Abstract
To test if otoliths can be used to track fish migration in polluted areas, fish sampled from Onondaga Lake, heavily polluted with mercury, were used in an assay to determine whether mercury was detectable in the fishes’ otoliths using synchrotron-based scanning X-ray fluorescence microscopy (SXFM). Mercury was undetectable, but selenium, rarely reported in otoliths and known as a physiological antagonist to mercury, was. Strontium was also present but appeared to be taken up independently of selenium, and thus these serve as independent biogeochemical markers. Both selenium and mercury were detected in fish tissues, but selenium was below levels considered toxic. Selenium was low in otoliths of fishes collected in nearby Oneida Lake. Synoptic surveys of water chemistry revealed that Se is regionally highest in Onondaga Lake and in particular its main inlet, Onondaga Creek. SXFM appears to be a sensitive method for detecting selenium in otoliths.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Within the fisheries sciences, the use of otoliths (earstones, organs that form part of the hearing and balance system in teleost fishes) has become an essential component of quantitative research. As aragonitic structures that grow incrementally, otoliths serve as natural chronometers, permitting accurate age and growth assessments. Increasingly, otolith chemistry is studied to link the temporal information with environmental signals. A suite of trace elements (mainly Sr, Ba, and Mn) as well as isotopes of C, O, and sometimes Sr and S, have been found to be meaningful markers of environmental conditions (e.g., Campana 1999; Campana and Thorrold 2001; Limburg et al. 2001; Kennedy et al. 2002; Weber et al. 2002). A number of studies have investigated factors that affect incorporation of Sr, Ba, and Mn into otoliths (e.g., Bath et al. 2000; Elsdon and Gillanders 2004; Kraus and Secor 2004; Martin and Thorrold 2005), but there is still much work to be done to understand both what trace elements can be taken up, and the conditions that affect their uptake and their usefulness as natural tags (Elsdon et al. 2008).
For the past few years, we have been experimenting with the use of synchrotron-based scanning X-ray fluorescence microscopy (SXFM) to map out trace elements in thin sections of otoliths (Limburg et al. 2007). This non-destructive method, using monocapillary optics to produce incident x-ray beams at the micro scale, can detect multiple elements simultaneously at levels as low as femtograms/μm2 under ideal conditions (Bilderback et al. 2003). Mapping elemental concentrations with micro-scale resolution permits us to detect visually whether measured elemental concentrations have biological meaning (i.e., are incorporated into the ring structure) or not (e.g., appear as random noise in the elemental map, or as obvious contamination).
Some of our initial explorations of applying SXFM to otolith chemistry were focused on fishes of Onondaga Lake in central New York State (Fig. 4), which became heavily polluted as a result of urbanization and industrial development (Effler 1996). It is particularly contaminated with mercury, which was used in manufacturing products for the Allied Chemical Company. In 2004, we undertook a pilot survey to quantify mercury in otoliths of fish from Onondaga Lake, and assayed fishes across a range of trophic levels as well as in benthic vs. pelagic habitats. We instead detected selenium, an element we were not expecting to find. Mercury and selenium are well known as physiological antagonists (Cuvin-Aralar and Furness 1991; Yang et al. 2008). The most likely mechanism underlying this antagonism is thought to be the formation of a stable and biologically inert complexation of the two elements (Yoneda and Suzuki 1997). Because of such interactions, we hypothesized that the presence of Hg in the water would affect in some way Se incorporation into fish otoliths. Hence, in this study, we have the following objectives:
-
Objective 1: We report here on preliminary, novel mapping of otolith selenium, in addition to other trace elements, from fish otoliths collected primarily from Onondaga Lake (heavily contaminated with Hg) and Oneida Lake, a lake nearby, which does not contain high mercury levels. Both elements bioaccumulate through aquatic and terrestrial food webs, but they are sequestered differently in different tissues (Yang et al. 2010).
-
Objective 2: We analyzed tissues for Se and Hg in four of the Onondaga Lake fish to examine the fate of these two elements in somatic tissues.
-
Objective 3: Finally, we surveyed water courses and lakes in the region to determine whether Se was uniquely elevated in Onondaga Lake relative to other water bodies. We present these preliminary results and our interpretations.
