Summary
Background The inhibition of insulin-like growth factor receptor-1 (IGF-1R) induces cell cycle arrest and enhancing the effect of castration by delay of progression of human prostate cancer models. Linsitinib is a small molecule and potent dual inhibitor of IGF-1R and insulin receptor tyrosine kinase activity. We report results of a single-arm, phase II study evaluating the safety and efficacy of linsitinib in men with chemotherapy-naïve asymptomatic or mildly symptomatic metastatic castration resistant prostate cancer (mCRPC). Methods Patients received at 150 mg orally twice daily on a 28-day cycle. The primary endpoint was prostate specific (PSA) response at 12 weeks and correlative studies included circulating tumor cells (CTCs) and circulating endothelial cells (CECs). Results Seventeen patients, median age 68 (55–78) and pre-treatment PSA of 55.23 (2.46–277.60) were enrolled and completed 12 weeks of therapy. All but two patients discontinued therapy secondary to PSA progression, which met the predefined futility criteria and led to early termination of this study. Overall best response (RECIST v1.1) included a partial response in 1 patient and stable disease in 8 patients. Higher baseline CTCs were associated with higher pre-treatment PSA levels (Spearman r = 0.49, p = 0.04) but no correlation between PSA progression and CTCs/CECs were observed. Most common adverse events included fatigue, nausea/vomiting, AST/ALT changes and prolonged QT interval. Conclusions Single-agent linsitinib was safe and well tolerated but failed to show activity in men with mCRPC. These results highlight the complexity of using IGF-1R as a therapeutic target in this patient population. ClinicalTrials.gov NCT01533246.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second-leading cause of death in men in the United States with estimated 161,360 new cases and 26,730 deaths in 2017as per SEER data [1]. Although more than 90% of metastatic PCa patients initially respond to androgen deprivation therapy, most tumors became refractory and progress to a castration- resistant state. Management of mCRPC manifested as rising prostate-specific antigen, worsening symptomatic disease or progressive disease on imaging studies has always been area of interest.
In the last 11 years, the treatment landscape of mCRPC has dramatically changed with the approval of six different life-prolonging therapies by the Food and Drug Administration (FDA) [2]. Unfortunately, most patients experience disease progression, thus it is critical to gain a better understanding of the underlying pathways contributing to drugs resistance and mechanisms to target. More recently, with the advent of tumor molecular profiling, a number of targeted therapies have been investigated in men with mCRPC, but results have been overall modest and tumor invariably develops resistance to these therapies as well [3,4,5,6].
The insulin-like growth factor receptor-1 (IGF-1R) is a tetrameric transmembrane receptor tyrosine kinase that is widely expressed in normal human tissues and required for embryonic development and postnatal growth. IGF-1R and its ligands, IGF-1 and IGF-2, are up regulated in a variety of human cancers [7]. The IGF axis activation ligands IGF-1 and IGF-2 have shown to be associated with cellular mitogenesis, angiogenesis, tumor cell survival and tumerogenesis in various cell lines [8,9,10]. The IGF-1R and its ligands, IGF-1 and IGF-2, play a key role in regulating growth, resistance to apoptosis, and invasion in a variety of human cancers [11,12,13]. Epidemiological studies have shown that increased circulating IGF-1 levels and decreased insulin-like growth factor binding protein-3 (IGFBP-3) levels are associated with higher risk of developing prostate cancer [14]. In addition, IGF-1R is often overexpressed in prostate tumors and can mediate prostate cancer cell proliferation and resistance to androgen ablation therapy [15, 16]. Preclinical models have shown evidence of chemo sensitization of androgen independent human prostate cancer cells when IGF-IR blockade is combined with cisplatin, mitoxantrone, or paclitaxel [17]. Thus, inactivation of the IGF-I axis represents a potential target to treat androgen independent prostate cancer. Treatment strategies involving monoclonal antibodies against IGF-1R have been studied in recent years in different settings of CRPC [18,19,20].
