Abstract
Parasites may be an important component of early life mortality in fishes, but assigning part of total mortality to parasites is difficult. The Chapman-Robson mortality estimator is a robust and potentially valuable way to quantify the added mortality of parasites when age data are available. We used daily age data and the Chapman-Robson catch-curve procedure to estimate daily mortality for 15 years in juvenile age-0 shortnose suckers (Chasmistes brevirostris), and for 6 years, the daily mortality of fish with and without black spot infection, a trematode whose final host is a piscivorous bird. Infected fish always had higher mortality rates than uninfected fish, and for 3 years when those differences were significant, the added daily mortality for infected fish was 3.6–3.7 %. Based on the proportion infected each year, and for durations of 15–50 d, juvenile populations were 18.3–38.6 % lower than they would have been without black spot infections. There were no significant differences in growth between infected and uninfected fish in most years and little indication of a direct metabolic impact of infections. Thus, this added mortality, primarily in July and August, seemed unlikely to be an indirect result of infection and was most likely due to predation. The source of that predation is unknown but the parasite’s final hosts, piscivorous birds, seemed the most obvious candidate for this added mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early life-history losses in fishes are thought to be caused by predation, advective loss from nursery areas, and starvation (Sinclair 1988; Bailey and Houde 1989; Houde 2002), and parasites can potentially exacerbate all three mechanisms (Bourque et al. 2006). Strathmann et al. (2002) have suggested that the pelagic larval stage, common in many fishes and invertebrates, is an evolutionary mechanism to reduce parasite infections by reducing host densities relative to benthic habitats. In general, the effect of parasites on early life stages of fish will be greater than that on adults, in part due to their small size relative to the parasite’s size and the added metabolic demands of parasites.
Despite long recognition that parasites might be an important source of early life mortality in fishes, there is relatively little research on their effects on growth, condition, or survival of early life stages (Grutter et al. 2009), or on the demographic impact on adult recruitment. For cases where demographic impacts have been evaluated, parasite-mediated mortality may be significant. For example, Bourque et al. (2006) suggested that interannual variability in a cestode infection in larval and juvenile smelt could drive recruitment variability, and Ferguson et al. (2011) demonstrated that up to 75 % of overwinter mortality in one group of coho salmon parr could be attributed to heavy infections of several parasites. These studies employed commonly used analytical methods (Anderson and Gordon 1982; Lester 1984) to estimate mortality from relatively high rates of infections (100 s to 1,000 s of parasites per fish). These methods assume that parasites have direct lethal effects and that low intensity infections are less significant than high intensity infections. Because parasites use their host’s nutritional reserves, a direct relationship between intensity and mortality is to be expected, but in small fishes, the difference between prevalence and intensity may be small. Grutter et al. (2009) have shown a single ectoparasite, such as a gnathiid isopod, can kill larval and small juvenile fishes as well as negatively affect fish performance and increase mortality indirectly. Planes et al. (2009) found that only parasite-free juvenile sparids survived in their study.
Digenetic trematodes (parasitic flatworms) can be a significant cause of fish mortality because they increase the probability of the fish host being consumed (Barber et al. 2000; Ferguson et al. 2011) or reduce survival during times of stress such as over winter (Pracheil and Muzzall 2010). Digenetic trematodes have complex life cycles with a final vertebrate host and one or more intermediate hosts. In the three-host life cycle, the first intermediate host is usually a mollusc, often a snail, which releases infectious cercariae that encyst as a metacercaria in a second intermediate host, such as a fish. Successful metacercariae do not kill the fish host directly but increase the probability of predation by the final host, often a bird. Metacercariae of some trematodes are easily visible as external cysts. One group of these was given the genus name, Neascus, and cause black spot infections in fishes. Subsequent work showed that several adult trematode genera had neascus-like metacercariae and the adult taxonomy and nomenclature, rather than the larval nomenclature, are now used. As is the case with the larval fish genus, Leptocephalus, we use the genus as a descriptive life history stage term, and refer to neascus-like metacercaria. This is a common and convenient terminology (Ferguson et al. 2010), because all neascus-like metacercaria are known to exhibit low host specificity (Cairns et al. 2005) and typically have a life cycle with snails as the first intermediate host, fish as the second intermediate hosts, and fish-eating birds as the final host (Cairns et al. 2005). For example, Hoffman and Putz (1965) recorded 25 species of fish hosts for the black spot trematode, Uvulifer ambloplitis.