Materials and methods
Fish collections and tissue analyses
Five species of fish (one Gizzard Shad (Dorosoma cepedianum), two Channel Catfish, (Ictalurus punctatus), one White Sucker (Catostomus commersoni), one Smallmouth Bass (Micropterus dolomieu), and two Walleye (Sander vitreus)) were collected by trap nets in 2003 in routine monitoring surveys of Onondaga Lake. Sagittal otoliths were extracted, rinsed in de-ionized water, and stored dry until preparation. Otoliths of four of these fish (one each of Gizzard Shad, White Sucker, Channel Catfish, and Walleye) were analyzed for the current study (see methods below). Tissues from these fish were collected for analysis of mercury and selenium. Additionally, three fish (one Gizzard Shad, one Atlantic Salmon [Salmo salar], and one Walleye) were collected from Oneida Lake, and their otoliths were similarly extracted. We note that results from two of these species—Walleye and Channel Catfish—were previously presented in a paper describing development of the SXFM method (Limburg et al. 2007), and hence in this current study we are adding replication.
Fish tissues (muscle, liver, and ovaries if available) and a benthic sample composed of sediment and zebra mussels, all collected from Onondaga Lake, were analyzed for Se and Hg by neutron activation analysis at the University of Missouri-Columbia Research Reactor Center. Samples were irradiated for 50 hours, decayed for at least 10 days, and counted for 4 hours. Certified standards for 75Se and 203Hg (oyster tissue SRM 1566a and orchard leaves SRM 1571) were counted for one hour, and were also run as quality controls. Results (ppm wet weight) were converted to mMol/L.
Otolith elemental analysis
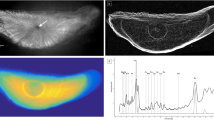
Otoliths were dissected, cleaned, sectioned in either the transverse or sagittal plane (depending on the size), and mounted on pressed silica (ultra-pure) rounds for grinding, polishing, and subsequent analysis. All elemental analyses were conducted at the Cornell High-Energy Synchrotron Source (CHESS). These were conducted either on the D-Line (by RH in 2004) or the F3 (by DD and KL in 2006 and 2007) bending magnet beamlines. Detailed methods are described in Limburg et al. (2007, 2010), but briefly, multilayer monochromators (0.6–1% bandwidth) produced X-ray beams at energies ranging from 10 to 20 keV. The beams were focused with single-bounce glass capillary optics (Bilderback et al. 2003; Cornaby 2008) to produce a photon flux on the order of 1011 counts per second at spot sizes ranging from 15–100 microns, depending on the size of the otolith specimen. The beam was used to illuminate a spot on the sample and a fluorescence spectrum integrated for 5–45 s; an energy dispersive XFlash™ detector (Röntec) was used on the D-Line and a Vortex (SII) detector was used on the F3 line. At both stations, an aluminum foil attenuator was placed over the detector to reduce the high calcium counts, in order to increase sensitivity to trace elements. A NIST standard (SRM-8704, Buffalo River Sediments) was used for calibration of the 2006 and 2007 analyses. In the earlier analyses (done by RH), concentrations reported in Limburg et al. (2007) were estimated using the method of Vincze et al. (1995); here, we present previously unpublished data for two fish (Fig. 1), and for these we only present the intensities rather than concentrations, due to absence of calibrations. Limits of detection were sub-ppm for Se; limits of detection for Sr and Ca were well below the concentrations presented here. Data are visualized as elemental maps of intensities (Fig. 1) and Se:Ca and Sr:Ca ratio maps (Figs. 2 and 3).
Early scans of Onondaga Lake fish otoliths showing different patterns in Sr and Se incorporation. Top: Channel Catfish from Onondaga Lake, September 2003, age 19 yr, 569 mm TL, analyzed with 100 micron steps. Sr is scaled to 3.5 × 104 counts and Se is scaled to 4.5 × 103 counts. Bottom: Gizzard Shad, age 5 yr, 374 mm, 50 micron steps. Photo not available. Sr is scaled to 1.6 × 104 counts and Se is scaled to 2 × 103 counts. The two otoliths were analyzed on different dates, so count rates differed; in fact, the results are qualitatively similar (i.e., calibrations would have yielded similar results, but these runs preceded our calibrations). Bars equal 1 mm
Optical images (left), Se:Ca ×1,000 (center), and Sr:Ca ×1000 (right) mass fraction ratios in fishes captured in Oneida Lake. Top: blueback herring (posterior and antirostrum); middle: Walleye young-of-year; bottom: Atlantic salmon. Se:Ca and Sr:Ca scales are as in Fig. 2. Bars equal 1 mm
Geographic region and synoptic survey
Following the discovery of selenium in Onondaga Lake fish otoliths and tissues, we surveyed water chemistry during summer months in 2007 and 2008 in a 70-km region of central New York State along a series of connected waterways (from west to east the Seneca River, Oneida River, Chittenago Creek, Oneida Creek, Erie Canal, and Fish Creek) and lakes (Cross Lake, Onondaga Lake, and Oneida Lake); Fig. 4.