Linsitinib (OSI-906) is a small molecule that is a highly selective dual inhibitor of IGF-1R and insulin receptor tyrosine kinase activity. The IGF-1R is activated by its cognate ligands, IGF-1 and IGF-2, and also by insulin at a much lower affinity. Ligand binding to the receptor activates intrinsic protein tyrosine kinase activity, resulting in β subunit phosphorylation and the stimulation of signaling cascades that include the PI3K/AKT and Ras/Raf/MAPK pathways [7]. Linsitinib has been reported to be well tolerated in patients with advanced solid tumors. The majority of adverse effects reported were grade 1–2 nausea, vomiting, fatigue, and diarrhea [21, 22]. Dose-limiting toxicities (DLTs) observed in early phase studies were QTc prolongation, hyperglycemia and elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (all grade 3). Based on phase 1 studies the recommended phase II dose (RP2D) was 150 mg twice daily [22]. To determine the activity of linsitinib in men with mCRPC, a Simon two-staged phase II study was conducted.
Patient and methods
The study was open at Cleveland Clinic and Case Comprehensive Cancer Center. The Cleveland Clinic Institutional Review Board (IRB) and Case Comprehensive IRB reviewed and approved the trial in accordance with cancer therapy evaluation program (CTEP) multicenter guidelines. The study drug was provided under a Collaborative Agreement with CTEP, Division of Cancer Treatment and Diagnosis (DCTD), NCI. This study was registered in ClinicalTrials.gov with number NCT01533246.
Eligibility criteria
Patients with asymptomatic or mildly symptomatic mCRPC, defined by a score of 3 or less on Brief Pain Inventory-short form (BPI-SF) Question #3 (worst pain in last 24 h), were enrolled in the study [23]. Eligibility criteria included histologically confirmed PCa with mCRPC documented by positive whole body bone scan or soft tissue (lymph node or visceral) metastasis on imaging studies (CT, MRI scans). If lymph node metastasis was the only evidence of metastasis, it was required to be ≥2 cm in diameter (long axis). Additional eligibility criteria included Eastern Cooperative Group Performance Status (ECOG PS) [24] of 0–1, surgically or medically castrated testosterone levels of ≤50 ng/dL (<2.0 nM), adequate hepatic, renal and bone marrow function (Hemoglobin ≥10.0 g/dL, absolute neutrophil count >1500/μL, platelet count ≥100,000/μL, serum albumin ≥3.5 g/dL, serum creatinine <1.5 x ULN or a calculated creatinine clearance ≥60 mL/min, serum potassium ≥3.5 mmol/L, serum bilirubin <1.5 x ULN, AST or ALT <2.5 x UL). Patients who had received prior chemotherapy for CRPC were excluded. However, prior neoadjuvant or adjuvant chemotherapy was allowed as long it was completed 1 year prior to study entry. Prior investigational agents (including adrenal inhibitors, antiadrogens and Sipuleucel-T) or other hormonal therapy, were allowed as long as discontinued within a specified time prior to enrollment. Prior treatment with luteinizing hormone releasing hormone (LHRH) agonists must have been initiated at least 4 weeks prior to Cycle 1 Day 1 and was continued throughout the study. Patients on stable doses of bisphosphonates were allowed to continue on this medication, but patients were not allowed to initiate this therapy within 4 weeks prior to starting linsitinib or throughout the study. Other exclusion criteria included current use of opiate analgesic for cancer related pain, prior radiotherapy for cancer related pain within 4 weeks, prior IGF-1R inhibitor, other concurrent malignancy, concurrent administration of CYP1A2 inhibitors/inducers, prolonged QTc >470 millisecond (mean QTc with Bazett’s correction [25]), history of familiar long QT syndrome, known brain metastasis, insulin dependent diabetes mellitus, known HIV and hepatitis A, B, C.
