Abstract
Understanding how spatial ecology varies between life stages, and whether there is an overlap of critical areas (e.g., nursery areas, breeding sites), may provide significant benefits to conservation planning. The present work examined the space use and residency of shark-like batoids (families Rhynchobatidae and Rhinobatidae) in a nearshore system. An array of 63 acoustic receivers deployed in Cleveland Bay, north Queensland, Australia, passively tracked 15 G. typus and 20 Rhynchobatus spp. between 2009 and 2011. Glaucostegus typus were monitored between 1 and 766 days (mean = 333 ± 69 days) and were present in the site from 1 to 198 days (mean 73 ± 25 days). Both adult male and female G. typus exhibited philopatric behaviour patterns, leaving the bay and returning after periods of about 9–12 months to use the same areas where they were detected in previous years. Individuals with lower residency had larger activity spaces. Rhynchobatus spp. were monitored for 1 to 707 days (mean = 231 ± 50 days) and were present in the site from 1 to 350 days (mean 82 ± 24 days). Rhynchobatus spp. exhibited no synchronicity in use of the bay. Both G. typus male and female residency changed with size of individuals, in comparison size had no effect on the residency of Rhynchobatus spp. The present study improves our understanding of shark-like batoid spatial ecology in nearshore waters and may provide useful information for the management of these populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding spatial ecology is essential for quantifying vulnerability to exploitation and to understand the benefits of conservation management (Simpfendorfer et al. 2010, 2011a; Farrugia et al. 2011). Thus identification of critical areas (e.g., nursery or mating areas), understanding the seasonality of their use, and vulnerability of these habitats to anthropogenic impacts, all contribute to the development of appropriate management strategies (Simpfendorfer et al. 2011a; Yates et al. 2012). If species utilise specific habitats during key life history stages or exhibit strong site fidelity, then localized impacts (e.g., fisheries and habitat alteration) could have significant consequences for populations (Knip et al. 2012a) (Fig. 1).
The current understanding of elasmobranch spatial ecology has largely come from research on shark species, and more specifically those with a fusiform body form such as the carchariniformes and lamniformes (Conrath and Musick 2010; Heupel et al. 2010; Speed et al. 2010; Knip et al. 2011a). Despite a surge in acoustic monitoring studies (Voegeli et al. 2001; Heupel et al. 2006) and application of this approach to numerous elasmobranch species (Heupel and Webber 2012), the spatial ecology of batoids remains poorly understood (Vaudo and Heithaus 2012). One group of batoids—the shark-like batoids (i.e. families Rhynchobatidae, Rhinoabatidae, Rhinidae and Pristidae) which are morphologically similar to sharks in having an elongate body and well developed caudal fin—have been particualrly poorly studied. There is little information describing shark-like batoid habitat preferences and movements and how these behaviours change with life history stage. What is known largley comes from fisheries dependent catch and effort data (White et al. 2013) or visual surveys (Vaudo and Heithaus 2009, 2012). However, spatial regulation of fishing effort and gear selectivity in addition to poor taxnomic resolution of bycatch species limit the utility of these data for assessing distribution and habitat preference of non-target species.
Nearshore areas provide critical habitat for elasmobranch species (Heupel et al. 2007; Knip et al. 2010) and function similarly for at least some shark-like batoids (Simpfendorfer et al. 2010). Although multiple shark species may inhabitat the same nearshore region (Simpfendorfer and Milward 1993), use may be partitioned by habitat or prey community composition (White and Potter 2004; Pikitch et al. 2005; DeAngelis et al. 2008). Futher, there often is a temporal component to partitioning with changes between seasons, cohorts or between life history stages (Knip et al. 2011a, b). Thus understanding the use of nearshore areas by shark-like batoids will be important for designing effective conservation strategies where they are needed.
