Plecoglossus altivelis), an amphidromous fish with a life span of only 1 year, spawns in the lower reaches of rivers in autumn. Newly hatched larvae drift down into estuaries and coastal waters, where they remain throughout their larval and early juvenile stages, and then in spring juveniles ascend the rivers. They grow and mature in rivers, and spawn in the following autumn (Senta and Kinoshita 1985; Tsukamoto et al. 1989; Takahashi et al. 1990). This species had been divided into two subspecies, i.e., the Ayu (Plecoglossus altivelis altivelis) distributed in the Japan Islands and the Ryukyu-ayu (Plecoglossus altivelis ryukyuensis) in the Ryukyu Islands (Nishida 1988).
Studies on the early life history of Ayu have progressed since the 1980s when it was discovered their main nursery ground in shallow waters such as the surf zone (Senta and Kinoshita 1985). Recently, it has been shown that a number of Ayu larvae and juveniles remain in estuaries, and these displayed higher growth rates (Tsukamoto et al. 1989; Takahashi et al. 1990; Tran et al. 2012). It was reported that early development and growth of Ayu vary with spawning period (Tsukamoto et al. 1989; Takahashi et al. 1990, 2000), and show a relationship to water temperature (Takahashi et al. 1999; Kishino et al. 2008; Iguchi and Takeshima 2011).
Tran et al. (2012) found that the Ayu larvae appeared in the Kalong River, northern Vietnam, which is probably their southernmost distributional area, thus this suggests that Ayu are the most latitudinally-expanded of amphidromous fish from cool temperate to tropical waters. In spite of this study, little is known of the growth rate of the Vietnamese Ayu and differences in development among latitudinal larvae.
It has been suggested that the early life history characteristics determine the distribution and population genetic structure of this fish species (Kwan et al. 2012), and for adaptation to each local environment the Ayu has remarkable genetic variations in reproductive and life history traits (Nishida 1986). However, differences in early stages traits of Ayu among latitudinal cohorts have been poorly reported. In this paper, to elucidate the distributional biodiversity of Ayu, we examined and compared development and growth of Ayu larvae collected from the Kalong estuary (ca. 21°30′N) facing the Gulf of Tonkin, and the Shimanto estuary (ca. 33°N) facing Tosa Bay, the Muko estuary (ca. 35°N) facing the Seto Inland Sea and the Niigata coast (ca. 38°N) facing the Sea of Japan.
Materials and methods
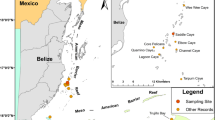
Fish larvae were monthly and semi-monthly sampled from the Kalong River in Vietnam and from three waters in Japan from October 2010 to February 2011 (Fig. 1, Table 1). Details of the sampling methods and sites in the Kalong River system are available in Tran et al. (2012). In Japan, collections were carried out in wadeable depths of the Niigata coast, the Muko and Shimanto estuaries (Fig. 1b–d). Samplings were made along the shore of the Shimanto estuary and along the bank of the Kalong River by seine net (1 × 4 m, 1 mm mesh-aperture) (Kinoshita et al. 1988). In the Muko estuary, collections were made by a different seine net (modified after Kinoshita et al. 1988), with net bag length 1.5 m, mesh-aperture 0.5 mm (Fig. 1c). From the Niigata coast, a seine net used to sample was notably different from the other sites; the structure and towing methods of this net are described in Iseki et al. (2012). All Japan specimens were fixed in 10 % formalin, and sorted larvae were transferred to 80 % ethanol. Water temperature (°C) was measured at the surface and bottom of each station during the sampling periods.
Sampling sites of Ayu larvae: a Kalong River system, northern Vietnam facing the Gulf of Tonkin; monthly and bimonthly collections were made from November 2010 to February 2011. b Niigata coast facing the Sea of Japan from October to December 2010. c Muko estuary facing the Seto Inland Sea from November to December 2010. d Shimanto estuary facing the Pacific from November 2010 to February 2011. Solid and open circles indicate the stations where Ayu larvae were collected by a larva net and a small seine net, respectively
Ayu larvae divided into developmental stages were in accordance with Kendall et al. 1984, and larval body length and other body parts were measured to the nearest 0.1 mm using an ocular micrometer attached to a binocular microscope. In this study, unlabeled lengths indicate BL (notochord length for preflexion and flexion larvae and standard length for postflexion larvae). Proportional measurements, including pre-anal length (PAL), body depth (BD), head length (HL), eye diameter (ED) and snout length (SnL), were made for all specimens collected from the Kalong River and the Muko estuary, and 100 random specimens of each collection date from the Shimanto estuary and the Niigata coast (Table 1). Because larvae smaller than 7 mm BL could not be collected from the bank waters in the Niigata coast and the Shimanto estuary, in the latter, the smaller larvae taken by the simultaneous larva net sampling were used (Fig. 1d; Table 1).
