Summary
Genomic studies have established a set of three core-signaling pathways, receptor tyrosine kinase (RTK), p53 and retinoblastoma (Rb) signaling pathways, contributing glioblastoma (GBM) and revealed that dysregulation of at least two pathways is required for GBM progression. In the present study, we investigate efficacy of combination of palbociclib, cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, and erlotinib, epidermal growth factor receptor (EGFR) inhibitor in GBM cell systems with different p53 status. Cell proliferation and colony formation assays showed that the combination treatment synergistically suppressed GBM cell proliferation. LN229 cells with mutant p53 and wild-type PTEN were more sensitive to the combination treatment. Further studies indicated that the synergetic anti-GBM effects were due to cell apoptosis induction and cell cycle arrest at G1 phase. Signaling examination indicated that levels of p-Rb and p-4E-BP1 significantly decreased by the combination treatment; however, Akt and MAPK signaling were differentially suppressed among the three GBM cell lines. Hence, our data demonstrate that palbociclib and erlotinib exert synergistic anti-GBM activity, providing pre-clinical evidence and a proof-ofconcept that usage of the combination of EGFR and CDK4/6 inhibitors for GBM treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most aggressive and the most common gliomas in humans [18]. Combination therapy for treating glioblastomas consists of surgery, radiotherapy, and chemotherapy [16]. However, even such a radical treatment strategy gives rise to mean patient survival time reaching only 14.6 months [36]. Patients with high grade tumors typically suffer poor prognosis due to the resistant nature to available therapies [31]. Comprehensive efforts by The Cancer Genome Atlas Research Network (TCGA) revealed therapeutic targets in up to 90% of GBM samples including targets of Receptor Tyrosine Kinase (RTK), PI3-Kinase, and Rb1 pathway [4]. There is a statistical tendency towards mutual exclusivity of alterations of components within each pathway, consistent with the thesis that deregulation of one component relieves the selective pressure for additional ones [5]. In other words, dysregulation of the at least two pathways among the three is a core requirement for glioblastoma pathogenesis.
Recent progress in understanding the molecular events that occur during GBM development has drawn interest in developing signal pathway-specific small-molecule kinase inhibitors for treatment [24]. Among these inhibitors are erlotinib and gefitinib, which target the epidermal growth factor receptor (EGFR) [33]. Erlotinib is the well-studied compound for GBM treatment [13, 28]. Although EGFR inhibitors have shown good preclinical inhibitory activity, the monotherapy is not sufficient to suppress tumor growth and have shown disappointing results in patients with GBM [3]. Thus, the targeted approach against EGFR signaling likely requires a synergistic drug combination strategy to become successful in cancer ultimately [7, 15, 27]. Cell cycle progression is frequently unbalanced in cancer and its inhibitors also attract plenty of attention for therapeutic application [1]. Palbociclib (PD0332991) is a selective cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, leading to inhibition of Rb1 phosphorylation and eventually to cell cycle blockage [10]. It has been reported having anti-tumor activity in hepatocellular carcinoma [2], synovial sarcoma [34], head and neck squamous cell carcinoma [22], liposarcoma [8], and non-small cell lung cancers [32]. Recently, it is approved for breast cancer treatment by the U.S. Food and Drug Administration [9]. Here, we investigate the efficacy a combination treatment with erlotinib and palbociclib in various GBM cells. Data indicates that palbociclib and erlotinib exert synergistic inhibition in human GBM cells, which due to stronger apoptosis inducing and cell cycle arresting activity. Further investigations show that synergistic inhibiting Rb and mTOR signaling contributing the efficacy of the combined treatment. Collectively, our data suggest that combined inhibition of both EGFR and CDK4/6 may serve as a therapeutic strategy for GBM treatment.
