Summary
Preclinical studies in small cell lung cancer (SCLC) have shown that hyaluronic acid (HA) can be effectively used to deliver chemotherapy and selectively decrease CD44 expressing (stem cell-like) tumour cells. The current study aimed to replicate these findings and obtain data on safety and activity of HA-irinotecan (HA-IR). Eligible patients with extensive stage SCLC were consented. A safety cohort (n = 5) was treated with HA-IR and Carboplatin (C). Subsequently, the patients were randomised 1:1 to receive experimental (HA-IR + C) or standard (IR + C) treatment, to a maximum of 6 cycles. The second line patients were added to the study and treated with open label HA-IR + C. Tumour response was measured after every 2 cycles. Baseline tumour specimens were stained for CD44s and CD44v6 expression. Circulating tumour cells (CTCs) were enumerated before each treatment cycle. Out of 39 patients screened, 34 were evaluable for the study. The median age was 66 (range 39–83). The overall response rates were 69% and 75% for experimental and standard arms respectively. Median progression free survival was 42 and 28 weeks, respectively (p = 0.892). The treatments were well tolerated. The incidence of grade III/IV diarrhea was more common in the standard arm, while anaemia was more common in the experimental arm. IHC analysis suggested that the patients with CD44s positive tumours may gain survival benefit from HA-IR. HA-IR is well tolerated and active in ES-SCLC. The effect of HA-IR on CD44s + cancer stem-like cells provide an early hint towards a potential novel target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small cell lung cancer (SCLC) is often sensitive to platinum based chemotherapy, but early relapses limit the effectiveness of this form of therapy [11, 23, 43, 46, 48]. The current standard of care to treat extensive stage small cell lung cancer (ES-SCLC) is a combination of platinum analogues (either cisplatin or carboplatin) [43, 46] and etoposide [14, 39]. The addition of a third agent, such as paclitaxel to a platinum-etoposide doublet has been associated with increased toxicity without improvement in survival [36], whereas alternatives to etoposide have been combined with platinum agents in various studies. Several studies have shown high response rates with irinotecan-based regimens [4, 26, 27, 41]. Noda et al. in [31] reported a clinical trial comparing the use of cisplatin with either irinotecan or etoposide in patients with ES-SCLC showing significant improvement in median survival and toxicity profile in favour of irinotecan [31]. Subsequently, another phase III also showed improved survival with irinotecan [20]; however, several other trials failed to confirm positive results due to reasons unexplained [19, 24, 33, 42, 45, 51]. A recent meta-analysis of 8 clinical trials did confirm the superiority of irinotecan over etoposide [18]. A related compound topotecan has also shown to have activity in this disease in a first-line and relapse setting, but so far there are no randomised clinical trials suggesting an improved outcome with this agent [5, 8].
Currently there is no well-accepted standard second line therapy for advanced SCLC. Anthracyclines, taxanes and alkylating agents have all been trialed with very little benefit and at the cost of significant toxicity. A clinical trial evaluated the activity of irinotecan in combination with carboplatin in patients with ES-SCLC who were chemotherapy-naïve or previously treated [10]. The data showed that this combination of chemotherapy drugs was promising for the treatment of relapsed ES-SCLC. A recent meta-analysis confirmed these findings [53]. Therefore, irinotecan in combination with a platinum agent serves as a reasonable alternative to platinum-etoposide to treat patients with ES-SCLC.
The existence of cancer stem cells (CSC) is thought to explain the failure of chemotherapy and other treatments to eradicate metastatic disease. CD44, in combination with other biomarkers, is often used as a marker of CSCs in solid tumours [21, 49, 55]. The expression of CD44 in lung cancer is well described [40, 47], though the frequency of expression may vary according to the histological subtypes [29, 50]. The human CD44 gene is alternatively spliced into many different isoforms with tissue and differentiation-specific expression [34]. The standard isoform of CD44 (CD44s) is the smallest isoform and lacks all of the 9 variable exons, whereas the variant isoforms (CD44v) include variable number of exon insertions (v2-v10) and are expressed mostly by cancer cells (exon v1 is not expressed in humans) [34]. The CD44v6 isoform appears to be the most commonly reported marker of stem cell phenotype in lung cancer [25, 54]. The CD44-hyaluronic interaction plays an important role in tumorigenesis as well as invasion and migration of malignant cells [32, 34]. Recent data from preclinical studies suggest that CD44-Hyalurunon interaction could be an attractive therapeutic target in lung cancer [28, 37, 44].