Age determinations using sagittal otolith (Tsukamoto and Kajihara 1987) were performed only for specimens ranging from 10 to 30 mm BL in size (Takahashi et al. 1999) taken from the bank waters (Table 1). Fifty specimens from each collection date were used for age in days estimation, except in the one case when less than 50 specimens were collected. In this case, all specimens were used for age determination, except for samples in the Muko estuary because of the otolith being damaged (Table 1). The hatching dates were estimated from the age (days) and back-calculating from the dates of capture. Growth rate (mm/day) of each fish was calculated by dividing the difference between body length at sampling and mean body length at hatching (5.1 mm; Wakui et al. 2009) with age in days estimated.
Although a detailed description of the Vietnamese Ayu larvae was given in the previous work, the identification to the subspecies level of the Vietnamese Ayu was still hesitant (Tran et al. 2012). Therefore, Plecoglossus altivelis is used as the scientific name in the present study.
Results
Seasonal changes of water temperature
Seasonal changes in mean water temperature at sampling stations from the bank waters of the four sites are shown in Fig. 2. Temperature gradually fell from around 20 °C in November to below 15 °C in January and February from most sites. The temperature was highest in the Kalong water in most of seasons except for mid January which was lower than in the Shimanto estuary. From the three Japan waters, the thermal condition showed a somewhat similarity until middle December, thereafter it was highest in the Shimanto estuary, and lowest in the Muko estuary in early January. In contrast to the Kalong estuary, the temperature was higher in January than February in the Shimanto estuary. The mean water temperature was ca. 23 °C in October 2010 from the Niigata coast and the Muko estuary (http://www1.kaiho.mlit.go.jp/KAN5/kaisyo/backn-id.htm; the present study), and 24 °C in the Shimanto estuary in October 2008, 2009 and 2011, but no data in 2010 due to poor weather (the present study).
Development
Figure 3 represents the sequence changes in ratio of five measured parts to the body length of Ayu larvae collected at the four sites. There were significant differences (p < 0.05) in the allometry regressions of all body parts between sites, except for PAL/BL among the three Japan sites (p > 0.05), BD/BL between the Muko and Shimanto estuaries (p > 0.05), and HL/BL in the Shimanto and Muko estuaries as well in the Niigata coast and Muko estuary (p > 0.05). The ratio of pre-anal length to BL seemed to be constant as size increase, with a mean of 76 % in the Vietnamese larvae (Fig. 3a). In the Shimanto and Muko estuaries, this value gradually increased up to 10–15 mm BL, and then gradually decreased until 30 mm BL (Fig. 3a).
In the Shimanto estuary and Muko estuary, initially, the BD, HL and ED were ca. 7, 14 and 6 % BL, and gradually decreased to ca. 6, 13 and 4 % BL, respectively, until ca. 10 mm, after that they all began to increase, and reached ca. 9, 17 and 6 % BL, respectively, at ca. 24 mm (Fig. 3b–d). For the Vietnamese larvae, the above three ratios revealed the same tendency, but the turning point occurred at ca. 12 mm BL (Fig. 3b–d). In the Niigata coast, in spite of limited size ranges between 5 and 8 mm BL larvae, the proportions of PAL/BL, BD/BL, HL/BL and ED/BL showed a turning point, at ca. 10 mm (Fig. 3a–d).
The ratio of snout to the body length increased with growth in the Vietnamese Ayu larvae ranging from 5 to 25 mm in size (Fig. 3e). In the larvae of P. a. altivelis, this value increased markedly from ca. 1 to 4 %, until ca. 10 mm BL, then became constant up to 30 mm BL (Fig. 3e). Between 10 and 25 mm BL in size, the snout tended to be longer in the Japanese larvae (Fig. 3e).
