Abstract
The cellulase activities of bacterial strains in the intestine of grass carp were analyzed, using filter paper and absorbent cotton as substrates and measuring the concentration of glucose by calorimetry. Six strains were isolated and determined high cellulase activity in all grass carp. Strains showed different abilities to produce cellulase, which suggests that they interact in the grass carp intestine to digest cellulose. The presence of cellulose activity suggests that grass carp have the ability to digest cellulose in the diet. The cellulase enzymatic activity increased dramatically after 6 days of culture and reached its peak at the 7th day. Microbes are probably the main source of cellulase in grass carp diets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulose is produced by plants and is recycled by microbes. It is a sustainable energy resource, as one of the main components of plants and also the most abundant organic materials in the world. The structure of cellulose in plants is very complicated and difficult to degrade because of its unique molecular structure. Cellulose can not be degraded directly by animals, but only by some microbes such as bacteria and fungi (Xiao et al 2002). Cellulose is thought to be indigestible and therefore of little nutritional value in formulated fish feeds. It is vitally important to develop methods to improve enzymatic hydrolysis of cellulose, since developments in fishery nutrition and aquaculture technology have encouraged the use of cheaper feed ingredients, including cellulose. Enzymatic hydrolysis of cellulose has been extensively reported since the utilization of cellulosic biomass as a renewable resource has great potential for reducing emissions of carbon dioxide to help prevent global warming (Claassen et al 1999; Li et al 2004a, 2004b, 2005). However, despite the fact that many studies have been conducted worldwide, the significance of cellulose in fish diets is not yet fully elucidated. Fishes cannot produce cellulase and thus, they can not utilize cellulose directly.
Some reports have suggested that soluble, high molecular weight non-starch polysaccharides such as those common in cellulose increase digestive viscosity and reduce digestive enzyme access to other nutrients (Castanon et al. 1997; Bedford 2000; Francis et al. 2001). In fishes this can result in poor growth and low feed efficiency, depending on ingredient type and proportion, in cultured fishes (Watanabe 2002).
Inclusion of exogenous enzymes as additives in plant-based feeds has greatly improved feed utilization in terrestrial animals (Bedford 1995; Castanon et al. 1997; Bedford 2000). Cellulase isolated from grass carp must be adapted to the internal environment of the fish intestine and have the ability to effectively digest cellulose. In this study, bacterial strains with cellulase-producing ability were identified from the intestine of grass carp. The capacity of cellulase to hydrolyze chemically defined cellulosic substrates was evaluated and it was determined that it might play a significant role in the utilization of plant materials in fish diets.
Materials and methods
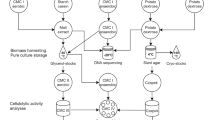
Isolation and screening of microbes
Sample collection: Ten 10 grass carp were purchased from a retail market, having been fed mainly grass. Their individual live weights were 3.91, 3.94, 3.97, 3.97, 3.99, 4.00, 4.02, 4.03, 4.05, and 4.08 kg, respectively. The full length of the intestine from each fish was removed and food collected. Intestine lengths ranged from 79 to 144 cm.
In the first round of screening (Horikoshi et al. 1984), 1 gm of food from the intestine was ground and 9 ml of de-ionized (DI) water added to dissolve the material. This solution was diluted by 10 times to 10–1, 10–2, 10–3, 10–4, 10–5, 10–6 and then 0.1 ml of the diluted solutions was inoculated onto plates and cultured at 37°C. Na-CMC was used to detect endo-β-1,4-glucanase activity. 0.1 ml of the screening strain suspension was added to the cellulose medium and mixed it at 50°C. The clearance area around colonies of strains of cellulase producing microbes was recorded as white medium.
In the second round of screening (Qi 2003), the diameter of the clear area was measured around each circle. The ratio of the diameter of the circle and the clearance area for each strain were calculated. Those with high clearance cycle/strain cycle ratios were selected for further testing.