The giant shovelnose ray, Glaucostegus typus and whitespotted guitarfish, Rhynchobatus spp. are shark-like batoids listed in threatened categories in the International Union for Conservation of Nature (IUCN) Red List assessments. Intensive fishing pressure has resulted in population declines in South-East Asia (White and McAuley 2003). The morphology of these species has implications for their capture and retention in commercial fisheries where interaction are more akin to those of sharks species then typical dorsal ventrally flattened batoids species. It is currently unclear whether the shark-like morphology of these species will also affect how they use space within an ecosystem. In Australia G. typus has been classified as ‘high risk’ in ecological risk assessments due to distributional overlap with multiple fisheries (notably gill-net and prawn trawl) and assumed low productivity (Salini et al. 2007). The Rhynchobatus spp. complex in Australia is comprised of three distinct species, Rhynchobatus australiae, R. laevis and R. palpebratus that have consistently been confused in the literature (Last and Stevens 2009). The species complex in Australian waters has made assessing the level of threat to this group challenging. Current management strategies within Queensland waters treat the species complex as a single group due to difficulties in identification. Thus we have treated all individuals as a group that will herein be referred to as Rhynchobatus spp. Although fishing effort in Australia is not as intense as South-East Asia they are taken in fisheries, and development is altering the habitat, hydrology and water quality of nearshore areas (U.N. 2012). If nearshore areas are critical habitat for shark-like batoids then significant development in these regions may have long-term implications for the stability of these populations.
Fisheries dependent data suggest both juvenile and adult G. typus and Rhynchobatus spp. occur within the same nearshore areas in northern Queensland (White et al. 2013). However, habitat utilisation by these morphologically similar species, and whether these nearshore areas represent important habitats, remains unclear. The purpose of the present study was to examine: 1) residency of two shark-like batoids within a nearshore region; 2) compare activity space size between and within species; and 3) investigate changes in spatial ecology based on size and sex within species.
Materials and methods
Study location
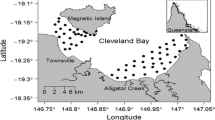
Cleveland Bay (19o12′3″S, 146o54′4″E) is a shallow water embayment situated in the central region of the Great Barrier Reef World Heritage Area (GBRWHA). The bay is approximately 27 km wide and covers an area of 225 km2. The majority of the bay is less than 10 m deep with a maximum tidal range reaching 4.2 m and encompasses a diverse range of habitat types including mangroves, fringing coral reefs and seagrass beds.
Field methods
A series of 63 VR2W acoustic receivers (Vemco Ltd.; www.vemco.ca) were deployed in November 2008 throughout the Conservation Park Zone (gillnet and trawling prohibited, bait netting and line fishing permitted) of Cleveland Bay to passively monitor the movement of a range of inshore predators, including two shark-like batoids. Acoustic receivers recorded time, date and identity of tagged individuals that swam within detection range of the units. Receivers were deployed in a grid arrangement and extended across all habitat types present including mangroves, seagrass, fringing reef, sand and mud. Receivers had a detection range of approximately 900 m (Heupel unpubl. data). Receivers were serviced quarterly to download data, change batteries and remove biofouling.
Glaucostegus typus and Rhynchobatus spp. were caught between October 2009 and January 2011 using long lines (500 m bottom set mainline—6 mm nylon rope) and gill nets (length 200 m, mesh size 114 mm). Hooks were attached to the long line on gangions composed of a 1 m section of nylon cord, a swivel and 1 m of wire trace. Sizes 10/0, 14/0, 16/0 Mustard tuna circle hooks, 10/0 Gamakatsu octopus hooks, circle (Offset-Point) and 10/0 Eagle claw wide gap hooks were used and baited with either squid (Loligo opalescens), blue threadfin salmon (Eleutheronema tetradactylum) or butterfly bream (Nemipteris spp.). Hook size and type was varied to reduce any size selectivity bias associated with the long lines. Captured shark-like batoids were secured to the boat using a tail rope and then placed ventral side up. Once individuals were in a state of tonic immobility measurements and transmitter deployment commenced. Individuals were sexed, stretch total length (STL) was measured to the nearest mm, a genetic sample was taken and individuals were tagged with a rototag in the first dorsal fin. Individual maturity was classified as either juvenile or adult according to known size at maturity estimates (Last and Stevens 2009). Transmitters were surgically implanted into the abdominal cavity to ensure long-term retention and mitigate against biofouling (JCU animal ethics permit #A1566). Individuals with stretch total lengths less than 700 mm were fitted with V13 transmitters (13 × 36 mm) and larger individuals were fitted with larger V16 transmitters (16 × 68 mm). All transmitters were coded to allow individual identification and were set to pulse randomly once every 45—75 s at 69 kHz. Random repeat rates allowed multiple individuals to be monitored simultaneously without the signals continuously overlapping.