The shortnose sucker (Chasmistes brevirostris Cope 1879) is an endangered species endemic to the Klamath Basin of Oregon and California (USFWS 1988). The initial listing was based, in part, on the perception of recruitment failure and subsequent work has confirmed intermittent and low adult recruitment (Janney et al. 2008). Upper Klamath Lake, the primary habitat of the species, is hypereuthrophic (Eilers et al. 2004) and poor water quality is also a conservation concern.
Here we used a large time series to explore the use of catch-curve data to estimate daily mortality in juvenile shortnose suckers with and without black spot infection, the external metacercarial cysts of a neascus-like trematode. Our purpose was to examine the effect of trematode metacercariae on growth of juvenile shortnose suckers, describe 15 years of interannual variation in parasite incidence, quantify the effect of parasites on mortality, and speculate on the potential impact of bird predation on age-0 survival in late summer.
Methods
Sample collection and specimen data
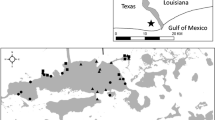
Juvenile (=age-0) suckers were collected from June through October, 1995–2009, in the Upper Klamath Lake system by beach seine, cast net, and otter trawl. The primary inflow to Upper Klamath Lake is the Williamson River and the outlet is the Link River that flows into Lake Ewauna. In 2007 and 2008 levees around the Williamson River delta were breached as part of habitat restoration (Wood et al. 2012), and effectively broadened the connection between Agency Lake and Upper Klamath Lake (Fig. 1).
With few exceptions, sample effort and timing were constant from year to year. Fixed-site beach seine sampling began in early June and continued every third week through early August. The beach seine was 6.1 m long with a 2 × 2 × 2 m bag, and 4.8 mm bar mesh. Each sample was collected as a quarter circle arc from shore, normally from water less than 1.5 m deep and over non-vegetated or sparsely vegetated shorelines. Two samples were collected at each site in Upper Klamath Lake (10 sites) and Agency Lakes (5 sites) and one sample at each site in Lake Ewauna (6 sites), River Bend (2 sites), and South Marsh (4 sites). Dense vegetation in late summer prevented some collections from River Bend and South Marsh. After delta restoration the site at the former mouth of the Williamson River was retained but a new site, at the new mouth of the Williamson River, was added.
Three stratified random cast net surveys were conducted each year, three weeks apart, in late August, mid September, and early October, with 140 samples per survey (420 samples per year), except for 1995 when there were only September (150 samples) and October (88 samples) surveys. In 2001, September sampling was delayed 1 week because of the terrorist attack on September 11. We used eight substrate categories as strata. A hand-held GPS unit and maps were used to locate each site. The cast net was a 5-m-diameter net with 6.3-mm bar mesh and sampling was always within 10 m of the shoreline. Cast net samples were collected from non-vegetated or sparsely vegetated locations and were collected along the edge of vegetation wherever the shoreline was densely vegetated. Typical sampling depths were 0.5–1.0 m, and rarely exceeded 1.5 m. Substrate type at each site was verified and bottom water quality (temperature, dissolved oxygen, and pH) measured at each site with a Hydrolab Reporter Multiprobe and Surveyor 3 Display Logger or a YSI 650 MDS Display with a 600QS Sonde.
Three random otter trawl surveys were conducted concurrently with the cast net surveys. We used a 5-m semi-balloon otter trawl with 16-mm bar mesh and 6-mm bar mesh liner with attached tickler chain. Trawl locations were randomly selected from a grid of coordinates at 1-km intervals covering Upper Klamath Lake. No site began within 1 km of the shoreline, and trawl direction was typically parallel with the prevailing wind. Each survey consisted of 15 tows, each tow lasted 20 min, and exact distance of each tow was calculated with differentially corrected GPS data. Bottom water quality (temperature, dissolved oxygen, and pH) was measured at the conclusion of each trawl with a Hydrolab Reporter Multiprobe and Surveyor 3 Display Logger or a YSI 650 MDS Display with a 600QS Sonde.