Water chemistry analyses
Filtered, acidified water samples were analyzed for Ca, Sr, Rb, Mg, Mn, and Ba with inductively coupled optical emission spectrometry (ICP-OES). Only Sr and Ca results are reported here. At least two wavelengths were monitored for each element and averaged. Samples were eliminated if the coefficient of variation exceeded 10%.
Dissolved selenium was analyzed on acidified samples using a Perkin Elmer Elan DRC-e ICP-MS. All samples and standards contained 2% OPTIMA grade nitric acid and 1% methanol (to enhance the sensitivity for Se) (Moellmer et al. 2007). Calibration curves were developed using 0 (blank), 0.5, 1, and 2 ppb standards. All calibration curve correlation coefficients exceeded 0.999. The ELAN DRC (Dynamic Reaction Cell) with oxygen as a reaction gas was optimized to eliminate interferences from the Ar2+ dimer and ArCa+ which occur at the same mass-to-charge ratio as Se78 and Se80. To ensure elimination of these interference problems, several Se isotopes were measured. Operating conditions were: RF power = 1,400 W; nebulizer flow = 1.06 L·min-1; O2 reaction gas flow = 0.9 m L·min-1; and DRC bandpass controlling parameters RPq = 0.8; RPa = 0.001.
Results
We originally hypothesized that mercury might be discovered in Onondaga Lake fish otoliths, given the elevated concentrations of mercury in sediments and biota of the lake. We did not find any mercury, but instead found selenium (Limburg et al. 2007; see Fig. 4 therein). Ultimately, we were able to map Se in four different fish species in Onondaga Lake (this study, Figs. 1 and 2; cf. Limburg et al. 2007). In all of these fishes, Se was in low concentrations in the inner regions of the otoliths (corresponding to early life), and increased in the outer annuli. In contrast, strontium was elevated in the core regions of most of these fish (Figs. 1 and 2). Fishes from Oneida Lake, roughly 20 km away, had low Se compared to Onondaga Lake fish, and varying levels of Sr:Ca (Fig. 3).
All Onondaga Lake fish tissues assayed by neutron activation analysis had measurable concentrations of both selenium and mercury (Table 1). Mercury concentrations were elevated in predatory fishes (Walleye, Smallmouth Bass, and Channel Catfish) compared to White Sucker and a zebra mussel/sediment composite sample. Selenium:mercury ratios were highest in ovarian tissues of both Channel Catfish and White Sucker, and relative to the predatory fishes, White Sucker had higher Se:Hg in muscle and liver tissues.
Water collected in 2007 and 2008 showed regional differences in Se (Fig. 5, ANOVA p < 0.05), with highest concentrations in the Onondaga Lake basin and second highest to the west. Although concentrations were mostly lower in 2008, inter-annual differences were not statistically different (ANOVA, p = 0.27). On the other hand, strontium-to-calcium ratios were elevated both in the Onondaga (2007 mean 17.1 × 10-3 ± 5.7 × 10-3 s.d.; 2008 mean 20.6 × 10-3 ± 9.2 × 10-3 s.d.) and Oneida lake basins (2007 mean 21.0 × 10-3 ± 4.2 × 10-3 s.d.; 2008 mean 22.5 × 10-3 ± 3.9 × 10-3 s.d) (Fig. 6).
Mean (± s.d.) strontium:calcium ratios in water during June, July, and August 2007 across the synoptic survey region. Numbers correspond to sites on the map in Fig. 4
Discussion
Onondaga Lake fish were found to have differing patterns of selenium and strontium in their otoliths, and the Se was elevated relative to concentrations found in fishes from Oneida Lake. Given that Se was present in tissues and otoliths, but that it appeared elevated in the outer annuli of all Onondaga Lake fish otoliths assayed, we hypothesized that these fish had moved into the lake from unknown areas that had lower dissolved Se concentrations. We also note that Sr was elevated in the core regions of all Onondaga Lake fishes and in some Oneida Lake fishes (Fig. 3), and that Oneida Lake water chemistry stands out with Sr and Sr:Ca ratios on par with Onondaga Lake (Fig. 6), although its inlet streams to the north and east are Sr-depleted (Limburg and Siegel 2006), which may explain the varying levels of Sr:Ca in otoliths of fish caught in Oneida Lake. Thus, Sr:Ca alone may not distinguish fish originating from one lake or the other, but the combination of Sr and Se appears to mark residency in Onondaga Lake.