Data analysis
Data collected from acoustic receivers were analyzed to examine presence, residency and movement patterns of shark-like batoids within Cleveland Bay. The locations of monitored individuals within the receiver array were estimated every 30 min using a mean position algorithm that provided an individual’s center of activity (COA) (Simpfendorfer et al. 2002). Data analyses for this study were conducted in the R environment (R Development Core Team 2009).
Residency
The daily presence of shark-like batoids was defined by at least two detections of an individual for that day on any receiver within the array. Daily presence was plotted to provide a visually interpretable timeline of occurrence within Cleveland Bay throughout the study period. One-way ANOVAs were used to compare the influence of sex on total days monitored and total days detected. A Residency Index (RI) was calculated for each individual following methods described by Simpfendorfer et al. (2011b) where the ratio between the number of days an animal was detected to the number of days from the first to the last detection was determined. A value of one indicated an individual was always present, while zero indicated an individual was not detected after release. Residency index values were compared between species with size, sex and total number of days monitored using analysis of covariance (ANCOVA). A post-hoc Tukeys unequal N Honest Significant Difference (HSD) test was used to identify groups that were significantly different from each other.
Activity space
Activity spaces of shark-like batoids were calculated based on COA estimates using 50 and 95 % kernel utilization distributions (KUD) calculated with the adehabitat package in R (Calenge 2006). Activity spaces were calculated at monthly intervals, plotted in R using Maptools and subsequently plotted using ARCmap. One-way ANOVA was used to test for differences in 50 and 95 % KUDs within and between species, sexes and size classes. Size of KUD was compared between years for individual’s for which philopatry was observed using one-way ANOVA.
Results
A total of 15 G. typus were fitted with acoustic transmitters and included 7 males and 8 females representing comparable length ranges (Table 1). With the exception of one female (Transmitter 56316; STL = 2,660 mm), all tagged and released G. typus provided detection data. Twenty Rhynchobatus spp. were fitted with acoustic transmitters. Females dominated this sample (n = 18) and ranged from 860 to 2,650 mm STL. Only two males (975 and 1,500 mm STL) were captured, fitted with transmitters and released. One female Rhynchobatus spp. (Transmitter 56319; STL = 2,260 mm) released with a transmitter 1.3 km from the outer line of the eastern side of the array and one female (Transmitter 56312; STL = 1,420 mm) released close to the western boundary were never detected.
Residency
Glaucostegus typus were monitored between 1 and 766 days (mean = 333 ± 69 days) and were present in the site from 1 to 198 days (mean 73 ± 25 days). There was no significant difference between sexes for either total days monitored (Table 2: ANOVA, F 1,30 = 0.66, P = 0 · 42) or days present (Table 2; ANOVA, F 1,13 = 0.8237, P = 0.38). The RI did not differ significantly between sexes (Table 2; ANOVA, F 1,13 = 0.23, P = 0 · 63). Residency of both sexes changed with individual size (ANOVA, F 1,13 = 8.86, P < 0.05). Juveniles (STL < 1,000 mm) had very low residency indexes. Residency increased in sub adult individuals (STL 1,000–1,500) and then decreased for adults (STL > 1,500 mm). Philopatric behavior (returning to the same location in subsequent years) was exhibited by adult G. typus. Adult females were observed to leave the bay in the first weeks of December prior the wet-season and returned in October the next year. Six of nine G. typus females ranging in size from 2,110 to 2,670 mm exhibited philopatry (Fig. 2a). Periods of absence ranged from 284 to 704 days (mean = 391 days). Two of these individuals returned in two consecutive years; 56,311 was absent between 5/11/2009 and 7/11/2010 (367 days) and again between 13/12/2010 and 28/10/2011 (319 days). Similarly, 56,536 was absent between 14/12/2009 and 4/10/2010 (294 days) and again from 21/12/2010 to 2/10/2011 (285 days). The remaining four females had a single philopatric event during the monitoring period with absences of 284, 309, 383 and 704 days respectively. Three male G. typus were also observed to leave and return to the bay with periods of absence of 155, 286 and 333 days (Fig. 2a). Males returned to the bay earlier than females, typical during August and September.