Cellulase enzymatic activity measurement
The glucose concentrations in enzyme assays (see Wave length for the production after cellobiose, cellotriose and glucose oxidized by DNS) was determined relative to a standard curve for glucose in sodium acetate buffer (Liu 2002). Selected strains obtained were inoculated into 10 ml of Na-CMC medium and cultured them at 37°C for 5, 6 and 7 days. 1 ml of the supernatant was used to measure β-1,4-glucosidase activity, 0.5 ml of which was used to measure exo-β-1,4-glucanase and endo-β-1,4-glucanase activity. Control tubes were heated in boiling water for 5 min to inactivate them. After allowing the samples to cool, 2 ml of acetic acid/sodium acetate buffer and 2 ml of Na-CMC were added to the β-1,4-glucosidase activity assay. 2 ml of acetic acid/sodium acetate buffer and 2 ml of absorbent cotton were added to the assay for exo-β-1,4-glucanase activity. 2 ml of acetic acid/sodium acetate buffer was added to a sample of 1 cm × 3 cm filter paper to assay for endo-β-1,4-glucanase activity. Tubes were incubated at 50°C for 5 min and then cooled with ambient temperature water. 2 ml of 3,5-dinitrosalicylic acid (DNS) was added to the tubes, which were then placed tubes into 100°C bath for 5 min, allowed to cool with tap water and made the final volume to 10 ml. Optical density (OD) was measured at 490 nm.
where B is the weight (μg) obtained from the standard curve above.
Wave length for the production after cellobiose, cellotriose and glucose oxidized by DNS
The most commonly-used wavelengths for detecting oxidized and reduced DNS by cellobiose, cellotriose and glucose are 550 nm, 540 nm 520 nm and 490 nm, respectively. The OD value obtained was different from wavelengths for the detection of reduced DNS and the resultant enzymatic activity was measured. The absorption properties of the deoxidized DNS by cellobiose, cellotriose and glucose were studied. Brown-red products had strong absorption between the wave lengths of 540–470 nm with a peak at 490 nm. Thus a wave length of 490 nm was used to detect cellulase activity.
Results
Screening of cellulase producing microbes
After the first round of screening with cellulase selective medium, positive strains were transferred to plates for the second round of screening at 37°C to culture for 48 h. Strains were picked out by colony morphology observation. Strains obtained were transferred from the second round screening to new plates to purify them. 31 strains were screened and 2 strains with cellulase activity were obtained.
The strains obtained were further inoculated to a liquid fermentation medium. The strains were cultured and levels of β-1,4-glucosidase, endo-β-1,4-glucanase and exo-β-1,4-glucanase activity were measured. 6 strains of microbes with relatively high cellulase activity were collected and designated as: X1, X2, X3, X4, X5 and X6 (shown in Table 1).
The effect of pH on absorbance
Two milliliters of acetic acid/sodium acetate buffer with different pH was added to the samples. 1 ml of enzymatic solution with 0.5% CMC-Na Two milliliters was then added, after complete hydrolysis at 50°C, DNS was then added and the absorbance was measured. The absorbance was the highest at pH 4.8 which was why the acetic acid/sodium acetate solution was chosen for the experiment.
The effect of culturing time on cellulase production
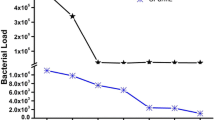
The X1 microbe was chosen to study the effects of culturing time on cellulase production. Cultures on filter paper fermentation medium were obtained and then analyzed for enzymatic activity. From the 3rd day on, enzymatic activity was measured every 24 h (Fig. 1).
Enzymatic activity increased dramatically after 6 days of culture and reached its peak at the 7th day (Fig. 1). The enzymatic activity of the other microbe strains obtained in this study was also studied. Similar results for the enzymatic activity were obtained, with the highest values around the 7th day. Therefore cellulase activity was measured during the period between 5 and 8 days.
Cellulase activities and X-ray dispersion
According to the results obtained for the OD490/glucose concentration above, the highest OD490, which represented the cellulase activity, was measured between 5 and 7 days culture of all the microbes to calculate the enzymatic activity (Table 1).