We tentatively hypothesize that the Onondaga fishes whose otoliths we analyzed by SXFM may have originated in Oneida Lake. Fishes move between Onondaga Lake and other areas via connected waterways (Siniscal 2009). Siniscal reported re-capturing fish that had been tagged in Onondaga Lake in Oneida Lake, in between the two lakes, but also west of Onondaga Lake as well as in Onondaga Lake tributaries.
Selenium is an essential trace element for vertebrate nutrition, but can be toxic at levels exceeding 2 μg/L in dissolved form (Lemly 1999). The Se concentrations we measured were well below this, and in no case did we observe tissue concentrations approaching toxic levels (8 ppm dry weight (d.w.) in muscle tissue, 12 ppm d.w. in liver, or 10 ppm d.w. in ovaries, Lemly 2000). On the other hand, Ralston et al. (2007) note that Se:Hg ratios (Table 1) can be a diagnostic of mercury toxicity. Also, our observations on Walleye uptake of Se and Hg in different tissues are in agreement with Yang et al. (2010), who found that Se:Hg was 2–7-fold greater in liver vs. muscle tissue in young-of-year Walleye.
As mentioned in the Introduction, mercury and selenium are physiological antagonists. Selenium inhibits the toxicity of inorganic and methylmercury (Cuvin-Aralar and Furness 1991; Yang et al. 2008). Because Onondaga Lake contains high levels of mercury and because Se was found at relatively high concentrations in fish otoliths, we at first hypothesized that elevated mercury in fish would somehow facilitate the incorporation of Se into otoliths. However, an experiment that treated fish with various combinations of Se and Hg showed that increased Hg resulted in decreased incorporation of Se into otoliths (Lochet et al. 2009). Thus, despite moderately elevated Se concentrations in Onondaga Lake compared to other areas within the region, we cannot explain why Se appears to be elevated in otoliths of Onondaga Lake fish compared to Oneida Lake fish. It is possible that other, as yet unknown, facilitating factors are involved, such as food chain bioaccumulation. Further experimentation, including in situ caging studies, could be helpful in this regard.
The data presented here should be viewed as a preliminary analysis of how a novel trace element, selenium, could be used as a tracer of environmental history. Although selenium can be measured in otoliths with ICP mass spectrometry (Palace et al. 2007), it is very difficult. Synchrotron-based SXFM is sensitive to Se and has few interferences, making it an ideal instrument for Se quantification. The current drawback is that these analyses are time-intensive; typical whole otolith maps, such as those presented in Figs. 1, 2 and 3 require 5–24+ hours, at current data collecting rates. A new generation of X-ray fluorescence detectors that incorporate multiple units (elements) should become available in the near horizon. Once these are in use, data collecting time should drop, and a more comprehensive study could be undertaken.
References
Bath GE, Thorrold SR, Jones CM, Campana SE, McLaren JW, Lam JWH (2000) Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim Cosmochim Acta 64:1705–1714
Bilderback DH, Huang R, Kazimirov A, Kriksunov IA, Limburg K, Fontes E (2003) Monocapillary optics developments and applications. Adv X-ray Anal 46:320–325
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Campana SE, Thorrold SR (2001) Otoliths, increments, and elements: key to a comprehensive understanding of fish populations. Can J Fish Aquat Sci 58:30–38
Cornaby SW (2008) The handbook of x-ray single-bounce monocapillary optics, including optical design and synchrotron applications. Thesis (Ph. D.)--Cornell University, Ithaca, NY
Cuvin-Aralar MA, Furness RW (1991) Mercury and selenium interaction: a review. Ecotoxicol Environ Safety 21:348–364
Effler SW (ed) (1996) Limnological and engineering analysis of a polluted urban lake: prelude to environmental management of Onondaga Lake, New York. Springer, New York
Elsdon TS, Gillanders BM (2004) Fish otolith chemistry influenced by exposure to multiple environmental variables. J Exp Mar Biol Ecol 313:269–284
Elsdon TS, Wells BK, Campana SE, Gillanders BM, Jones CM, Limburg KE, Secor DH, Thorrold SR, Walther BD (2008) Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations, and inferences. Oceanogr Mar Biol Annu Rev 46:297–330
Kennedy BP, Klaue A, Blum JD, Folt CL, Nislow KH (2002) Reconstructing the lives of fish using Sr isotopes in otoliths. Can J Fish Aquat Sci 59:1–5
Kraus RT, Secor DH (2004) Incorporation of strontium in otoliths of an estuarine fish. J Exp Mar Biol Ecol 302:85–106
Lemly AD (1999) Selenium impacts on fish: an insidious time bomb. Hum Ecol Risk Assess 5:1139–1151
Lemly AD (2000) Guidelines for conducting TMDL consultations on selenium. Technical assistance report to the U.S. Fish and Wildlife Service, Division of Environmental Contaminants, Washington, D.C.