Age-0 suckers were preserved in 95 % ethanol, x-rayed in the lab and identified to species following Markle et al. (2005). There are three species of suckers present in the Upper Klamath Basin, but only specimens identified as shortnose suckers were included in this analysis. Each specimen was identified, measured, and externally examined under a dissecting microscope for presence of encysted, neascus-like metacercariae (“black spot”). We evaluated the location and color (pale, brown, black) of metacercariae on each fish. Cyst color changes from pale to brown to blue-black as metacercariae develop, and thus color can be used to evaluate relative timing of infections and heterogeneity of infections relative to fish size. For convenience, we use the word, “infected”, to refer to fish with black spot infections, recognizing that other parasites may be present when black spot is absent.
The median values of temperature, dissolved oxygen, and pH were compared between infected and uninfected suckers. Differences were evaluated at α =0.01 with a Mann–Whitney test and significant differences illustrated with a quantile plot.
A subset of 752 shortnose suckers >30 mm SL (standard length) was assigned daily ages based on the otolith ageing procedures in Terwilliger et al. (2003). Age was estimated in days based on the median number of otolith increments from three independent, blind reads. Smaller fish were excluded because the age at length relationship in this species becomes nonlinear when fish less than 30 mm SL are included. Aged suckers were randomly selected from size-stratified samples and age at length regressions calculated for infected and uninfected fish for each year. If there were no significant differences in intercepts or slopes, all fish for the year were pooled and a single regression calculated. All regressions assumed a hatch length of 9 mm. For mortality estimates, specimens were grouped into 5 day age groups based on the estimated age.
Newly infected fish were defined as those fish with pale cysts only. The pattern of new infections was examined by age groups for all years combined and for each year separately. Mortality estimates were first calculated with newly infected fish in the infected group, but because newly infected fish have most likely experienced the mortality regime of uninfected fish, an adjusted mortality was calculated with newly infected fish in the uninfected group. All analyses were performed with Statgraphics Centurion XVI and Excel.
Mortality estimation
For wild fish populations where an individual’s size or other constraints preclude tagging, catch-curve data are commonly used to estimate mortality (Chapman and Robson 1960; Robson and Chapman 1961; Dunn et al. 2002). Catch curves have an ascending limb representing fish that are not fully vulnerable to the sampling gear and a descending limb representing vulnerable fish. Only the descending limb is used to estimate Z. Assumptions behind this approach are: populations are closed to immigration and emigration, mortality on the descending limb of the catch curve is constant, vulnerability of all ages on the descending limb of the catch curve is constant, fish ages are accurate, and the age class can be accurately sampled over the season.
Because there is largely unknown sampling bias, especially in the fixed site beach seine samples, we focused on the difference in mortality between infected and uninfected fish. Although this bias would affect the accuracy of the background mortality estimate, we assume that any bias or inaccuracy in vulnerability, ageing, or tracking year classes due to the sampling protocol applies equally to both infected and uninfected suckers. We also think that, because of the restricted age range analyzed, the assumption of constant mortality is also reasonable. However, the closed population assumption is clearly violated for comparisons of the difference between infected and uninfected fish because the infected fish population is recruiting from the uninfected population. The closed population assumption will be most closely approximated when the proportion of new infections is low and individuals with new infections are grouped with uninfected fish.
The instantaneous total mortality (Z) for all fish (Za), infected fish (Zi), and un-infected fish (Zu) was estimated by the Chapman and Robson (1960) catch-curve estimator. For convenience, we present results as percent daily mortality (D) where
The difference in mortality between infected and uninfected fish (ΔD) was
where Di is the daily mortality rate of infected fish and Du is the daily mortality rate of uninfected fish. Compared to regression estimators, the Chapman-Robson estimator has been shown to have higher precision and lower bias and perform better at lower sample sizes in simulations with combined mortality, recruitment and ageing errors (Dunn et al. 2002).
The Chapman-Robson estimator requires that the initial, youngest, age of the declining limb be re-coded as age 0 and subsequent age groups recoded based on that initial age (Robson and Chapman 1961). The Chapman-Robson estimator of survival, \( \widehat{S} \), is:
where T is the weighted sum of the recoded ages with the weights equal to catch at age, and n is the total number of fish on the descending limb of the catch curve.