As shown in Table 2 and Table 3, the enzymatic activity of the same strain of microbe was different on different substrates. There were also differences in enzymatic activity among different strains. Among all the 6 strains, CMC enzymatic activity was higher in X3, X5 and X6, with X5 obtaining the highest level of activity. This indicates that this strain has a strong ability to decompose water-soluble substrates. For filter paper enzymatic activity, X2, X4 and X5 demonstrated high activity, with X2 obtaining the highest. X2 had the greatest ability to decompose multi-component cellulose substrates. Higher exo-β-1,4-glucanase activity appeared in X1, X2 and X3, with the highest level of activity being observed in X3.
Discussion
There are a few reports concerning microbial flora and microbial cellulase production in the intestinal tracts of fishes (Stickney and Shumway 1974; Lesel et al. 1986; Das and Tripathi 1991; Stellwag et al. 1995; Saha and Ray 1998; Bairagi et al. 2002). Grass carp is a fish that feeds only on hydrophytes in natural waters. Therefore, there must be some kind of mechanism for them to use plant resources effectively, which suggests that cellulase plays an important role in their digestive system. Because grass carp can not produce cellulase by itself, there should be some cellulase-producing microbes in the grass carp's intestine.
Grass carp with high intestinal microbial activity may be supplied with additional carbohydrate energy through alternative routes. Cellulase activity has been observed in several fish species indicating that fish may be able to utilize cellulose (Chakrabarti et al. 1995). Our experiment has demonstrated that cellulase-producing microbes exist in the intestine of grass carp.
Appropriate amounts of dietary fibre are important for digestion in fishes because fibre can enhance the peristaltic movements of the intestine, stimulate the secretion of digestive enzymes and enhance the contact surface between food and enzymes. Previous reports have demonstrated that microbes in the fish intestine could secrete cellulase, which could help fish to digest cellulose into cellobiose, cellotriose and other oligosaccharides and eventually into glucose to be utilized by the fish. The grass carp gut is three times the length of body. Because cellulose is difficult to digest and the fish intestine is relatively long, food passes through the intestine relatively slowly. Therefore, it is necessary to add cellulase to fish feed to increase gut absorption. Until now, few reports are available concerning the application of cellulase in fish feed.
All of the six strains with high cellulase-producing ability were obtained from the middle or rear part of the intestine, while the strains screened from the front part of the intestine had relatively low cellulase activity. This suggests that all these high cellulase-producing strains were established and stable strains. The cellulase activities for different strains were found to be quite different. Some of them had high CMC activity while some of them had high endo-β-1,4-glucanase. This indicates that these strains might work together to decompose cellulose in the grass carp intestine.
As the grass carp do not have a stomach to digest cellulose, it can only digest cellulose in its intestine. Shcherbina and Kazlawlene (1971) suggested that some portion of dietary cellulose is digested in the anterior portion of the gut while the remaining portion of the cellulose is digested in the posterior portion of the digestive tract, indicating the probable presence of microbial cellulase in the posterior region. Analysis of the residual food obtained from grass carp intestine showed that the ability for cellulose digestion was very limited. Large amounts of cellulose were still found even in the rear part of its intestine.
Bacteria in the diet of the fish may adapt themselves to the environment of the gastrointestinal tract and form a symbiotic association. In our study, a considerable population of bacterial symbionts was isolated from the alimentary tracts of grass carp and some of the strains were shown to exhibit significant cellulolytic activity.