Results
Palbociclib/erlotinib attenuates cell proliferation in human glioblastoma cells
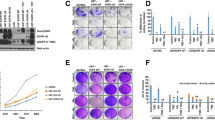
To study the efficacy of combination therapy of CDK4/6 and EGFR inhibitors, we firstly investigated inhibitive effects of palbociclib and erlotinib in three human glioblastoma cell lines: U87MG (PTEN−/−), LN229 (p53 mutant), and LNZ308 (TP53 −/− & PTEN −/−). The three cell lines were treated with various concentrations of palbociclib/erlotinib for three days followed by proliferation examination using MTT assay. Proliferations of all the three GBM cell lines were suppressed by both palbociclib and erlotinib in a dose-dependent manner (Fig. 1a and b). However, their sensitivities to the drugs were distinct: LN299 cells were the most sensitive cells to both palbociclib and erlotinib, with IC50 values 2.7 and 2.3 μM, respectively. U87MG and LNZ308 were less sensitive and LNZ308 was the lest sensitive cell line among them. Furthermore, we conducted time-course experiment to confirm the anti-GBM activity of palbociclib/erlotinib with the concentration of IC50 indicated above. Data showed that both palbociclib and erlotinib suppressed GBM cell proliferation in a time-dependent manner. Consistent with the dose-response data, LN299 and LNZ308 are the most and least sensitive cell lines to the two drugs, respectively (Fig. 1c and d).
Effect of palbociclib and erlotinib in human glioblastoma cells. U87MG, LN229, and LNZ308 cells were seeded in 96-well plate (3000 cells/well) and treated with indicated concentrations of palbociclib (a) and erlotinib (b) for 72 h. For time-course study, cells were prepared as described above and treated with palbociclib (c) and erlotinib (d) for indicated times. Cell proliferation of each treatment group was assessed by MTT assay as described in Materials and Methods. Data were expressed as mean ± SD
Combination of palbociclib and erlotinib synergistically suppresses GBM cell proliferation
Based on the data described above, we further examined the effects of the combined treatment of palbociclib and erlotinib in the GBM cells. Cells were treated with palbociclib and erlotinib at different concentrations for 72 h. Compared to palbociclib alone, treatment with combination of erlotinib significantly decreased cell proliferation in all three GBM cell line, even with the low concentration of erlotinib (Fig. 2a-c). Combined treatment showed similar pattern of inhibition in LNZ308 and U87MG cells: with erlotinib, the effects of various concentrations of palbociclib (0.5~2 μM) are not significant different (Fig. 2a and c). Higher concentrations of erlotinib showed stronger effects than those of lower concentrations when combined with palbociclib. In LN229 cells, treatment with low concentrations of palbociclib (0.5 μM) and erlotinib (1.25 μM) dramatically suppressed cell growth compared to control cells. However, the inhibition was not increased as the concentration increased (Fig. 2b). To further confirm the observed synergistic effect, the combined effect of palbociclib and erlotinib (palbociclib: erlotinib molar ratio was 1:2) was determined using the combination index (CI) method developed by Chou et al. [6]. The CI value for each combination was <1 (Fig. 2d), indicating there is a synergistic inhibitory effect. Still, the differences of the synergistic patterns among the three GBM cell lines were noticed. In LN229 cells, the fraction affected values ranged from 0.10 ~ 0.82, whereas in LNZ308 and U87MG cells, the fraction affected values ranged from 0.12 ~ 0.41 and 0.16 ~ 0.86, respectively, indicating that LN229 cells were more sensitivity to the combination treatment. Collectively, the data indicate that combination of palbociclib and erlotinib dramatically potentiates that anti-proliferative activity against human GBM cells.