Treatment planning
Patients received linsitinib at 150 mg orally twice daily on a 28-day cycle with plan to continue on study until progressive disease, drug intolerability, or consent withdrawal. Regardless of the reason, the maximum time off linsitinib allowed was 14 consecutive days.
Evaluation
Study endpoints included PSA response at 12 weeks, safety, RECIST-defined response, time to PSA progression, and overall survival (OS). Blood correlative studies were collected to assess the effect of target molecule on circulating tumor cells (CTC) and circulating endothelial cells (CEC), on C1D1 prior to receiving study drug, on C2D1, C4D1 and at the end of treatment. To evaluate the effect of linsitinib in circulating endothelial cells (CEC) ad circulating tumor cells (CTC), blood samples were collected using CellSearch® System from Veridex LLC, in two 10 mL cell saver tubes of peripheral blood.
Progressive disease was documented by PSA progression and/or radiographic progression according to modified RECIST 1.1. PSA progression was defined by a 25% or greater increase in PSA value and an absolute increase of 2 ng/mL or more from the nadir, which was confirmed by a second value obtained 3 or more weeks, per prostate cancer working group 2 (PCWG-2) criteria [26]. Radiographic response and progression were evaluated using the new international criteria proposed by the revised Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1) [27]. Changes in the largest diameter (one-dimensional measurement) of the tumor lesions and the shortest diameter in the case of malignant lymph nodes were used per RECIST 1.1. For toxicity assessments, descriptions and grading scales found in the revised national cancer institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 were utilized. Study drug was discontinued permanently if any grade (G)4 toxicity was reported or study was interrupted for more than 14 consecutive days. Dose reduction was permitted for subsequent cycles depending on the severity of the toxicity to the dose level of 100 mg and 75 mg BID.
Statistical evaluation
The primary endpoint of the trial was PSA response at 12 weeks. Secondary endpoints included safety based on CTCAE version 4.0, RECIST-based response in patients with bi-dimensional measurable disease, time to PSA progression, and overall survival. A two-stage accrual design was employed to test the hypothesis that linsitinib has an underlying probability of PSA response of 30%. With a plan to initially accrue 17 eligible and evaluable patients, a futility rule was included to terminate the trial if six or fewer PSA responses were observed (PSA response rate < 35%). If more than six PSA responses were observed, then an additional 18 eligible and evaluable patients would be treated. Linsitinib would be accepted as a potentially active therapy if 14 or more patients have had a PSA response. With this design, the overall type I and II errors were 9% each; and the likelihood of stopping the trial early was <8% if linsitinib was active (>55% PSA response) but >78% if it was not (<30% PSA response).
Categorical data was summarized as frequency counts and proportions; measured data was summarized using means, standard deviations, medians, and ranges; and time to event data such as time to PSA progression and overall survival was calculated by using the method of Kaplan Meier. This study was monitored by the Clinical Data Update System (CDUS) version 3.0. Cumulative CDUS data was submitted quarterly to CTEP by electronic means.
Results
Patient characteristics
From February 2012 to April 2012, 18 patients were entered into the trial between 2/2012 and 4/2012. One patient was considered ineligible and has been excluded from all analyses. Table 1 summarizes patient and disease characteristics. Median age at on-study was 68 (55–78); all patients had good ECOG performance status (76% ECOG 0, 24% ECOG 1); and all patients achieved castration levels of testosterone chemically. Forty-one percent of patients had bone-only disease, 29% had only soft-tissue metastasis, and 29% had both. With the exception of one patient with liver disease, all metastases were to lymph nodes. Median pre-treatment PSA was 55.23 ng/mL (range 2.46–277.60).
Treatment administration
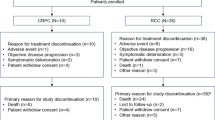
Fifteen out of the 17 patients discontinued treatment: 13 patients due to disease progression, one patient withdrew consent and one due to adverse events (AEs). Patients received a median of 3, 28-day treatment cycles (range 2–6+). Three patients had dose reductions to 100 mg bid; one patient had the second of two cycles held due to a hospitalization, and one patient consistently missed several doses per cycle while on-study (3 cycles). Patients took a median of 97.5% of the prescribed doses (range 75%–108%); with 82% of patients being >90% compliant.