Hyaluronic Acid ChemoTransport (HyACT®) is a novel anti-cancer technology (Hyaluronan Laboratory, Monash University) specifically designed to target and transport chemotherapeutic agents to the activated intra-tumoral CD44. This technology involves the unique biologic and physiochemical properties of hyaluronic acid as a macromolecular carrier of the chemotherapy agent [7]. Preclinical studies have strongly correlated the efficacy of the HyACT platform with enhanced therapeutic responsiveness in CD44 positive tumour cells [7]. Safety of this combination has been demonstrated in early phase clinical trials [16, 38]. A randomised phase II study of HA-Irinotecan (HA-IR) showed improvement if progression free survival compared to irinotecan in metastatic colorectal cancer [17]. A subsequent phase III trial however, did not reach the primary endpoint of statistically significant improvement in progression free survival. Our aim was to obtain clinical evidence to determine whether HA-IR, is capable of targeting CD44-expressing lung cancer stem cells by demonstrating that HA-IR/carboplatin (HA-IR + C) provides a clinically significant benefit (safety and efficacy) over IR + C, in ES-SCLC patients.
Methods
Study design
Study population
Patients eligible for enrolment were ≥18 years old with ES-SCLC (confirmed on histology or cytology) with measurable disease on computed tomography (CT) scans. Additional inclusion criteria were as follows: Eastern Co-operative Oncology Group (ECOG) performance status (PS) of 0 or 1; life expectancy ≥3 months; asymptomatic/controlled brain metastasis; adequate haematological, renal and hepatic function. Patients with concomitant medical illness, poor performance status (ECOG ≥2), and inflammatory bowel disease or concurrent active malignancy other than non-melanoma skin cancers were excluded from the study. Prior radiotherapy was allowed, provided it was delivered more than 4 weeks prior to study treatment. The study was approved by the human research and ethics committee at Monash Medical Centre, Melbourne, Australia and Monash University, Melbourne, Australia. Informed consent was obtained from all individual participants included in the study.
Study design and evaluation
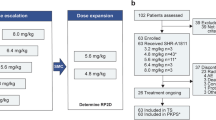
All first line patients were assigned to treatment using a central 1:1 randomisation system prior to the first dose of study treatment. Patients randomised to the experimental arm received HA-IR + C, once every 3 weeks for up to six treatment cycles. Patients randomised to the standard arm received six cycles of IR + C at the same dose and schedule. The first five patients were treated on the experimental arm (HA-IR + C), to establish the safety of the combination. Once the safety was established, the remaining patients were randomised to one of the two treatment arms. Due to slow initial recruitment, a third arm of second line patients (patients previously treated with only one line of chemotherapy for ES-SCLC) was subsequently added. All second line patients received HA-IR + C at the similar doses (Fig. 1).
Chemotherapy schedule
With a 3-weekly schedule, patients on the experimental arm received IR at the dose of 150 mg/m2 in combination with HA at the dose of 1000 mg/m2, over a 90-min intravenous infusion. This was followed by carboplatin dosed to an area under the curve (AUC) of 5 mg/mL per minute, intravenously over 60 min. Carboplatin was calculated using the formula of Calvert et al., with the creatinine clearance estimated by the Cockcroft and Gault method [9, 12]. Patients randomised to the standard arm, received similar treatment without HA.
Assessment of toxicities and response
Patients continued to receive treatment according to the protocol specifications if there was no evidence of excessive toxicity or progression of the disease, or a patient request to discontinue therapy. Toxicities (based on the National Cancer Institute Common Terminology Criteria for Adverse Events [version 3.0] grading criteria) were recorded. All toxicities were recorded before each treatment cycle. Any grade 3 or above toxicity prompted dose reduction by 25%. A maximum of 2 dose reductions was allowed for excessive toxicity. Patients requiring more than 2 dose reductions were discontinued from the study. Treatment was to be withheld on day 1 of each treatment cycle if the absolute neutrophil count was <1.2 × 10^9/L or the platelet count <85 × 10^9/L, until the counts rose above these levels. For non-haematological toxicities the treatment was to be withheld on day 1 of each treatment cycle until the severity returned to < grade 2.
All patients underwent a clinical examination, tumour evaluation with a CT scan of chest, abdomen and pelvis, a complete blood count (white blood cell count, platelet count and haemoglobin) and a serum chemistry (creatinine, sodium, potassium, bilirubin, AST and ALT). Physical examination, blood counts, chemistry and toxicity evaluation were performed before each cycle of treatment until treatment was discontinued. Tumour response was evaluated using RECIST criteria (version 1.1) every 6 weeks during treatment.