Synergistic effect of palbociclib and erlotinib against glioblastoma cells. LNZ308 (a), LN229 (b), and U87MG (c) cells were seeded in 96-well plate (3000 cells/well) and treated with indicated concentrations of palbociclib with various concentrations of erlotinib for 72 h. Cell proliferation of each treatment group was assessed by MTT assay as described in Materials and Methods. Data were expressed as mean ± SD. d. Isobologram analysis and combination index analysis of the proliferation inhibition in GBM cells treated with the combination of palbociclib and erlotinib. CI < 1 indicates a synergistic effect
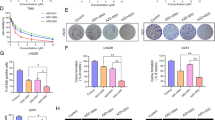
Combined treatment of palbociclib and erlotinib suppresses GBM colony formation
To further determine the synergistic activity of palbociclib and erlotinib against GBM. Soft agar colony formation assay, a cellular anchorage-independent grow assay that examines tumorigenic potential of transformed cells, was employed. Different from cell proliferation assay (Fig. 1), the inhibitive activity of the combination on colony formation among U87MG, LN229, and LNZ308 cell lines are similar. Combined treatment with palbociclib and erlotinib significantly suppressed colony formation compared to single drug treatment (Fig. 3a-c). However, among the three cell lines, LN229 was the most sensitive one respond to the combined treatment. The synergistic effects were further analyzed by calculating the CI values. As shown in Fig. 3d, unlike the distinct CI curve patterns from cell proliferation assay (Fig. 2d), patterns of the CI curves wee similar among the tree GBM cell lines (Fig. 3d). Combined treatment synergistically suppressed colony formation in all GBM cell lines. However, the beginning value of the fraction affected in LNZ308, U87MG, and LN229 were 0.21, 0.31, and 0.37, showing the different sensitivities of the cell lines to the combination therapy. These data confirm that combination of palbociclib and erlotinib exerts synergistic inhibition in GBM progression.
Efficacy of combination of palbociclib and erlotinib in GBM colony formation. LNZ308 (a), LN229 (b), and U87MG (c) cells were employed for colony formation assay as described in Materials and Methods. Data were expressed as mean ± SD. D. Isobologram analysis and combination index analysis of the colony inhibition in GBM cells treated with the combination of palbociclib and erlotinib. CI < 1 indicates a synergistic effect
Combined treatment of palbociclib and erlotinib induces apoptosis and cell cycle arrest in GBM cells
Palbociclib arrests cell cycle at G1 phase. It is also reported that cell cycle can be arrested by erlotinib in lung cancer [20] and hepatocellular carcinoma cells [17]. In addition, effect of erlotinib against cancer is also due to its apoptosis-inducing activity [23, 29]. Our data have demonstrated that combined treatment with palbociclib and erlotinib significant suppresses cell proliferation and tumorigenesis in human GBM cells. To determine whether cell cycle arrest is responsible for the synergistic effects, we carried our cell cycle analysis using flow cytometry. Data showed that palbociclib induced cell arrest at G1 phase in all GBM cell lines and LN229 cells were more sensitive to palbociclib (Supplemental Fig. 1, Fig. 4a). In addition, erlotinib blocked cell cycle progression at G1 phase in LN229 and U87MG cells (Supplemental Fig. 1, Fig. 4a). Treatment with both palbociclib and erlotinib significantly increased the cell number ratio in G1 phase compared to single drug treatment (Fig. 4a). To examine whether apoptosis was responsible for the suppression of cell proliferation and colony formation, we examined levels of an apoptosis marker by staining with active-caspase 3 in LN229 cells. As shown in Fig. 4b, the positive cells can be observed in erlotinib treated cells and treating with combination of palbociclib and erlotinib induced more apoptotic cells. Quantification by flow cytometric indicated that combination therapy synergistically enhanced the apoptosis-inducing effect compared to treatment with single agent (Fig. 4c). Moreover, PARP cleavages were notable in cells treated by the combination (Fig. 5a). Collectively, these data indicate that combined treatment with palbociclib and erlotinib blocks cell cycle at G1 phase and induces apoptosis in GBM cells.