The estimate of the standard error of \( \widehat{S} \) is:
The estimate of Z is:
and the approximation of the standard error of Z is:
For interannual comparisons among all individuals (Za), we calculated mortality for each year that the coded age-0 group had >15 specimens and there were at least four consecutive age groups with at least one specimen. For intra-annual comparisons of infected (Zi) and uninfected (Zu) individuals, we calculated mortality for each year that the coded age-0 group of infected individuals had >15 specimens and there were at least four consecutive age groups with at least one specimen. The age groups and number of age groups for uninfected fish were constrained to match the infected fish that year so that the coded age-0 group and number of age groups in the declining limb were the same.
Finally, we applied the proportion infected, survival estimates for infected (\( {{\widehat{S}}_i} \)) and uninfected (\( {{\widehat{S}}_u} \)) fish, and the duration of the declining limb each year to a hypothetical coded age-0 population of 1,000,000. We estimated the number of survivors (Nu) if all fish experienced a survival rate of \( {{\widehat{S}}_u} \) and the number of survivors (Ni) if the proportion infected were subjected to \( {{\widehat{S}}_i} \) and the proportion uninfected were subjected to \( {{\widehat{S}}_u} \) for the duration. These estimates were used to illustrate the percent lost from the final population due to presence of the parasite:
Results
Digenea infection patterns
We examined 9,028 age-0 shortnose suckers, 12.6–90.2 mm standard length (SL), of which 2090 (13.6–80.5 mm SL) had external black spot infections. Pale cysts were found on 468 (22.4 %) of all infected shortnose suckers. Individuals that only had pale cysts (302, 13.6–80.5 mm SL) were considered to be newly infected and these declined from 5.7 % of all fish in the 20–25 mm size group to 0 infected fish in the 55–60 mm size group, before increasing to 6.1 % in the 75–80 mm size group (Fig. 2a).
Patterns of black spot infections in shortnose suckers, 1995–2009. Points show mean and vertical bars show 95 % confidence intervals. a Newly infected shortnose suckers in 5 mm size groups as a proportion of all individuals in the size group. All size groups with N > 30. Newly infected fish are those with pale cysts only. b Annual pattern of prevalence. c Seasonal pattern of prevalence
On an annual basis, prevalence of black spot was variable, ranging from 11.2 to 32.8 % (Fig. 2b). The three highest years of prevalence occurred since 2005. The seasonal pattern showed a rise through August followed by a decline (Fig. 2c). Black spot intensity on infected fish was relatively stable at between one and two parasites per infected fish (\( \widehat{x}=1.6 \)). Median water quality values for uninfected (N = 2823) and infected (N = 1219) shortnose suckers >30 mm SL were, respectively: temperature (20.9 C, 21.1 C), dissolved oxygen (8.13 mgL-1, 8.13 mgL−1), and pH (9.31, 9.32). Only median pH was significantly different with infected fish found at slightly higher pH than uninfected fish (P < 0.0001, Fig. 3).
Effect on growth
The regression of otolith age on standard length for fish >30 mm SL was examined for each year (Table 1). For the years 1995–2007, there were no differences in regression slopes or intercepts between infected and uninfected fish and a common regression was used for both groups each year. For 2008 and 2009, there were differences and separate regressions were used for each group in each year (Table 1).
In all years there was a tendency for otolith-aged infected fish to be slightly, but not significantly, older for their size than uninfected fish. Median studentized residuals from the common regressions from 1995 to 2007 were 0.01 for uninfected fish (N = 511) and 0.08 for infected fish (N = 171) (Kruskal Wallis P = 0.13).
Mortality
Annual daily survival of juveniles >30 mm SL ranged from 0.786 to 0.951 (Table 2). Small sample sizes precluded estimates in 4 years. As might be expected, estimated mortality rates were related to number of age groups in the declining limb; 2 years with only four age groups (1998 and 2002) had much higher daily estimated mortality rates (19.7–21.4 %) than years with 7–13 age groups (4.9–9.6 %).