Rhynchobatus spp. were monitor for 1 to 707 days (mean = 231 ± 50 days) and were present in the site from 1 to 350 days (mean 82 ± 24 days) (Table 2: Fig. 2b). There was no significant difference between sexes in total days monitored (Table 2: ANOVA, F 1,15 = 0.01, P = 0 · 91) and total days present (Table 2: ANOVA, F 1,15 = 0.12, P = 0.72). However, given the low number of males monitored these results are inconclusive. There was no significant difference in RI between sexes (Table 2: ANOVA, F 1,15 = 0.06, P = 0 · 80) or size of individuals (ANCOVA F 3,12 = 0.8, P = 0 · 51). Individuals were observed to leave Cleveland Bay and return again with absences ranging from days to months. The longest absence was by a female (Transmitter 46986; STL = 1,960 mm) between 20/4/2011 and 13/11/2011 (207 days). However, synchronous philopatric behavior was not evident for Rhynchobatus spp. individuals. Individuals of all sizes monitored intermittently left the array for short periods (days-weeks) prior to returning. With the exception of individual 46,986 no individuals were observed to return to the bay once they had been absent for more than 200 days.
Activity space
When detected within the array Glaucostegus typus predominantly remained within the area of capture and displayed small core activity spaces (Fig. 3). Only two individuals moved between the eastern and western side of the array, both were adults: one female (STL = 2,670 mm) and one male (STL = 2,450 mm). Glaucostegus typus were found to have monthly 50 % KUDs that ranged from 2.4 to 18.2 km2 (mean = 9.57 km2) and monthly 95 % KUDs that ranged from 6.3 to 63.9 km2 (mean = 43.38 km2). Females and males had similarly sized activity spaces (Table 3; ANOVA, 50 %: F 1,13 = 0.46, P = 0 · 5; ANOVA, 95 %: F 1,13 = 0.00, P = 0 · 95). Glaucostegus typus with lower residency indices had larger activity spaces (Fig. 4a and b; ANCOVA 50 %: F 1,13 = 8.43, P < 0.05; 95 %: F 1,13 = 14.95, P < 0.001) and activity space varied with the size of individual (Fig. 4c and d; 50 %: F 1,13 = 9.11, P < 0.05; 95 %: F 1,13 = 19.14, P < 0 · 001). Juveniles (STL < 1,500 mm) had activity spaces that were concentrated in the shallow regions of Cleveland Bay while adults used shallow areas in addition to deeper regions further from the coast (Fig. 4). Glaucostegus typus returning to the bay annually used the same areas where they had been detected in previous years (Fig. 5) and activity space was similar among years (Table 3; ANOVA. 50 %:F 3,3 = 2.178, P = 0.2696; 95 %: F 3,3 = 4.42, P = 0 · 12).
Glaucostegus typus. Activity space of 4 adult G. typus that returned to Cleveland Bay inter-annually including; (a–c) female (STL = 2670 mm), d–f female (STL = 2650 mm), g–h male (STL = 2,450 mm), and (i–j) male (STL = 2,630 mm). Panels are 95 % Kernel Utilization distributions (KUDs)(solid line) and 50 % KUDs (black fill)
Rhynchobatus spp. activity space within Cleveland Bay tended to be localized within the western side of the bay (Fig. 6). Rhynchobatus spp. 50 % KUDs ranged from 4.0 to 20.6 km2 (mean = 7.03 km2) and 95 % KUDs ranged from 18.6 to 76.4 km2 (mean = 41.04 km2). There was no significant difference in KUD size between sexes (Table 3; ANOVA, 50 %: F 1,15 = 0.45, P = 0 · 5; ANOVA, 95 %: F 1,15 = 0.82, P = 0 · 38). Activity space size of Rhynchobatus spp. was not related to either RI (Fig. 6a and b. 50 %: ANOVA F 1,15 = 0.62, P = 0.44; 95 %: F 1,15 = 0.36, P = 0.55) or size of individual (Fig. 6c and d. 50 %: ANCOVA F 1,14 = 0.24, P = 0 · 62; 95 %: F 1,14 = 0.82, P = 0 · 37). Although Rhynchobatus spp. preferred different regions of Cleveland Bay to G. typus, activity space size was similar (Table 2; ANOVA, 50 %: F 1,32 = 1.31, P = 0 · 26; ANOVA, 95 %: F 1,32 = 2.03, P = 0 · 16).