Combined treatment with palbociclib and erlotinib induces G1 phase arrest and cell apoptosis. a Combined treatment enhanced G1 phase arrest in GBM cells. Cells treated with palbociclib (0.5 μM), erlotinib (2 μM) alone or combination for 24 h. Ratios of cells at the G1 phase were analyzed compared to control (as 1-fold). Data represents mean ± SD. *P < 0.05, **P < 0.05, compared to control respectively. b Combination of palbociclib and erlotinib synergistically induced cell apoptosis. LN229 cells were treated with palbociclib/erlotinib as describe above following by staining with active-caspase-3. Bar scale, 50 μm. c Following Annexin V/PI staining, quantification of apoptosis was accessed by flow cytometry analysis with cells treated with palbociclib, erlotinib alone or combination. Data represents mean ± SD. *P < 0.05, **P < 0.05, compared to control, respectively
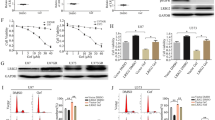
Effect of palbociclib, erlotinib alone or combination on Rb, Akt/mTOR signaling in GBM cells. a Following treatment with palbociclib, erlotinib alone or combination at the concentration described in Fig. 4, cell lysates were collected and analyzed western blotting using indicated antibodies. b Quantification of expressions of p-Rb, p-Akt, p-4E-BP1, and p-ERK normalized by corresponding total proteins, respectively. Data represents mean ± SD. c Inhibition rate of the combination treatment on the expressions of proteins as indicated compared to control respectively. Data represents mean ± SD. **P < 0.01
Involvement of Rb and Akt/mTOR signaling in palbociclib and erlotinib induced synergism against glioblastoma
Our data suggest that combination of palbociclib and erlotinib synergistically suppressed GBM tumorigenesis due to cell cycle arresting and apoptosis inducing activities. It is notable that the potencies of the combination are different among various cell systems. To define the molecular mechanism by which the combination treatment exerts synergistic activity against GBM, we further examined the cell survival, growth, as well as cell cycle regulation signalings with the lysates collected from the cells treated by palbociclib, erlotinib, and the combination. As shown in Fig. 5a and b, levels of p-Rb were all decreased in cells treated by palbociclib, which was consisted with the activity of CDK4/6 inhibition. It was also showed that erlotinib decreased p-Rb expression in LN229 cells. Expression levels of Rb signaling upstream regulators, CDK4/6 and Cyclin D1, were not affected by the drugs (Supplemental fig. 2). In LN229 and U87MG cells, both erlotinib and palbociclib suppressed and showed synergistic inhibition on Akt signaling, while the inhibitive effects were not notable in LNZ308 cells (Fig. 5a and b). However, combination treatment led to synergistic inhibition on p-4E-BP1 that is a readout of mTOR signaling pathway, downstream of Akt activity. Since MAPK/ERK signaling regulates cell functions including proliferation, gene expression, differentiation, and apoptosis [26], we further examined levels of p-ERK in cells treated by drugs. Data showed that erlotinib treatment induced p-ERK down-regulation in LN229 cells, while it had no cooperation effect in the presence of palbociclib (Fig. 5a and b). To further analyze the different synergistic efficacy in cell lines with distinct genomic background, we calculated the inhibition ratio of signalings described above. As shown in Fig. 5c, in LN229 cells, signaling of Rb, Akt/mTOR, as well as ERK were all significantly suppressed compared to U87MG and LNZ308 cells. In U87MG and LNZ308 cells, the synergistic effects on the signaling described above were similar except for Akt signaling. Following the combined treatment, the inhibition rate of p-Akt in U87MG was dramatically higher than that in LNZ308 cells (Fig. 5c). Together, these data indicate that combination of palbociclib and erlotinib exerts synergetic anti-GBM activity through blocking the Rb and Akt/mTOR signaling pathway.
Discussion
Since cancers establish themselves by multiple steps and their cellular signaling is executed through complex networks rather than linear pathways, it may not be straightforward for defining the drug target. Extent genomic studies have demonstrated that malignant tumors are induced by a combination of gene mutations, as well as mutation regulated signaling alteration. Accumulating data has shown that suppressing one signaling or blocking one specific signaling pathway by monotherapy is not enough for cancer treatment. Herein, utilization of combination treatment including combination of various chemotherapeutic agents has been well accepted for treating various cancers. GBM is the most common primary malignant brain tumor with highly infiltrative and invasive features. Molecular studies have identified three key genetic events contributing its development, including dysregulation of growth factor signaling induced by amplification and mutational activation of RTK genes, inactivation of the p53 and Rb tumor suppressor pathways [11], providing potential targets for conquer the most malignant cancer.