In the 6 years where intra-annual comparisons could be made, survival of infected fish was always lower than uninfected fish regardless of whether newly infected fish were included (Table 3) or excluded from the infected group (Table 4). These differences were significant for three of 6 years: 1995, 1999 and 2000. The additional daily adjusted mortality estimates for fish with black spot infections for these 3 years was 3.6–3.7 % (Table 4). Most of the mortality represented by these analyses occurred in July and August (Table 5). At the end of the 15–50 d period of these mortality rates, the final population size was 18.3–38.6 % smaller because of parasite mortality (Table 5).
Discussion
Trematode cysts and the melanocytes around them are presumed to be persistent, not shed by the fish host (Hoffman and Putz 1965; Ferguson et al. 2011), and consequently, are a useful biological tag. In juvenile shortnose suckers in Upper Klamath Lake, neascus-like cysts show a strong seasonal pattern with prevalence peaking in August (Fig. 2c) and infected fish more likely to be collected at slightly higher pH than uninfected fish (Fig. 3). Annual patterns show relatively high prevalence in the last 2 years after delta restoration (Fig. 2b). Marshlands around the delta have been restored and have helped retain sucker larvae in Upper Klamath Lake (Markle et al 2005; Wood et al. 2012), but the heavily vegetated delta may also have had a positive effect on the trematode’s intermediate snail host. In a restored salt marsh in California, Huspeni and Lafferty (2004) found that prevalence of larval trematodes that parasitize snails quadrupled after restoration. These types of responses of agricultural land to marsh restoration may be due in part to reductions in herbicide use (Griggs and Belden 2008) or to increasing complexity of food webs after restoration (Huspeni and Lafferty 2004). Coupled with the nearshore habitat of juvenile shortnose suckers, the density of snails and shallow water may greatly increase the density of infectious cercariae and the chance of infection. Alternatively, predation could be heavier, more efficient, or more selective for infected fish in some areas.
Metacercariae persist in their hosts for months to years (Hoffman and Putz 1965; Ferguson et al. 2010). Consequently, a standard indication of differential mortality caused by a parasite is a decline in prevalence within a population over time (Lester 1984) and we saw this pattern after August (Fig. 2c). The presence of cysts also had a slightly negative, though not significant, effect on juvenile shortnose sucker growth and in most years we could not detect an effect on growth (Table 2). Thus, we did not see a strong effect on metabolic demands of these parasites on small juveniles.
Metacercariae can also change a fish’s behavior to increase its probability of succumbing to the final host, often an avian predator (Lafferty and Morris 1996). The only evidence we saw of behavioral differences was that infected fish were caught at slightly higher pH (Fig. 3), though the size of this effect was very small. The Chapman-Robson catch-curve parameter is a potentially valuable way to quantify the added mortality of parasites that may be present in low numbers but which cause indirect mortality by increasing vulnerability to the parasite’s final host. Our approach compared survival of infected and uninfected fish and offered a way to evaluate even these low infection rates. However, the approach had several challenges, some related to parasite-host systems and some to the target species.
The primary challenge is the lack of independence of the two populations, with the uninfected population contributing to the infected population. Effectively, any measure of mortality of the uninfected population includes mortality and loss to the infected group while any measure of mortality of the infected population includes mortality and gain from the uninfected group. Mortality can be artificially increased or decreased in either group based on whether the coded age-0 group or an older group is affected. The pattern of newly infected individuals, those with only pale cysts (Fig. 2a), indicated heterogeneity in new infections with age. We, therefore, assumed that newly infected fish were experiencing the mortality rate of the uninfected group and created adjusted estimates in which newly infected fish were grouped with uninfected fish. In other parasite-host systems, there may or may not be other ways to reduce the effect of this problem.