Clinical efficacy
All 17 patients were evaluable for PSA response. One patient achieved a partial response (63% decline from 2.46–0.90 ng/ml) that lasted 1.9 months; and 7 others had transient PSA declines ranging from 3 to 42% (median of 24%). All but two patients demonstrated PSA progression with a median time to progression of 1.8 months.
Seventeen patients were evaluable for radiographic response using RECIST 1.1 criteria (Table 2). One patient had a partial response and 8 others had stable disease; these responses lasted for 2.8 months (range 0.8–5.0). Eight patients had progressed on therapy.
With a median follow up of 51 months (range 25.2–77.0), 82% of deaths were reported. Median overall survival was 33 months (95% CI, 30.7–34.6).
Safety
Table 3 summarizes all treatment related toxicities that occurred in at least three (>15%) patients. The most commonly reported toxicities were mild to moderate transaminase elevations (59%, 10/17; 3 G2, 7 G1), fatigue (59%, 10/17; 2 G2, 8 G1), hyperglycemia (47%, 8/17; 2 G2, 6 G1), prolonged QT interval (35%; 6/17; 3 G2 and 3 G1), and nausea/vomiting (35%; 6/17, 1 G2, 5 G1). No G4 toxicities have been reported. One patient reported G3 duodenitis that was considered at least possibly related to treatment. All other toxicities considered at least possibly related to treatment were coded mild or moderate.
CTC and CEC
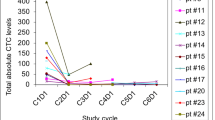
CTC and CEC samples were received at baseline, cycle 2 and 4, for all 17 patients (Table 4). Among these, 94% had evaluable CTC/CEC levels at baseline: A median of 1 CTC/7.5 mL and 20 CECs/4 mL of blood were detected. An association between pre-treatment PSA and pre-treatment CTC was observed (Spearman r = 0.49, p = 0.04). At week 12, fifteen patients had evaluable CTC/CEC levels. No significant change in the number of CTC and CEC counts was observed, over time. No correlation between CTC changes and PSA progression was observed.
Discussion
The IGF pathway has become an attractive therapeutic target for drug development in many solid tumors as it represents a key proliferative and pro-survival signaling pathway in a variety of malignancies and plays a crucial role in the development of resistance to a variety of useful cancer therapies [28, 29]. Monoclonal antibodies against IGF-R have previously been studies in early phase studies in variety of epithelial malignancies [19, 20, 30].
To determine the clinical role of IGF-R antibody in men with mCRPC a phase 2 study of linsitinib, a small molecule potent dual inhibitor of IGF-1R and insulin receptor TK, was conducted. In this study, linsitinib was well tolerated with most AEs experienced by patients were G1/2 and only few patients required a dose reduction due to toxicity.
The findings of this study failed to confirm the preclinical data supporting the inhibition of IGF-IR in this setting, even though the primary endpoint of PSA response at 12 weeks might not fully capture this agent’s activity. While an objective response was seen in only 1 patient with partial response, almost half of the population had a RECIST-defined SD and the overall survival reported is in line with the current standard of care life-prolonging therapies including abiraterone acetate and enzalutamide [31, 32] in the same setting. Nonetheless, in the absence of a control arm not exposed to IGF-pathway inhibition, no definitive assumptions can be made. Plus, we should highlight the positive impact that patient selection (visceral disease excluded) and the subsequent life-prolonging therapies after coming off trial may have caused in the overall survival of the study population.
The choice of this short end-point was based on Prostate Cancer Clinical Trials Working Group2 (PCWG2) [33] criteria with the purpose to detect a “proof-of-concept” signal and decide to go for a larger randomized clinical trial. Some of the advantages of using PSA as a primary endpoint include avoiding the treatment of larger numbers of patients and commitment of resources to potentially inactive therapies.