Among cancer therapies via blocking signaling pathways, targeting EGFR signaling has been well studies and a large number of drugs have been developed for treatment of glioma, such as monoclonal antibodies, tumor-antigen specific vaccines, tyrosine kinase inhibitors (TKI) [12, 14]. TKIs (erlotinib, gefitinib, lapatinib, and canertinib) are small molecules that mechanistically compete for the ATP-binding site in the TK domain of EGFR, blocking phosphorylation dependent downstream signaling [19]. Among them, erlotinib is an orally active and represents the best explored TK inhibitors in the clinic for the treatment of GBM [19]. However, most studies have shown very modest or no significant survival benefit from TKIs due to the recurrent problem of resistance caused by mutations in EGFR or tumor heterogeneity [12, 37]. Hence, with TKIs, combination treatment is required for achieving a better efficacy against GBM. It is reported that erlotinib can be used in combination with radiotherapy and temozolomide, prolonging survival of GBM patents [19]. The Rb pathway plays critical roles in the regulation of cell cycle progression and cell death. CDK4/6 contributes proliferation via suppressing the Rb1 tumor suppressor and by sequestering p27Kip1 and p21Cip1, thereby promoting E2F- and Cdk2- dependent cell cycle progression [30]. Amplification of chromosome 12q13-q15 [Cyclin-dependent kinase 4 (CDK4) amplicon] is frequently observed in numerous human cancers including GBM. The CDK4, CDK6 and CCND2 genes are amplified in 18%, 1% and 2% of the glioblastoma tumors examined [5]. Therefore, the frequent alterations of components of the Rb-pathway in cancer, such as CDK4, raise the possibility for rationally designed therapeutic strategies that targeting this pathway. Palbociclib, an orally active small molecule that potently and specifically inhibits cyclin D kinase 4/6, has been developed as chemotherapeutic agent for breast cancer treatment. It has synergistic anti-tumor activities in combination with other drugs in breast carcinoma, multiple myeloma, and other tumors [21, 25], indicating the therapeutic activity of palbociclib in vivo.
Since both EGFR and Rb signaling pathways play important roles in GBM progression, it is promising that blocking the two pathways by combination of erlotinib and palbociclib provides strong efficacy for GBM treatment. Since p53 pathway is the third key regulator of GBM development, we employ three cell systems (LN229, p53 mutant; U87MG, PETN deletion; and LNZ308, p53 and PTEN deletion) to study the effects of the combination treatment on GBM. Our data showed that erlotinib and palbociclib exert synergistic inhibition in all human GBM cell lines: LN229, U87MG, and LNZ308 (Figs. 2 and 3). Among the three cell lines, LN299 cells are more sensitive to the combined treatment compared to U87MG and LNZ308 cells. Further signaling examinations defined that the inhibition on the Rb and Akt/mTOR signaling was stronger than that of two other cell lines (Fig. 5c). These data indicate that in the presence of PTEN, GBM cells with p53 mutation are more sensitive to the combined treatment of erlotinib and palbociclib. Between U87MG and LNZ308 cell lines, it is notable that U87MG cells were more sensitive to the combined treatment, which is consistent with the inhibition on Akt signaling. Therefore, it is possible that in the absence of PTEN, a tumor suppressor and key regulator of PI3K/Akt pathway, p53 contributes the inhibition of Akt signaling through blocking EGRF and CDK4/6 activity. Except for CDK4/6, Rb is tightly regulated by CDK inhibitors (CKIs) such as p16INK4A(CDKN2A) and p18INK4C(CDKN2C) [35]. Among the three cell lines used, LN229 and U87MG are CDKN2A/CDKN2C deleted cell lines, while LNZ308 is a cell line with CDK4 amplification, which is the least sensitive one to either palbociclib or erlotinib, as well the combination.
Taken together, our study demonstrates that combination of EGFR inhibitor, erlotinib, and CDK4/6 inhibitor, palbociclib exhibits synergistic effects against human GBM, which stands a proof-of-concept of dual blocking the key signaling pathways contribute tumor suppression. Data presented here provides evidence that blocking EGFR and Rb signaling are a potential strategy for GBM treatment. Dosages of these approved anti-cancer drugs can be reduced when used in combination, which could avoid potential side effects and toxicities.