Age and body length relationships
The age-BL relationships of the Ayu ranging from 10 to 30 mm in size from the bank waters of the four sites are presented in Fig. 4. Linear regressions of BL (y; mm) on age (x; day) of the four sites were expressed as y = 0.3381x + 9.2597 (r = 0.98), y = 0.2069x + 8.6742 (r = 0.76), y = 0.2402x + 8.3369 (r = 0.93) and y = 0.2327x + 11.713 (r = 0.74) for Niigata coast, the Muko estuary, Shimanto estuary and Kalong estuary, respectively. These linear approximations indicated that growth rates of the larvae were fastest in Niigata coast and slowest in the Muko estuary. There was a small difference in the age-BL relationships between localities. Body length at a 40-day old larva collected from the Niigata coast, the Muko estuary, the Shimanto estuary and the Kalong estuary was estimated as 22.8, 16.9, 18.0 and 21.0 mm, respectively.
Growth rate and hatching date relationships
The relationships between growth rate and hatching date of Ayu larvae are given in Fig. 5. Growth rates showed differentiations among localities and hatching periods. Overall, the growth rates were highest for the larvae born in early October from the Niigata coast, and lowest in late October and early November from the Muko estuary (Fig. 5a–b). Mean growth rates (mm/day) of the Ayu collected from Niigata coast (0.54) were the highest, followed by the Kalong estuary (0.47), the Shimanto estuary (0.38) and the Muko estuary (0.34). From the Niigata coast, growth rates decreased significantly with hatching dates from early October to middle October and November (Fig. 5a). In the Muko estuary, on the other hand, this value was lowest in October and early November, and increased until middle November, subsequently tended to decrease with hatching dates (Fig. 5b). From the Shimanto estuary, the growth rate inclined to decrease with hatching dates (Fig. 5c). Although a few larvae were collected from the bank waters of the Kalong estuary, the larvae born in January-February exposed lower growth rates than those in December (Fig. 5d), and higher than those hatched in the same periods from the Shimanto estuary (Fig. 5c–d).
Discussion
When the proportional changes of the Vietnamese Ayu were compared with those of the P. a. altivelis collected from Japan, there were few differences in the morphometrics through ontogeny, e.g., the snout was shorter, and the ratio of pre-anal length to BL was constant in the Kalong larvae (Fig. 3a, e). In the larvae of P. a. altivelis (Tachihara and Kimura 1991; Takahashi et al. 2000) and P. a. ryukyuensis (Tachihara and Kawaguchi 2003), the percentages of pre-anal length to BL increased remarkably up to about 10–15 mm BL, subsequently decreased until about 30 mm BL, unlike those collected in the three Japan waters of the present study (Fig. 3a).
In this study, the proportional turning point of the P. a. altivelis occurred at ca. 10 mm BL, and that of the Vietnamese Ayu was at ca. 12 mm BL (Fig. 3b–e). This occurred faster than that in observation of Takahashi et al. (2000). In the case of reared Ryukyu-ayu, P. a. ryukyuensis, as the same size range of the Ayu in the present study, there are two turning points, at ca. 7–8 and 14–18 mm BL, occurring at the completion of yolk absorption and during the flexion period, respectively (Tachihara and Kawaguchi 2003). In the present specimens, however, only the latter turning point was observed at ca. 10–12 mm BL.
Particularly, Takahashi et al. (2000) demonstrated that the lower water temperature caused the proportional body depth in the larvae of P. a. altivelis to be more slender, with their size ranging from 10 to 65 mm BL. This correlation was not observed for the present larvae sizes measuring between 5 and 30 mm BL. The body proportions of Ayu larvae herein were similar in spite of difference in water temperatures among the four sites (Figs. 2 and 3). In agreement with the current result, it was reported that a distinct differentiation in proportions was detected only in larvae and juveniles over 30 mm BL (Takahashi et al. 2000). This evidence implies that water temperature would have had an influence on morphometrics of Ayu larvae after their experiences of residence.