References
Bairagi A, Sarkar GK, Sen SK, Ray AK (2002) Enzyme producing bacterial flora isolated from fish digestive tracts. Aquacult Int 10:109–121. doi:10.1023/A:1021355406412
Bedford MR (1995) Mechanism of action and potential environmental benefits from the use of feed enzymes. Anim Feed Sci Technol 53:145–155 doi:10.1016/0377-8401(95)02018-U
Bedford MR (2000) Exogenous enzymes in monogastric nutrition—their current value and future benefits. Anim Feed Sci Technol 86:1–13. doi:10.1016/S0377-8401(00)00155-3
Castanon JIR, Flores MP, Pettersson D (1997) Mode of degradation of non-starch polysaccharides by feed enzyme preparations. Anim Feed Sci Technol 68:361–365. doi:10.1016/S0377-8401(97)00046-1
Chakrabarti I, Gani MA, Chaki KK, Sur R, Misra KK (1995) Digestive enzymes in 11 freshwater teleost fish species in relation to food habit and niche segregation. Comp Biochem Physiol A 112:167–177. doi:10.1016/0300-9629(95)00072-F
Claassen PAM, Van Lier JB, Contreas AML (1999) Utilization of biomass for the supply of energy carriers. Appl Microbiol Biotechnol 52:741–755. doi:10.1007/s002530051586
Das KM, Tripathi SD (1991) Studies on the digestive enzymes of grass carp, Ctenopharyngodon idella (Val.). Aquaculture 92:21–32. doi:10.1016/0044-8486(91)90005-R
Francis G, Makkar HPS, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227. doi:10.1016/S0044-8486(01)00526-9
Horikoshi K, Nakao M, Kurono Y (1984) Cellulase of an alkalophilic Bacillus strain isolated from soil. Can J Microbiol 30:774–779
Lesel R, Fromageot C, Lesel M (1986) Cellulose digestibility in grass carp, Ctenopharyngodon idella and in goldfish, Carassius auratus. Aquaculture 54:11–17. doi:10.1016/0044-8486(86)90249-8
Li C, Yoshimoto M, Fukunaga K, Nakao K (2004a) Preparation and characterization of cellulase-containing liposomes for their immobilization suitable for enzymatic hydrolysis of cellulose. J Chem Eng of Jpn 37:680–684. doi:10.1252/jcej.37.680
Li C, Seki K, Matsunaga T, Yoshimoto M, Fukunaga K, Nakao K (2004b) Enzymatic hydrolysis of waste paper in an external loop airlift bubble column with continuous ultrasonic irradiation. J Chem Eng of Jpn 37:1041–1049. doi:10.1252/jcej.37.1041
Li C, Yoshimoto M, Ogata H, Tsukuda N, Fukunaga K, Nakao K (2005) Effects of ultrasonic intensity and reactor scale on kinetics of enzymatic saccharification of various waste papers in continuous irradiated stirred tanks. Ultrason Sonochem 12:373–384. doi:10.1016/j.ultsonch.2004.02.004
Liu DH (2002) Methods for cellulose activity measurement. Chin Feed 17:27–28
Qi Y (2003) A study on isolation and characterization of cellulase producing microbes. Nat Prod Res Dev 15:510–513
Saha AK, Ray AK (1998) Cellulase activity in rohu fingerlings. Aquacult Int 6:281–291. doi:10.1023/A:1009210929594
Shcherbina MA, Kazlawlene OP (1971) The reaction of the medium and the rate of absorption of nutrients in the intestine of carp. J Ichthyol 11:81–85
Stellwag EJ, Smith TD, Luczkovich JJ (1995) Characterization and ecology of carboxymethylcellulase-producing anaerobic bacterial communities associated with the intestinal tract of the pinfish, Logodon rhomboides. Appl Environ Microbiol 61:813–816
Stickney RR, Shumway SE (1974) Occurrence of cellulase activity in the stomachs of fish. J Fish Biol 6:779–790. doi:10.1111/j.1095-8649.1974.tb05120.x
Watanabe T (2002) Strategies for further development of aquatic feeds. Fish Sci 68:242–252. doi:10.1046/j.1444-2906.2002.00418.x
Xiao CL, Xu CX (2002) A study on the application of cellulose from microbes. J Microbiol 22:33–35
Acknowledgements
This study was funded by an Excellent Project for Scholars Returned from Abroad award, Ministry of Human Resource (2005) and the Key Laboratory of Freshwater Fish Germplasm Resource and Biotechnology, Chinese Academy of Fish Sciences (CAFS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Zheng, Z., Cong-xin, X. et al. Isolation of cellulose—producing microbes from the intestine of grass carp (Ctenopharyngodon idellus). Environ Biol Fish 86, 131–135 (2009). https://doi.org/10.1007/s10641-008-9384-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-008-9384-0