Discussion
Using long-term movement data, this study found that despite being morphologically similar Glaucostegus typus and Rhynchobatus spp. use space in nearshore waters differently. Rhynchobatus spp. tended to be present for longer continuous periods while G. typus were present for shorter, predictable periods. Philopatry has been observed in a number of elasmobranch species (see review by Hueter et al. 2005) but the present study is the first to quantify the repetitive seasonal use of nearshore areas by a shark-like batoid. Individuals returned annually to use the same regions suggesting strong site fidelity. The spatial ecology of male G. typus changed with the ontogenetic shift to maturity, with resident sub adult individuals (STL = 1,000–1,500 mm) becoming transient adults (STL = 2,450–2,650 mm). Reduced transmitter detection associated with very shallow habitats may have contributed to the low residency index values for individuals with stretch total lengths less than 1,000 mm. Fisheries dependent (e.g., catch data; R. productus; Marquez-Farias 2005) and independent (e.g., belt transects; G. typus; Vaudo and Heithaus 2009) surveys have previously documented seasonal movement of shovelnose ray species into nearshore areas. However these studies did not quantify how individuals used space and the synchronous manner of the philopatry. Rhynchobatus spp. residency was highly variable with no relationship between individual size and presence within the bay. It is possible that any patterns of spatial ecology have been masked by monitoring individuals from all three species of the complex.
Glaucostegus typus showed both seasonality and site fidelity in the use of Cleveland Bay. Adult females arrived in October and left in the first weeks of December prior the wet-season. Returning females inhabited the same regions of the bay and had similar sized activity spaces between years. Adult males returned to the bay several weeks prior to the return of females. Activity space of adult males and females overlapped during periods of presence within the bay. Fisheries independent sampling found adult males had sperm running and females of lengths over 2,200 mm had mid- to late-term embryos between September to November (White, unpubl. data), suggesting that presence of adult G. typus within the bay may have been associated with pupping and possibly mating. Observation of neonates within mangrove habitats of the bay, after the wet-season further supports the link between use of the bay as a mating and/or pupping area. Other species of shovelnose ray (e.g., Rhinobatos productus) have been found in nearshore areas of California (Talent 1985) and Baja California (Salazar-Hermoso and Villavicencio-Garayzar 1999) during summer months suggesting that these species may seasonally migrate into these habitats. However, the longevity and intensity of the shovelnose ray fishery that operates in the area suggests the Baja California population are resident year round and not philopatric (Farrugia et al. 2011). Traditional mark-recapture and acoustic monitoring of juvenile R. productus found no inter-annual site fidelity (Farrugia et al. 2011), similar to the present study in which only adults were observed to return to the study site. Strong site fidelity observed in adult G. typus suggests that nearshore areas are a key component of the species spatial ecology, and may form critical habitat. Identification of critical habitats can greatly improve process of species management, through the use of spatial and seasonal regulations to protect both the habitats themselves and the species that use them.
The core activity space of G. typus juveniles was typically centered in shallow regions on the eastern side of the bay close to sand beaches and mangrove fringed coastline. Acoustic tracking of Pristis pectinata and P. microdon revealed similar behaviour with neonate sawfish inhabiting extremely shallow waters (Whitty et al. 2009; Simpfendorfer et al. 2010). The occurrence of G. typus in shallow waters may be related to predator avoidance, optimising growth or as a consequence of foraging behaviour (Sims 2003; Matern et al. 2004; Wetheree et al. 2007). Vaudo and Heithaus (2009) suggested that G. typus preference for shallow habitats in Shark Bay, Western Australia, was driven by physiological gains attained through the exploitation of local thermal heterogeneity. Physiological gains may also be driving habitat use of G. typus in the present study, but this remains to be demonstrated. While shallow nearshore habitats may provide advantages for shark-like batoids, their proximity to shore (and hence human development and activities) also makes them more vulnerable to anthropogenic impacts, and may mean that the species is most vulnerable in these habitats.