Materials and methods
Cell lines and reagents
Human glioblastoma U87MG, LN299, and LNZ308 cells were cultured in DMEM with 10% FBS and 1 × Pen/Strep/glutamine. Erlotinib and palbociclib were from Elleckchem and dissolved in DMSO at 10 mM concentration as stocking solution. CaspGLOW™ red active caspase-3 staining kit was from Biovision. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazol-iumbromide (MTT) and antibody against β-actin (A2228) were purchased from Sigma. All other antibodies (p-Rb, #8181; Rb, #9309; p-Akt, #4060; Akt, #4685; p-4E-BP1, #2855; 4E–BP1, #9644; p-ERK, #4370, ERK, #4695; PARP, #9532; CDK4, #12790; CDK6, #13331; Cyclin D1, #2978) were obtained from Cell signaling (Beverly, MA). The horseradish peroxidase linked IgG secondary antibodies were purchased from GE healthcare.
Cell proliferation assay
Cells were seeded in 96-well plates (3000 cells/ well) for treatment. The next day, the cells were treated with compounds in fresh medium. Triplicate experiments were performed in a parallel manner for each concentration point. Following treatment, the culture medium was removed and the cells were washed twice with PBS. Then 0.5 mg/ml MTT solution was added to each well. The cells were further incubated at 37 °C for 4 h. The supernatant was discarded and 100 μl of dimethyl sulfoxide (DMSO) was added to each well. The mixture was shaken on a micro-vibrator for 5 min and the absorbance was measured at 570 nm.
Flow cytometric analysis
Cells were collected and washed twice with ice-cold PBS. Then the cells stored at −20 °C for over 24 h following fixing with 70% ethanol. Before staining, the fixed cells were centrifuged at 3000 rpm for 10 min and the supernatant was discarded. Cells were then washed with 5 ml PBS and incubated with propidium iodide (20 μg/ml) /RNase A (20 μg/ml) in PBS for 45 min. After incubation, samples were analyzed by flow cytometer with FlowJo software.
Colony formation assay
For colony formation assay, a base 0.6% agar gel with 10% FBS in DMEM was prepared and added to the wells of a six-well culture plate. Cells were seeded at a density of 5000 cells per well on top of the base agar for anchorage-independent growth in 0.4% agar gel with 10% FBS in DMEM supplemented with certain drug(s). The cells were maintained at 37 °C and allowed to grow for 3 weeks. Completed medium with drug(s) was replaced every two days. The colonies were scored following staining with MTT.
Determination of combination index
The synergistic effect of palbociclib and erlotinib was quantified by calculation of the combination index (CI), which was calculated according to the median-effect principle.
The equation for the isobologram was as follows: CI = (D)1/ (Dx)1 + (D)2/(Dx)2, where (Dx)1 and (Dx)2 indicate the individual dose of palbociclib and erlotinib required to inhibit a given level of cell growth, and (D)1 and (D)2 indicate the doses of palbociclib and erlotinib necessary to produce the same effect in combination, respectively. The combined effects were scored on the following scale: CI < 1, synergism; CI = 1, additive effect; and CI > 1, antagonism. Data analysis was performed with Calcusyn software.
Western blot analysis
Cells were collected and lysed in RIPA buffer and cell lysate were collected following 30 min incubation on ice and centrifuged for 15 min at 4 °C. Total protein was quantified using the Bradford reagent, and equal amounts of total protein were mixed with 4 × SDS sample buffer, incubated at 95 °C for 5 min, and loaded in SDS-PAGE gels. After electrophoresis, proteins were transferred to nitrocellulose filter membrane and blocked for 1 h in 5% milk prepared in TBST at room temperature. Each membrane was incubated with primary antibody at 4 °C for overnight. The blots were then washed and incubated with HRP-conjugated secondary antibodies for 1 h, washed three times with PBST, and visualized using the Immobilon Western Chemiluminescent HRP Substrate.
Statistical analysis
Data are presented as the mean ± S.D. of three independent experiments. Statistical evaluation was performed using the Student’s t-test or one-way ANOVA. Difference was statistically significant when P < 0.05. All statistical analyses were performed using Prism software (Graph Pad Software, La Jolla, CA, USA).