The IGF-IR is postulated to play a key role in metastasis by regulating cell adhesion, motility, migration and angiogenesis [34], thus the analysis of CTCs and CECs in clinical trials with IGF inhibitors is particularly appealing. Previous studies have shown that CTCs may be independent predictors of the time to disease progression as well as survival [35, 36]. In our study, though there was a possible association between pre-treatment CTCs and PSA, CTC counts did not change significantly following two cycles of treatment and no relationship was observed with regards to tumor burden and PSA correlation. The value of these findings is limited due to the relatively small number of patients with collected CTCs in addition to other known challenges with CTC-information, such as the timing of collection, the low sensitivity of the multiple CTC assays available and the existence of heterogeneous CTC populations [37].
While this study failed to show any significant PSA or objective response, the IGF-R pathway remains critical in CRPC progression and several growth factors including IGF-1R cross talk with androgen receptors (AR) in prostate cancer cells [38]. With this in mind, possible combinations using novel IGF-R inhibitors with current novel agents might be of clinical interest. Pre-clinical studies with different combinations of IGF-R1 with chemotherapy [39], antiandrogens [40, 41], PI3K/AKT/mTOR pathway [42], suggest synergistic effects in prostate cancer and may be may be a potential area of interest to explore, in the setting of CRPC.
In conclusion, this phase II study of linsitinib monotherapy in men with asymptomatic and mildly symptomatic mCRPC showed to be safe and well tolerated. Treatment with linsitinib however failed to show a significant objective and PSA response, any effect on CTCs or survival benefit. The combination of novel agents capable of blocking the IGFR axis with existing therapies in mCRPC may be a potential area of interest.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Food and Drug Administration (FDA) (2017) Hematology/oncology (cancer) approvals and safety notifications. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs. Accessed Dec 2017
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu Y-M, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS (2015) DNA-repair defects and Olaparib in metastatic prostate cancer. N Engl J Med 373(18):1697–1708. https://doi.org/10.1056/NEJMoa1506859
Chi KN, Higano CS, Blumenstein B, Ferrero JM, Reeves J, Feyerabend S, Gravis G, Merseburger AS, Stenzl A, Bergman AM, Mukherjee SD, Zalewski P, Saad F, Jacobs C, Gleave M, de Bono JS (2017) Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): a phase 3, multicentre, open-label, randomised trial. Lancet Oncol 18(4):473–485. https://doi.org/10.1016/s1470-2045(17)30168-7
Templeton AJ, Dutoit V, Cathomas R, Rothermundt C, Bärtschi D, Dröge C, Gautschi O, Borner M, Fechter E, Stenner F (2013) Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08). Eur Urol 64(1):150–158
Amato RJ, Wilding G, Bubley G, Loewy J, Haluska F, Gross ME (2012) Safety and preliminary efficacy analysis of the mTOR inhibitor ridaforolimus in patients with taxane-treated, castration-resistant prostate cancer. Clin Genitourin Cancer 10(4):232–238. https://doi.org/10.1016/j.clgc.2012.05.001
Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y (2009) Implications of insulin-like growth factor-I for prostate cancer therapies. International Journal of Urology: Official Journal of the Japanese Urological Association 16(2):161–167. https://doi.org/10.1111/j.1442-2042.2008.02224.x
Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA (2002) Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology 143(9):3259–3267. https://doi.org/10.1210/en.2001-211408
Kurmasheva RT, Houghton PJ (2006) IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta 1766(1):1–22. https://doi.org/10.1016/j.bbcan.2006.05.003