As shown in Fig. 5, the growth rate tended to decrease with hatching date from each site. This means that the earlier-hatched larvae achieved a faster growth than the later ones. This result is consistent with previous findings (Tsukamoto et al. 1989; Iseki et al. 2012). Generally, early life stages of fish can feed more and grow faster under longer day length, because they are daylight feeders (Shoji et al. 2011). In the present study, specimen sizes for otolith analysis as larger as 10 mm BL had completed the absorption of yolk (Takahashi et al. 1998), so they had to depend on external food supply for their energy source. Accordingly, the Ayu larvae born later would have grown slowly because of being under shorter daylength. In P. a. altivelis of Japan, growth rate fluctuations according to hatching date appears to be a factor leading to shifts in the term of residency, and the long-term residence of late-hatched larvae could be related to their slow growth (Takahashi et al. 2002; Azuma et al. 2003; Iseki et al. 2012). However, only 21 larvae were collected in the bank waters, thus the recruitment process of Vietnamese Ayu needs to be further affirmed.
In both the Niigata coast and Muko estuary, the larvae were born in October but displayed different growth rates (Fig. 5a–b). From the Niigata coast, the younger larvae (10–37 days old, mean 13) were born in early October (mode at 11–14), thus they had a longer period at higher temperature (ca. 23 °C in October). Conversely, from the Muko estuary, the older larvae (38–59 days old, 45) were predominantly born in 28–30 October, therefore they had a longer period at lower temperature (ca. 18 °C in November or even below 15 °C in December) (Fig. 2). Hence, higher growth in Niigata larvae was probably due to larvae majorly born in early October, when the temperature was still higher and the larvae were little born in the other three sites. However, the main hatching dates had been distributed from early to late January in the Kalong, from early November to middle January in the Shimanto, from late October to middle November in the Muko and from early October to early November in Niigata (Fig. 5). The experienced temperatures during the larval period of principal cohorts were ca. 15 °C in the Kalong and Shimanto, and 15–20 °C in the Muko and Niigata (Fig. 2), accordingly temperatures hardly caused local fluctuations in growth of Ayu larvae. Hence, in the present study, the relationship between water temperature and growth rate of Ayu larvae could not be completely understood probably because of other heterogeneous factors that may affect the growth of this fish larvae. It was generally stated that growth rates of fish larvae could be an understanding of the complex interactions (Claramunt and Wahl 2000). Therefore, to approach the problem in Ayu, a further laboratory experiment must be made among latitudinal samples.
It has been commonly shown that higher survival is usually attributed to higher growth rates in fish larvae. Accordingly, the present study could support the previous result that Ayu were distributed in the tropical region (Tran et al. 2012). Despite distribution across different water environments, Ayu could lead their early life history from the cool temperate to tropical regions, indicating the potential biodiversity of this fish in the world. A boarder distribution of Ayu has been also discussed based on the morphological and molecular characters (Nishida 1986; Kwan et al. 2012), but like the present study, a full comprehension has been not determined because of a limited number of geographical samples, especially in China. Hence, it is necessary to conduct further investigations over a wider area to confirm this.
References
Azuma K, Takahashi I, Fujita S, Kinoshita I (2003) Recruitment and movement of larval Ayu occurring in the surf zone of a sandy beach facing Tosa Bay. Fish Sci 69:355–360
Claramunt RM, Wahl DH (2000) The effects of abiotic and biotic factors in determining larval fish growth rates: a comparison across species and reservoirs. Trans Am Fish Soc 129:835–851
Iguchi K, Takeshima H (2011) Effect of saline water on early success of amphidromous fish. Ichthyol Res 58:33–37