There are also difficulties measuring background mortality rate. A time series of early life history stages cannot usually be collected by a single gear and there is almost no work on the effects of gear selectivity on catch-curve analyses (Dunn et al. 2002), yet some measure of multiple size classes is necessary to determine the region of the declining limb of the catch curve. Although the comparison of infected and uninfected populations produced an estimate of background mortality, that estimate has an unknown bias from sampling. We assume that bias is the same for infected and uninfected populations and the additive mortality due to the presence of black spot is accurate. It is worth pointing out, in this regard, that the overall daily mortality estimates, 4.9–21.4 % (Table 2) and background daily mortality estimates, 5.0–20.0 % (Table 4), are higher than Houde’s (2002) estimate for a “typical marine early juvenile fish” (4.7 %). Whereas, for freshwater fish, Wilde and Durham (2008) estimated daily survival of 0.920–0.964 for six species of Great Plains minnows. Seven of our 11 overall daily survival estimates, 0.786–0.951 (Table 2), were within their range and four were lower. In the 3 years when differences were significant, the additional daily mortality of fish with black spot infections was 3.6–3.7 %.
A small increase in daily mortality, in the ranges we identified for black spot infections, compounded over time can have a profound impact on survival (Houde 1987). The effect of these rates on the population also depends on the proportion infected. We used the coded age-0 population as a starting point for proportion infected and assumed some infected fish die for reasons not due to black spot. The estimated populations after 15–50 d of parasite-related mortality were 18.3–38.6 % lower than they would be without the added mortality of black spot infections (Table 5).
A long-lived, endangered species with a history of poor recruitment is a poor candidate for any study that requires good sample sizes. In 15 years—which collectively had very poor recruitment—we had only 6 years which met criteria for analysis of infections. Those criteria might be loosened, but our interest was in a conservative estimate of the mortality due to black spot infections.
A key unknown is the source of additional mortality for infected fish. In most years we could not detect an effect of parasite prevalence on fish growth and in 2 years the effect was small, thus predation seems the logical source of additional mortality rather than direct mortality from the infection. We cannot currently identify this predation source but avian predation should be a prime suspect. During a PIT tag study of age 1+ suckers in Upper Klamath Lake between 2009 and 2012, Burdick (2013) found 1.2–4.7 % of tag detections were on American white pelican or double-crested cormorant colonies. There are, however, over 50 species of water birds found in the Upper Klamath Lake basin (Shuford et al. 2004) and many are piscivorous, so this estimate of the effect of avian predation on juvenile sucker mortality is certainly low (Burdick 2013). It is possible that behavioral or other changes which can be caused by parasites (Lafferty and Morris 1996) make fish more vulnerable to numerous predators, in addition to the parasite’s final host. The slight preference for higher pH in infected fish may be such a mechanism, but it is not clear how that preference would increase non-avian predation.
The implication, of course, is that avian or other predation on an endangered fish species may be limiting its recovery and management options may be limited. There would be social, legal, and ethical hurdles to efforts to control snail populations or fish-eating birds (Lane and Morris 2000) in such a large system. Most efforts to control trematode infections in fish are in pond culture and there are currently no plans to supplement wild populations of shortnose suckers. Should culture of this species begin, treatment of fish with a trematodicide prior to release might be an effective option. A caveat in our interpretation is that our parasite examination was superficial and other parasites, including other trematodes, are common in shortnose suckers (C. Banner, pers. comm., November 2012). It is possible that black spot infection is simply a marker that indicates a greater number of infections of all parasites than in uninfected fish. Susceptibility to disease and infections can have a genetic component and individuals with black spot infection could have reduced genetic diversity of the major histocompatibility complex, which plays an important role in the adaptive immune response (Fraser and Neff 2010).
Finally, it is difficult to measure mortality in fishes, especially of early life stages (Houde 2002), and even more so to separate parasite-caused host mortality in the field (Pracheil and Muzzall 2010). It is clear that analytical methods perform well, but that “the absence of methods to control for departures from assumptions” requires use of the most robust estimator, such as the Chapman-Robson estimator (Dunn et al. 2002). In addition to ageing errors and gear selectivity (Dunn et al. 2002), we need better field sampling that allows stronger inference about abundance of an age class. Our sampling included attempts to control for ageing errors and both fixed site and stratified random sampling, the later an attempt to allow stronger inference about abundance. Unless our sampling differentially selected either infected or uninfected fish, a possibility given the host behavioral changes that trematodes can cause (Lafferty and Morris 1996), the added mortality seen in infected fish should be a minimum estimate of mortality due to black spot infections.