Samani AA, Yakar S, LeRoith D, Brodt P (2007) The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28(1):20–47. https://doi.org/10.1210/er.2006-0001
Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M (1998) Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet (London, England) 351(9113):1393–1396. https://doi.org/10.1016/s0140-6736(97)10384-1
Chang YS, Wang L, Liu D, Mao L, Hong WK, Khuri FR, Lee HY (2002) Correlation between insulin-like growth factor-binding protein-3 promoter methylation and prognosis of patients with stage I non-small cell lung cancer. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 8(12):3669–3675
Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8(12):915–928. https://doi.org/10.1038/nrc2536
Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M (2004) Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet (London, England) 363(9418):1346–1353. https://doi.org/10.1016/s0140-6736(04)16044-3
Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E (2002) Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst 94(14):1099–1106
Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME (2004) Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res 64(23):8620–8629. https://doi.org/10.1158/0008-5472.CAN-04-2446
Hellawell GO, Ferguson DJ, Brewster SF, Macaulay VM (2003) Chemosensitization of human prostate cancer using antisense agents targeting the type 1 insulin-like growth factor receptor. BJU Int 91(3):271–277
Ryan CJ, Harzstark AH, Rosenberg J, Lin A, Claros C, Goldfine ID, Kerner JF, Small EJ, Youngren JF (2008) A pilot dose-escalation study of the effects of nordihydroguareacetic acid on hormone and prostate specific antigen levels in patients with relapsed prostate cancer. BJU Int 101(4):436–439. https://doi.org/10.1111/j.1464-410X.2007.07330.x
Friedlander TW, Weinberg VK, Huang Y, Mi JT, Formaker CG, Small EJ, Harzstark AL, Lin AM, Fong L, Ryan CJ (2012) A phase II study of insulin-like growth factor receptor inhibition with nordihydroguaiaretic acid in men with non-metastatic hormone-sensitive prostate cancer. Oncol Rep 27(1):3–9. https://doi.org/10.3892/or.2011.1487
Molife LR, Fong PC, Paccagnella L, Reid AH, Shaw HM, Vidal L, Arkenau HT, Karavasilis V, Yap TA, Olmos D, Spicer J, Postel-Vinay S, Yin D, Lipton A, Demers L, Leitzel K, Gualberto A, de Bono JS (2010) The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer 103(3):332–339. https://doi.org/10.1038/sj.bjc.6605767
Jones RL, Kim ES, Nava-Parada P, Alam S, Johnson FM, Stephens AW, Simantov R, Poondru S, Gedrich R, Lippman SM, Kaye SB, Carden CP (2014) Phase I study of intermittent oral dosing of the insulin-like growth Factor-1 and insulin receptors inhibitor OSI-906 in patients with advanced solid tumors. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-14-0265
Puzanov I, Lindsay CR, Goff LW, Sosman JA, Gilbert J, Berlin J, Poondru S, Simantov R, Gedrich R, Stephens A, Chan E, Evans TR (2014) A phase I study of continuous oral dosing of OSI-906, a dual inhibitor of insulin-like growth Factor-1 and insulin receptors in patients with advanced solid tumors. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-14-0303
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 23(2):129–138
Sorensen JB, Klee M, Palshof T, Hansen HH (1993) Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer 67(4):773–775
Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM (2003) What clinicians should know about the QT interval. JAMA 289(16):2120–2127. https://doi.org/10.1001/jama.289.16.2120
Scher HI, Morris MJ, Basch E, Heller G (2011) End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol 29(27):3695–3704. https://doi.org/10.1200/JCO.2011.35.8648
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4(7):505–518. https://doi.org/10.1038/nrc1387
Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M (2001) In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR). Cancer Res 61(16):6276–6280
Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, Adjei AA, de Bono JS (2007) Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 13(19):5834–5840. https://doi.org/10.1158/1078-0432.CCR-07-1118
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim C-S, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin M-E, Venner PM, Tombal B (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371(5):424–433. https://doi.org/10.1056/NEJMoa1405095
Ryan CJ, Smith MR, Fizazi K, Miller K, Mulders P, Sternberg CN, Saad F, Griffin T, De Porre P, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf DE (2014) 753 final overall survival (OS) analysis of COU-AA-302, a randomized phase 3 study of Abiraterone acetate (AA) in metastatic castration-resistant prostate cancer (MCRPC) patients (pts) without prior chemoherapy. Ann Oncol 25(suppl 4):iv255. https://doi.org/10.1093/annonc/mdu336.1
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol Off J Am Soc Clin Oncol 26(7):1148–1159. https://doi.org/10.1200/jco.2007.12.4487
Bahr C, Groner B (2005) The IGF-1 receptor and its contributions to metastatic tumor growth-novel approaches to the inhibition of IGF-1R function. Growth Factors 23(1):1–14. https://doi.org/10.1080/08977190400020229
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 14(19):6302–6309. https://doi.org/10.1158/1078-0432.CCR-08-0872
Olmos D, Arkenau HT, Ang JE, Ledaki I, Attard G, Carden CP, Reid AH, A'Hern R, Fong PC, Oomen NB, Molife R, Dearnaley D, Parker C, Terstappen LW, de Bono JS (2009) Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO 20(1):27–33. https://doi.org/10.1093/annonc/mdn544
Liu W, Yin B, Wang X, Yu P, Duan X, Liu C, Wang B, Tao Z (2017) Circulating tumor cells in prostate cancer: precision diagnosis and therapy. Oncol Lett 14(2):1223–1232. https://doi.org/10.3892/ol.2017.6332
Zhu M-L, Kyprianou N (2008) Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer 15(4):841–849. https://doi.org/10.1677/ERC-08-0084
Wu JD, Haugk K, Coleman I, Woodke L, Vessella R, Nelson P, Montgomery RB, Ludwig DL, Plymate SR (2006) Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res 12(20 Pt 1):6153–6160. https://doi.org/10.1158/1078-0432.ccr-06-0443
Mancarella C, Casanova-Salas I, Calatrava A, Ventura S, Garofalo C, Rubio-Briones J, Magistroni V, Manara MC, Lopez-Guerrero JA, Scotlandi K (2015) ERG deregulation induces IGF-1R expression in prostate cancer cells and affects sensitivity to anti-IGF-1R agents. Oncotarget 6(18):16611–16622. https://doi.org/10.18632/oncotarget.3425
Yu EY, Li H, Higano CS, Agarwal N, Pal SK, Alva A, Heath EI, Lam ET, Gupta S, Lilly MB, Inoue Y, Chi KN, Vogelzang NJ, Quinn DI, Cheng HH, Plymate SR, Hussain M, Tangen CM, Thompson IM Jr (2015) SWOG S0925: a randomized phase ii study of androgen deprivation combined with cixutumumab versus androgen deprivation alone in patients with new metastatic hormone-sensitive prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol 33(14):1601–1608. https://doi.org/10.1200/jco.2014.59.4127
Rathkopf DE, Danila DC, Morris MJ, Slovin SF, Borwick LS, Momen L, Curley T, Arauz G, Larson SM, Fleisher M, Rosen N, Scher HI (2011) Anti-insulin-like growth factor-1 receptor (IGF-1R) monoclonal antibody cixutumumab (cix) plus mTOR inhibitor temsirolimus (tem) in metastatic castration-resistant prostate cancer (mCRPC): results of a phase I pilot study. J Clin Oncol 29(15_suppl):e15081–e15081. https://doi.org/10.1200/jco.2011.29.15_suppl.e15081
Funding
No funding was used for this study. This was the Cancer Therapy Evaluation Program (CTEP) Sponsored Trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Barata, P., Cooney, M., Tyler, A. et al. A phase 2 study of OSI-906 (linsitinib, an insulin-like growth factor receptor-1 inhibitor) in patients with asymptomatic or mildly symptomatic (non-opioid requiring) metastatic castrate resistant prostate cancer (CRPC). Invest New Drugs 36, 451–457 (2018). https://doi.org/10.1007/s10637-018-0574-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0574-0