Iseki T, Miyauchi Y, Fujii T (2012) Residence pattern of the Ayu Plecoglossus altivelis altivelis larvae and juveniles occurring in the surf zone of a sandy beach, Niigata Prefecture, northern Sea of Japan. Fish Sci 78:55–65
Kendall AW, Ahlstrom EH Jr, Moser HG (1984) Early life history stages of fishes and their characters. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL (eds) Ontogeny and systematic of fishes. Am Soc Ichthyol Herpetol, Spec Publ 1, pp 11–22
Kinoshita I, Fujita S, Takahashi I, Azuma K (1988) Occurrence of larval and juvenile Japanese snook, Lates japonicus, in the Shimanto estuary. Jpn J Ichthyol 34:462–467
Kishino T, Shinomiya A, Kotobuki H (2008) Survival rates of larval Ryukyu-Ayu Plecoglossus altivelis ryukyuensis under differing experimental conditions of temperature and salinity. Jpn J Ichthyol 55:1–8
Kwan YS, Song HK, Lee HJ, Lee WO, Won YJ (2012) Population genetic structure and evidence of demographic expansion of the Ayu (Plecoglossus altivelis) in East Asia. Anim Syst Evol Divers 28:279–290
Nishida M (1986) Geographic variation in the molecular, morphological and reproductive characters of the Ayu Plecoglossus altivelis (Plecoglossidae) in the Japan-Ryukyu Archipelago. Jpn J Ichthyol 33:232–248
Nishida M (1988) A new subspecies of the Ayu, Plecoglossus altivelis (Plecoglossidae) from the Ryukyu Islands. Jpn J Ichthyol 35:236–243
Senta T, Kinoshita I (1985) Larval and juvenile fishes occurring in surf zones of western Japan. Trans Am Fish Soc 114:609–618
Shoji J, Toshito S, Mizuno K, Kamimura Y, Hori A, Hirakawa K (2011) Possible effects of global warming on fish recruitment: shifts in spawning season and latitudinal distribution can alter growth of fish early life stages through changes in daylength. ICES J Mar Sci 68:1165–1169
Tachihara K, Kimura S (1991) Embryonic development and morphological changes with growth of the larval and juvenile Ayu Plecoglossus altivelis altivelis of Lake Ikeda in southern Kyushu. Nippon Suisan Gakkaishi 57:789–795
Tachihara K, Kawaguchi K (2003) Morphological development of eggs, larvae and juveniles of laboratory-reared Ryukyu-Ayu Plecoglossus altivelis ryukyuensis. Fish Sci 69:323–330
Takahashi I, Kinoshita I, Azuma K, Fujita S, Tanaka M (1990) Larval Ayu Plecoglossus altivelis occurring in the Shimanto estuary. Nippon Suisan Gakkaishi 56:871–878
Takahashi I, Azuma K, Fujita S, Kinoshita I (1998) Spatial distribution of larval Ayu Plecoglossus altivelis in the Shimanto estuary. Fish Sci 64:522–525
Takahashi I, Azuma K, Hiraga H, Fujita S (1999) Different mortality in larval stage of Ayu Plecoglossus altivelis by birth dates in the Shimanto estuary and adjacent coastal waters. Fish Sci 65:206–210
Takahashi I, Azuma K, Fujita S, Hiraga H (2000) Differences in larval and juvenile development among monthly cohorts of Ayu, Plecoglossus altivelis, in the Shimanto River, Japan. Ichthyol Res 47:385–391
Takahashi I, Azuma K, Fujita S, Kinoshita I (2002) Habitat shift of Ayu Plecoglossus altivelis altivelis in early stages from waters adjacent to the bank to the center of flow in the Shimanto Estuary. Fish Sci 68:554–559
Tran HD, Kinoshita I, Ta TT, Azuma K (2012) Occurrence of Ayu (Plecoglossus altivelis) larvae in northern Vietnam. Ichthyol Res 59:169–178
Tsukamoto K, Kajihara R (1987) Age determination of Ayu with otolith. Nippon Suisan Gakkaishi 53:1985–1997
Tsukamoto K, Mochizuki K, Ohtake T, Yamasaki Y (1989) Distribution, migration and growth of Ayu larvae at the mouth of River Kumano. Fish Eng 50:47–57
Wakui K, Yagi Y, Yamanaka T, Kinoshita I (2009) A homing and comparison of their early life history of the Ayu among rivers in Tosa Bay. Aquabiol 31:522–529
Acknowledgments
We express our gratitude to Todd Miller for critical comments on the manuscript. This work was performed as corporative study between Hanoi National University of Education and Kochi University, and supported by the grant of special project of Kochi University. The authors declare that all the surveys in the present study comply with the current laws of Vietnam and Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tran, H.D., Kinoshita, I., Azuma, K. et al. The potential biodiversity of Ayu, as evidenced by differences in its early development and growth between Vietnam and Japan. Environ Biol Fish 97, 1387–1396 (2014). https://doi.org/10.1007/s10641-014-0229-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0229-8