References
Anderson RM, Gordon DM (1982) Processes influencing the distribution of parasite numbers within host populations, with special emphasis on parasite induced host mortalities. Parasitology 85:373–398
Bailey KM, Houde ED (1989) Predation on eggs and larvae of marine fishes and the recruitment problem. Adv Mar Biol 25:1–83
Barber I, Hoare D, Krause J (2000) Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev Fish Biol Fish 10:131–165
Bourque J-F, Dodson JJ, Ryan DAJ, Marcogliese DJ (2006) Cestode parasitism as a regulator of early life-history survival in an estuarine population of rainbow smelt Osmerus mordax. Mar Ecol Prog Ser 314:295–307
Burdick SM (2013) Assessing movement and sources of mortality of juvenile catostomids using passive integrated transponder tags, Upper Klamath Lake, Oregon—Summary of 2012 effort: U.S. Geological Survey Open-File Report 2013–1062, 12 pp
Cairns MA, Ebersole JL, Baker JP, Wigington PJ Jr, Lavigne HR, Davis SM (2005) Influence of summer stream temperatures on black spot infestation of juvenile coho salmon in the Oregon Coast Range. Trans Am Fish Soc 134:1471–1479
Chapman DG, Robson DS (1960) The analysis of a catch curve. Biometrics 16:354–368
Dunn A, Francis RICC, Doonan IJ (2002) Comparison of the Chapman-Robson and regression estimators of Z from catch-curve data when non-sampling stochastic error is present. Fish Res 59:149–159
Eilers JM, Kann J, Cornett J, Moser K, St Amand A (2004) Paleolimnological evidence of change in a shallow, hypereutrophic lake: Upper Klamath Lake, Oregon, USA. Hydrobiologia 520:7–18
Ferguson JA, Schreck CB, Chitwood R, Kent ML (2010) Persistence of infection by metacercariae of Apophallus sp, Neascus sp., and Nanoiphyteus salmincola plus two myxozoans (Myxobolus insidiosus and Myxobolus fryeri) in coho salmon Oncorhynchus kisutch. J Parasitology 96:340–347
Ferguson JA, Koketsu W, Ninomiya I, Rossignol PA, Jacobson KC, Kent ML (2011) Mortality of coho salmon (Oncorhynchus kisutch) associated with burdens of multiple parasite species. Int J Parasitol 41:1197–1205
Fraser BA, Neff BD (2010) Parasite mediated homogenizing selection at the MHC in guppies. Genetica 138:273–278
Griggs JL, Belden LK (2008) Effects of Atrazine and Metolachlor on the survivorship and infectivity of Echinostoma trivolvis trematode cercariae. Arch Environ Contam Toxicol:195–202
Grutter AS, Cribb TH, McCallum H, Pickering JL, McCormick MI (2009) Effects of parasites on larval and juvenile stages of the coral reef fish Pomacentrus moluccensis. Coral Reefs 29:31–40
Hoffman GL, Putz RE (1965) The black-spot (Uvulifer ambloplitis: Trematoda: Strigeoidea) of centrarchid fishes. Trans Am Fish Soc 94:143–152
Houde ED (1987) Fish early life dynamics and recruitment variability. Am Fish Soc Symp 2:17–29
Houde ED (2002) Chapter 3 Mortality. In: Fuiman LA, Werner RG (eds) Fishery science: the unique contributions of early life stages. Blackwell Publishing Co., Osney Mead, pp 64–87
Huspeni TC, Lafferty KD (2004) Using larval trematodes that parasitize snails to evaluate a saltmarsh restoration project. Ecol Appl 14:795–804
Janney EC, Shively RS, Hayes BS, Barry PM, Perkins D (2008) Demographic analysis of Lost River sucker and shortnose sucker populations in Upper Klamath Lake, Oregon. Trans Am Fish Soc 137:1812–1825
Lafferty KD, Morris K (1996) Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77:1390–1397
Lane RL, Morris JE (2000) Biology, prevention, and effects of common grubs (Digenetic trematodes) in freshwater fish. Technical Bulletin Series No. 115. Iowa State University, Ames, 6 pp
Lester RJG (1984) A review of methods for estimating mortality due to parasites in wild fish populations. Helgoländer Meeresun 37:53–64
Markle DF, Cavaluzzi M, Simon DC (2005) Morphology and taxonomy of Klamath Basin suckers (Catostomidae). Western North Am Nat 65:473–489