Glaucostegus typus and Rhynchobatus spp. are more mobile with larger activity spaces than other predominantly sedentary batoid species. With well-developed dorsal and caudal fins the body form of shark-like batoids falls between that of disc-shaped batoids and fusiform shark species. This morphology allows for greater swimming ability, which likely contributes to larger activity spaces than reported for disc-shaped batoids. The activity space of benthically associated disc-shaped rays (e.g., Dasyatis lata, Urobatis halleri; Cartamil et al. 2003; Vaudo and Lowe 2006), tend to be small (c. 1 km2), a consequence of spending long periods of time resting on the bottom. The fusiform shark species Carcharhinus amboinensis and C. sorrah monitored in Cleveland Bay had larger activity spaces (Knip et al. 2011a, 2012b) despite having smaller body sizes than the shark-like batoids examined. This suggests that shark-like batoids, while highly mobile, spend a portion of their time sedentary on the bottom and hence have moderate sized activity spaces. Glaucostegus typus and Rhynchobatus spp. spatial ecology is closest to morphologically similar species like the sawfish Pristis pectinata which has reported activity spaces (95 % KUD) between 4 km2 and 104 km2 (Simpfendorfer et al. 2010), and mid-water swimming batoid species like the myliobatid ray R. bonasus which reportedly has an activity space between 0.1 km2 and 62 km2 (Collins et al. 2007). Glaucostegus typus and Rhynchobatus spp. have smaller activity spaces sizes than highly mobile shark species, but larger than disc-shaped rays suggesting their behavior lies somewhere between these two groups.
The lack of correlation between Rhynchobatus spp. size and residency may be a result of the occurrence of three possible species in the species group. However, there were two clusters of individuals of similar size but differing residency within these data that may represent different species within the Rhynchobatus spp. complex. Varying size at maturity between species may explain differences in residency, with individuals with higher residency belonging to a species with larger size at maturity (possibly R. laevis) and and so monitored individuals would therefore be sub-adult. Large individuals with low residency may be adult R. australiae or R. palpebratus. The sample population was strongly skewed toward females, suggesting that habitat use may be partitioned by sex. With no general pattern of movement into or out of the bay it appears there is no synchronised philopatry as was the case for G. typus. Similar to Rhynchobatus spp. the fusiform shark species Rhizoprionodon terraenovae exhibited no consistent pattern of habitat use, had low residency and individuals moved into and out a bay frequently (Carlson et al. 2008). Like R. terraenovae, Rhynchobatus spp. may not be philopatric to specific nearshore areas but rather move between them.
The discrete use of nearshore areas has predominantly been described for fusiform shark species. Despite the ecological significance of shark-like batoids as mesopredators there is little understanding of how and why they utilize nearshore areas. The results of this study show that shark-like batoids with similar morphology have differing spatial ecologies. Inter-annual consistency in activity space size and location within the bay, coupled with the reproductive stage of individuals suggest Cleveland Bay provides critical habitat for G. typus. Rhynchobatus spp. had different habitat use and residency in the bay. Further research is needed to quantify the movements, habitat preferences and seasonality of shark-like batoids in other regions and habitats if the spatial ecology of these species is to be fully understood at the ecosystem scale. The present study improves our understanding of shark-like batoid spatial ecology in nearshore waters and may provide potentially useful information for the management of these populations.