Limburg KE, Siegel DI (2006) The hydrogeochemistry of connected waterways, and the potential for tracing fish migrations. Northeastern Geol Environ Sci 28:254–265
Limburg KE, Landergren P, Westin L, Elfman M, Kristiansson P (2001) Flexible modes of anadromy in Baltic sea trout: making the most of marginal spawning streams. J Fish Biol 59:682–695
Limburg KE, Huang R, Bilderback DH (2007) Fish otolith trace element maps: new approaches with synchrotron microbeam X-ray fluorescence. X-Ray Spectrom 36:336–342
Limburg KE, Høie H, Dale DS (2010) Bromine patterns in Norwegian coastal cod otoliths—a possible marker for distinguishing stocks? Environ Biol Fishes. doi:10.1007/s10641-010-9660-7
Lochet A, Limburg KE, Rudstam L, Montesdeoca M (2009) Selenium incorporation in fish otoliths: Effects of selenium and mercury from the water. Can J Fish Aquat Sci (in press)
Martin GM, Thorrold SR (2005) Temperature and salinity effects on magnesium, manganese, and barium incorporation in otoliths of larval and early juvenile spot Leiostomus xanthurus. Mar Ecol Prog Ser 293:223–232
Moellmer WO, Miller TG, Wilbur S, Soffey E (2007) ICP-MS analysis of trace selenium in the Great Salt Lake. Spectroscopy 1:1–6
Palace VP, Halden NM, Yang P, Evans RE, Sterling G (2007) Determining residence patterns of rainbow trout using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) analysis of selenium in otoliths. Environ Sci Technol 41:3679–3683
Ralston NVC, Blackwell JL III, Raymond LJ (2007) Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol Trace Elem Res 119:255–268
Siniscal AC (2009) Characterization of the fish community of recovering ecosystem, Onondaga Lake, New York. Ph.D. dissertation, State University of New York College of Environmental Science and Forestry, Syracuse, NY. p 116
Vincze L, Janssens K, Adams F, Rivers ML, Jones KW (1995) A general Monte Carlo simulation of ED-XRF spectrometers, II: Polarized monochromatic radiation, homogeneous samples. Spectrochim Acta 50B:127–147
Weber PK, Hutcheon ID, McKeegan KD, Ingram BL (2002) Otolith sulfur isotope method to reconstruct salmon (Oncorhynchus tshawytscha) life history. Can J Fish Aquat Sci 59:587–591
Yang D-Y, Chen Y-W, Gunn JM, Belzile N (2008) Selenium and mercury in organisms: interactions and mechanisms. Environ Rev 16:71–92
Yang D-Y, Xu Y, Chen Y-W, Belzile N (2010) Inverse relationships between selenium and mercury in tissues of young Walleye (Stizosedion vitreum) from Canadian boreal lakes. Sci Tot Env 408:1676–1683
Yoneda S, Suzuki KT (1997) Detoxification of mercury by selenium by binding of equimolar Hg-Se complex to a specific plasma protein. Toxicol Appl Pharmacol 143:274–280
Acknowledgments
We thank P. Thompson, D. Gefell, T. Brookings, and the Fish Creek Salmon Club for collecting fish for use in this study, and P Altman for conducting the neutron activation analysis. We thank the editors and two anonymous reviewers for helpful comments on an earlier draft. Partial support for the study came from the Great Lakes Protection Fund, as well as from the National Science Foundation (DEB-0238121 to KL) and a Fulbright fellowship (to AL). This work is based upon research conducted at the Cornell High Energy Synchrotron Source (CHESS) which is supported by the National Science Foundation and the National Institutes of Health/National Institute of General Medical Sciences under NSF award DMR-0225180.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Limburg, K.E., Lochet, A., Driscoll, D. et al. Selenium detected in fish otoliths: a novel tracer for a polluted lake?. Environ Biol Fish 89, 433–440 (2010). https://doi.org/10.1007/s10641-010-9671-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-010-9671-4