Markle DF, Reithel SA, Crandall J, Wood T, Tyler TJ, Terwilliger M, Simon DC (2009) Larval fish transport and retention and the importance of location for juvenile fish recruitment in Upper Klamath Lake, Oregon. Trans Am Fish Soc 138:328–347
Planes S, Lecchini D, Romans P, Mills SC (2009) Effects of post-settlement mortality on size and parasite load in juvenile Diplodus vulgaris and D. sargus in the Mediterranean. Aquat Biol 6:153–158
Pracheil BM, Muzzall PM (2010) Population dynamics of larval trematodes in juvenile bluegills from Three Lakes II, Michigan, and the potential for overwinter parasite-induced host mortality. Trans Am Fish Soc 139:652–659
Robson DS, Chapman DG (1961) Catch curves and mortality rates. Trans Am Fish Soc 90:181–189
Shuford WD, Thomson DL, Mauser DM, Beckstrand J (2004) Abundance, distribution, and phenology of nongame waterbirds in the Klamath Basin of Oregon and California in 2003. Final Report to U.S. Fish and Wildlife Service, Klamath Basin National Wildlife Refuge Complex
Sinclair M (1988) Marine populations: an essay on population regulation and speciation. University of Washington, Seattle
Strathmann RR, Hughes TP, Kuris AM, Lindeman KC, Morgan SG, Pandolfi JM, Warner RR (2002) Evolution of local recruitment and its consequences for marine populations. Bull Mar Sci 70:S377–S396
Terwilliger MR, Markle DF, Kann J (2003) Associations between water quality and daily growth of juvenile shortnose and Lost River suckers in Upper Klamath Lake, Oregon. Trans Am Fish Soc 132:691–708
United States Fish and Wildlife Service (1988) Endangered and threatened wildlife and plants; Determination of endangered status for the Shortnose Sucker and Lost River Sucker. Fed Regist 53:27130–27134
Wilde GR, Durham BW (2008) Daily survival rates for juveniles of six Great Plains cyprinid species. Trans Am Fish Soc 137:830–833
Wood TM, Hendrixson H, Markle DF, Erdman C, Burdick S, Ellsworth C, Buccola NL (2012) Dispersal of larval suckers at the Williamson River Delta, Upper Klamath Lake, Oregon, 2006–09. US Geol Surv Sci Investig Rep:2012–5016
Acknowledgments
Michael Kent and Craig Banner tentatively identified the larval trematode and they, Jayde Ferguson, and Ron Larson provided helpful discussions and feedback. We are grateful to T. Tyler (USBR) for logistic and other support. Numerous students, including Stacy Remple, Jes Kettretad, Kara Holem, Allison Estergard, Sara Akins, Justin Huff, Sheena Wheeler, Erin Beatty, Spencer Tejedas, Elizabeth Quimba, Gerardo Antonio Cordero, Kate Spangler, Kirsten Heesacker, Autumn Smith, Jenna Gelardi, Dax Strubb, Tamal Reece, Jake Godfrey, Kevin Stertz, Thomas Litwin, Kale Clausen, Andrea Norris, Michelle Maier, Mark Jansen, and Elaina Snyder provided invaluable help with field and laboratory work. This report was funded by the Bureau of Reclamation, U.S. Department of Interior, as part of Reclamation’s mission to manage, develop, and protect water and related resources in an environmentally and economically sound manner in the interest of the American public. Funding was provided through agreement USBR#2-FG-81-0813. The views in this report are the author’s and do not necessarily represent the views of Reclamation. Specimen collections were authorized under a series of Oregon scientific taking permits through Permit 6265, US Fish & Wildlife Service scientific taking permit TE006333-14, and OSU Institutional Animal Care and Use permit LAR-ID 4090.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markle, D.F., Terwilliger, M.R. & Simon, D.C. Estimates of daily mortality from a neascus trematode in age-0 shortnose sucker (Chasmistes brevirostris) and the potential impact of avian predation. Environ Biol Fish 97, 197–207 (2014). https://doi.org/10.1007/s10641-013-0141-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-013-0141-7