References
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Carlson J, Heupel M, Bethea D, Hollensead L (2008) Coastal habitat use and residency of juvenile atlantic sharpnose sharks (Rhizoprionodon terraenovae). Estuar Coast 31(5):931–940. doi:10.1007/s12237-008-9075-2
Cartamil DP, Vaudo JJ, Lowe CG, Wetherbee BM, Holland K (2003) Diel movement patterns of the Hawaiian stingray, Dasyatis lata: implications for ecological interactions between sympatric elasmobranch species. Mar Biol 142:841–847
Collins AB, Heupel MR, Motta PJ (2007) Residence and movement patterns of cownose rays Rhinoptera bonasus within a south-west Florida estuary. J Fish Biol 71:1159–1178
Conrath CL, Musick JA (2010) Residency, space use and movement patterns of juvenile sandbar sharks (Carcharhinus plumbeus) within a Virginia summer nursery area. Mar Freshw Res 61:223–235
DeAngelis BM, McCandless CT, Kohler NE, Recksiek CW, Skomal GB (2008) First characterization of shark nursery habitat in the United States Virgin Islands: evidence of habitat partitioning by two species. Mar Ecol Prog Ser 358:257–271
Farrugia TJ, Espinoza E, Lowe CG (2011) Abundance, habitat use and movement patterns of the shovelnose guitarfish (Rhinobatus productus) in a restored southern California estuary. Mar Freshw Res 62:648–657
Heupel MR, Webber DM (2012) Trends in fish tracking: where are the fish going and how will we follow them? Am Fish Soc Symp 76:219–231
Heupel MR, Semmens JM, Hobday AJ (2006) Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshw Res 57:1–13
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297
Heupel MR, Simpfendorfer CA, Fitzpatrick R (2010) Large-scale movement and reef fidelity of grey reef sharks. PLoS ONE 5(3):e9650
Hueter RE, Heupel MR, Heist KJ, Kneey DB (2005) Evidence of philopatry in sharks and implications for the management of shark fisheries. J Northwest Atl Fish Sci 35:239–247
Knip DM, Heupel MR, Simpfendorfer CA (2010) Sharks in nearshore environments: models importance, and consequences. Mar Ecol Prog Ser 402:1–11
Knip DM, Heupel MR, Simpfendorfer CA, Tobin A, Moloney J (2011a) Ontogenetic shifts in movement and habitat use of juvenile pigeye sharks Carcharhinus amboinensis in a tropical nearshore region. Mar Ecol Prog Ser 425:233–246
Knip DM, Heupel MR, Simpfendorfer CA, Tobin A, Moloney J (2011b) Wet-season effects on the distribution of juvenile pigeye sharks, Carcharhinus amboinensis, in tropical nearshore waters. Mar Freshw Res 62:658–667
Knip DM, Heupel MR, Simpfendorfer CA (2012a) To roam or to home: site fidelity in a tropical coastal shark. Mar Biol 159:1647–1657
Knip DM, Heupel MR, Simpfendorfer CA (2012b) Habitat use and spatial segregation of adult spottail sharks Carcharhinus sorrah in tropical nearshore waters. J Fish Biol 80(4):767–784
Last PR, Stevens J (2009) Sharks and rays of Australia vol 2. CSIRO Publishing, Melbourne
Marquez-Farias JF (2005) Gillnet mesh selectivity for the shovelnose guitarfish (Rhinobatos productus) from fishery-dependent data in the artisanal ray fishery of the Gulf of California, Mexico. J Northwest Atl Fish Sci 35:443–452
Matern SA, Cech JJ, Hopkins TE (2004) Diel movements of bat rays, Myliobatis californica, in Tomales Bay, California: evidence for behavioral thermoregulation? Environ Biol Fishes 58:173–182
Pikitch EK, Chapman DD, Babcock EA, Shivji M (2005) Habitat use and demogaphic population structure of elasmobranchs at a Caribbean atoll (Glovers Reef, Belize). Mar Ecol Prog Ser 302:187–197
Salazar-Hermoso F, Villavicencio-Garayzar C (1999) Relative abundance of the shovelnose guitarfish Rhinobatos productus (Ayres, 1856) (Pisces: Rhinobatidae) in Bahia Almejas, Baja California Sur, from 1991 to 1995. Cienc Mar 25:401–422
Salini J, Pillans R, Ovenden J, Buckworth R, Gribble N, McAuley R, Stevens J (2007) Northern Australian sharks and rays: the sustainability of target and bycatch species, phase 2. FRDC Project: 2002/064. CSIRO Marine and Atmospheric Research, Cleveland, Australia.