References
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES (2015) The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14:130–146
Bollard J, Miguela V, Ruiz de Galarreta M, Venkatesh A, Bian CB, Roberto MP, Tovar V, Sia D, Molina-Sanchez P, Nguyen CB, Nakagawa S, Llovet JM, Hoshida Y, Lujambio A (2016) Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut 66(7):1286–1296
Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R (2008) Epidermal growth factor receptor inhibitors in neuro-oncology: hopes and disappointments. Clin Cancer Res: Official J Am Assoc Cancer Res 14:957–960
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O'Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477
Cancer Genome Atlas Research, N (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Chou TC, Motzer RJ, Tong Y, Bosl GJ (1994) Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst 86:1517–1524
de Groot JF, Gilbert MR, Aldape K, Hess KR, Hanna TA, Ictech S, Groves MD, Conrad C, Colman H, Puduvalli VK, Levin V, Yung WK (2008) Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neuro-Oncol 90:89–97
Dickson MA, Tap WD, Keohan ML, D'Angelo SP, Gounder MM, Antonescu CR, Landa J, Qin LX, Rathbone DD, Condy MM, Ustoyev Y, Crago AM, Singer S, Schwartz GK (2013) Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol Off J Am Soc Clin Oncol 31:2024–2028
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16:25–35
Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3:1427–1438
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710
Gan HK, Kaye AH, Luwor RB (2009) The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci: Off J Neurosurg Soc Australas 16:748–754
Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H, Pesek M, Serwatowski P, Ramlau R, Janaskova T, Vansteenkiste J, Strausz J, Manikhas GM, Von Pawel J (2007) Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva lung cancer investigation trial. J Clin Oncol Off J Am Soc Clin Oncol 25:1545–1552
Hatanpaa KJ, Burma S, Zhao D, Habib AA (2010) Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 12:675–684
He Y, Su Z, Xue L, Xu H, Zhang C (2016) Co-delivery of erlotinib and doxorubicin by pH-sensitive charge conversion nanocarrier for synergistic therapy. J Control Release: Off Journal Control Release Soc 229:80–92
Huang J, Liu F, Liu Z, Tang H, Wu H, Gong Q, Chen J (2017) Immune checkpoint in glioblastoma: promising and challenging. Front Pharmacol 8:242
Huether A, Hopfner M, Sutter AP, Schuppan D, Scherubl H (2005) Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J Hepatol 43:661–669
Jovcevska I, Kocevar N, Komel R (2013) Glioma and glioblastoma - how much do we (not) know? Mol Clin Oncol 1:935–941
Karpel-Massler G, Schmidt U, Unterberg A, Halatsch ME (2009) Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: where do we stand? Mol Cancer Res: MCR 7:1000–1012
Ling YH, Li T, Yuan Z, Haigentz M Jr, Weber TK, Perez-Soler R (2007) Erlotinib, an effective epidermal growth factor receptor tyrosine kinase inhibitor, induces p27KIP1 up-regulation and nuclear translocation in association with cell growth inhibition and G1/S phase arrest in human non-small-cell lung cancer cell lines. Mol Pharmacol 72:248–258
McClendon AK, Dean JL, Rivadeneira DB, Yu JE, Reed CA, Gao E, Farber JL, Force T, Koch WJ, Knudsen ES (2012) CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle 11:2747–2755
Michel L, Ley J, Wildes TM, Schaffer A, Robinson A, Chun SE, Lee W, Lewis J Jr, Trinkaus K, Adkins D (2016) Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol 58:41–48
Nie P, Hu W, Zhang T, Yang Y, Hou B, Zou Z (2015) Synergistic induction of Erlotinib-mediated apoptosis by resveratrol in human non-small-cell lung cancer cells by down-regulating Survivin and up-regulating PUMA. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol 35:2255–2271
Omuro AM (2008) Exploring multi-targeting strategies for the treatment of gliomas. Curr Opin Investig Drugs 9:1287–1295
Palanisamy RP (2016) Palbociclib: a new hope in the treatment of breast cancer. J Cancer Res Ther 12:1220–1223
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183
Peereboom DM, Shepard DR, Ahluwalia MS, Brewer CJ, Agarwal N, Stevens GH, Suh JH, Toms SA, Vogelbaum MA, Weil RJ, Elson P, Barnett GH (2010) Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neuro-Oncol 98:93–99
Qaddoumi I, Kocak M, Pai Panandiker AS, Armstrong GT, Wetmore C, Crawford JR, Lin T, Boyett JM, Kun LE, Boop FA, Merchant TE, Ellison DW, Gajjar A, Broniscer A (2014) Phase II trial of Erlotinib during and after radiotherapy in children with newly diagnosed high-grade gliomas. Front Oncol 4:67
Shan F, Shao Z, Jiang S, Cheng Z (2016) Erlotinib induces the human non-small-cell lung cancer cells apoptosis via activating ROS-dependent JNK pathways. Cancer Med 5:3166–3175
Sherr CJ, Roberts JM (2004) Living with or without cyclins and cyclin-dependent kinases. Genes Dev 18:2699–2711
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29
Tao Z, Le Blanc JM, Wang C, Zhan T, Zhuang H, Wang P, Yuan Z, Lu B (2016) Coadministration of Trametinib and Palbociclib Radiosensitizes KRAS-mutant non-small cell lung cancers in vitro and in vivo. Clin Cancer Res: Off J Am Assoc Cancer Res 22:122–133
Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, Kubek S, Oldrini B, Chheda MG, Yannuzzi N, Tao H, Zhu S, Iwanami A, Kuga D, Dang J, Pedraza A, Brennan CW, Heguy A, Liau LM, Lieberman F, Yung WK, Gilbert MR, Reardon DA, Drappatz J, Wen PY, Lamborn KR, Chang SM, Prados MD, Fine HA, Horvath S, Wu N, Lassman AB, DeAngelis LM, Yong WH, Kuhn JG, Mischel PS, Mehta MP, Cloughesy TF, Mellinghoff IK (2012) Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov 2:458–471
Vlenterie M, Hillebrandt-Roeffen MH, Schaars EW, Flucke UE, Fleuren ED, Navis AC, Leenders WP, Versleijen-Jonkers YM, van der Graaf WT (2016) Targeting cyclin-dependent kinases in synovial sarcoma: Palbociclib as a potential treatment for synovial sarcoma patients. Ann Surg Oncol 23:2745–2752
Wiedemeyer WR, Dunn IF, Quayle SN, Zhang J, Chheda MG, Dunn GP, Zhuang L, Rosenbluh J, Chen S, Xiao Y, Shapiro GI, Hahn WC, Chin L (2010) Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci U S A 107:11501–11506
Wilson TA, Karajannis MA, Harter DH (2014) Glioblastoma multiforme: state of the art and future therapeutics. Surg Neurol Int 5:64
Zhu JJ, Wong ET (2013) Personalized medicine for glioblastoma: current challenges and future opportunities. Curr Mol Med 13:358–367
Funding
This work is supported by grants from Natural Science Foundation of Hunan (No.2016jj3169 and 2015jj4101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest and consent for the submission.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Supplemental figure 1
Effect of palbociclib, erlotinib alone or combination on cell cycle progression in LNZ308 (A), LN229 (B), and U87MG cells. Following treated with palbociclib (0.5 μM), erlotinib (2 μM) alone or combination for 24 h, cells were collected, fixed, and stained with PI as described followed by Flow cytometry analysis. (PDF 321 kb)
Supplemental figure 2
Effect of palbociclib, erlotinib alone or combination on expression levels of CDK4, CDK6, and Cyclin D1 proteins. Following treatment with palbociclib, erlotinib alone or combination at the concentration described in Fig. 4, cell lysates were collected and analyzed western blotting using indicated antibodies. (PDF 436 kb)
Rights and permissions
About this article
Cite this article
Liu, S., Tang, Y., Yuan, X. et al. Inhibition of Rb and mTOR signaling associates with synergistic anticancer effect of palbociclib and erlotinib in glioblastoma cells. Invest New Drugs 36, 961–969 (2018). https://doi.org/10.1007/s10637-018-0575-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0575-z