Simpfendorfer CA, Milward NE (1993) Utilisation of a tropical bay as a nursery area by sharks of the Families Carcharhinidae and Sphyrnidae. Environ Biol Fish 37(4):337–345
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci 59:23–32
Simpfendorfer CA, Wiley TR, Yeiser BG (2010) Improving conservation planning for an endangered sawfish using data from acoustic telemetry. Biol Conserv 143(6):1460–1469
Simpfendorfer CA, Heupel MR, White WT, Dulvy NK (2011a) The importance of research and public opinion to conservation management of sharks and rays: a synthesis. Mar Freshw Res 62:518–527
Simpfendorfer CA, Yeiser BG, Wiley TR, Poulakis GR, Stevens PW, Heupel MR (2011b) Environmental influences on the spatial ecology of juvenile smalltooth sawfish (Pristis pectinata): results from acoustic monitoring. PLoS ONE 6(2):e16918
Sims DW (2003) Tractable models for testing theories about natural strategies: foraging behaviour and habitat selection of free-ranging sharks. J Fish Biol 63:53–73
Speed CW, Field IC, Meekan MG, Bradshaw CJA (2010) Complexities of coastal shark movements and their implications for management. Mar Ecol Prog Ser 408:275–293
Talent JG (1985) The occurrence, seasonal distribution and reproductive condition of elasmobranch fishes in Elkhorn Slough, California. Calif Fish Game 71:210–219
Team RDC (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
U.N. (2012) Convention concerning the protection of the world cultural and natural heritage. United Nations Educational. Scientific and Cultural Organization, Saint-Petersburg, Russian Federation.
Vaudo JJ, Heithaus MR (2009) Spatiotemporal variability in a sandflat elasmobranch fauna in Shark Bay, Australia. Mar Biol 156:2579–2590
Vaudo JJ, Heithaus MR (2012) Diel and seasonal variation in the use of a nearshore sandflat by a ray community in a near pristine system. Mar Freshw Res 63(11):1077–1084. doi:10.1071/MF11226
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. J Fish Biol 68(6):1756–1766
Voegeli FA, Smale MJ, Webber DM, Andrade Y, O’Dor RK (2001) Ultrasonic telemetry, tracking and automated monitoring technology for sharks. Environ Biol Fishes 60:267–281
Wetheree BM, Gruber SH, Rosa RS (2007) Movement patterns of juvenile lemon sharks Negaprion brevirostris within Atol das Rocas, Brazil: a nursery characterized by tidal extremes. Mar Ecol Prog Ser 343:283–293
White WT, McAuley R (2003) Rhinobatos typus In: IUCN 2012. IUCN red list of threatened species. Version 2012.1. http://www.iucnredlist.org. Downloaded on 09 January 2012.
White WT, Potter IC (2004) Habitat partitioning among four elasmobranch species in near shore, shallow waters of a subtropical embayment in Western Australia. Mar Biol 145(5):1023–1032
White J, Heupel MR, Simpfendorfer CA, Tobin AJ (2013) Shark-like batoids in Pacific fisheries: prevalence and conservation concerns. ESR 19(3):277–284. doi:10.3354/esr00473
Whitty JM, Morgan DL, Peverell S, Thorburn DC, Beatty SJ (2009) Ontogenetic depth partitioning by juvenile freshwater sawfish (Pristis microdon; Pristidae) in a riverine environment. Mar Freshw Res 60:306–316
Yates PM, Heupel MR, Tobin A, Simpfendorfer CA (2012) Diversity in young shark habitats provides the potential for portfolio effects. Mar Ecol Prog Ser 458:269–281
Acknowledgments
We thank the staff and students of the Centre for Sustainable Tropical Fisheries and Aquaculture, including A. Chin, F. De Faria, A. Schlaff, O. Li, A. Harry and all volunteers for assistance with this project. Funding for this research was provided by the Australian Research Council (ARC) and Great Barrier Reef Marine Park Authority (GBRMPA) awarded to M.R. Heupel and C.A. Simpfendorfer. Additional research funding was granted to J. White from Sea World Research and Rescue Foundation (SWRRFI), James Cook University (JCU) School of Earth and Environmental Sciences (SEES). J. White was also supported by a JCU Postgraduate Research Scholarship and Smart State Futures Stipend. All research activities were conducted under GBRMPA permit # G09/29895.1 and Queensland Department of Primary Industries and Fisheries permit #90911. Treatment of all animals was conducted under ethical guidelines approved by JCU animal ethics #A1566. Spatial data was provided by the Commonwealth of Australia (Great Barrier Reef Marine Park Authority) 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
White, J., Simpfendorfer, C.A., Tobin, A.J. et al. Spatial ecology of shark-like batoids in a large coastal embayment. Environ Biol Fish 97, 773–786 (2014). https://doi.org/10.1007/s10641-013-0178-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-013-0178-7