Immunohistochemistry
Tumour specimens obtained before treatment were stained for CD44s and CD44v6 expression. The primary antibodies used for CD44s were, clone DB105, Miltenyl Biotech (dilution 1:100) and for CD44v6 it was clone ab78960 (Abcam, dilution1: 200). Tissue sections were deparaffinised in xylene and rehydrated in ethanol. Antigen retrieval was performed by microwaving the slides in citrate buffer (pH 6.0) for 10 min. Endogenous peroxidase activity was blocked by 15 min incubation in 1% hydrogen peroxide. A protein block with a 10% normal serum was performed for 30 min. Incubation with primary antibody was carried out at 4 °C overnight. The secondary antibody was applied for 30 min, after washing with tris-buffered saline (TBS). Diaminobezadine solution was used for colour detection, followed by counterstaining with haematoxylin. All staining runs were accompanied by appropriate control slides.
Scoring for CD44 expression
The stained slides were scanned into a digital slide scanner (APERIO Scan ScopeXT, San Diego®) and e-slides were created as described before [1, 2]. Two pathologists (BK & DNW) independently evaluated all the scanned sections in a blinded manner. Tumour specific expression of markers was evaluated and scored according to the method described earlier [2]. A cutoff point of 5% was selected to divide the specimens into positive and negative categories.
Circulating tumour cells (CTCs)
Blood samples for circulating tumour cells were collected prior to each treatment cycle and subsequently at each follow up visit. All samples were collected and stored at room temperature and were processed within 48 h for CTC counting. The CellSearch system was used for the CTC counting, the technical details of which have been previously described by Allard et al. [3]. CTCs were defined as EpCAM-isolated intact cells showing positive staining for cytokeratin and negative staining for CD45. The results were reported quantitatively as the number of CTCs per 8 ml of blood. The selected threshold to define patients into favourable and unfavorable groups was 8/8 ml of blood. Those with <8/8 ml of CTCs were categorized into the favourable group and vice versa. This threshold was selected based on a previous publication [30].
Statistical analysis
The primary endpoints of this study were to determine the objective response rate (ORR) and to estimate the expression of CD44s and CD44v6 in tumour samples. ORR was defined as the proportion of eligible patients whose disease met the RECIST 1.1 criteria for complete response (CR) or partial response (PR) on two consecutive evaluations at least 6 weeks apart. Secondary endpoints were the estimation of disease free survival, defined as the duration (in months) between the date of surgery and the date of first recurrence or death due to any cause and overall survival, defined as the duration (in months) between the date of surgery and the date of death due to any cause and to determine all the toxicities. An exploratory analysis to correlate CTC numbers (at baseline and during treatment) with clinical outcomes was also performed.
All analyses were performed using SPSS for windows version 20 (SPSS Inc., Chicago, IL). The survival curves were plotted using the Kaplan-Meier method and log-rank test was used to assess the statistical difference between the groups. Variables with p value 0.1 or less were entered into multivariable analysis and the Cox proportional hazard model was used to carryout group comparisons. The expression of CD44s and CD44v6 were dichotomised into either ‘low’ or ‘high’ scores according to the criteria described above. The correlation between CD44 expression and clinical-pathological characteristics and OTRR were then analyzed using a Chi-Square test. Statistical significance was set at a probability value of <0.05, with two-tailed p values.
Results
Patients characteristics
From September 2011 to September 2014, 39 patients were enrolled in the study, out of which 5 were excluded while 34 were analysed for efficacy analysis (Fig. 1). Patient characteristics of all 34 patients are shown in Table 1. Twenty-one (21) were male, 13 were female. The median age was 66 (range 39–83). The ECOG performance status was normal (0) in 11 out of 34 patients (32%), while it was impaired (1) in the rest (68%) of the population. Three patients had asymptomatic brain metastasis at the time of entry into the study. Twenty-one (21) patients were treated as first line, not having received any prior chemotherapy, while 13 patients were treated as second line, having progressed following first line chemotherapy. The first five patients (first line) were treated with HA-IR + C to establish the safety. Of the remaining 16 first line patients, 8 were treated on the experimental arm (HA-IR + C), while 8 were treated on the standard arm (IR + C). All of the second line patients (n = 13) received HA-IR + C. Patient characteristics and baseline demographics were similar in the treatment arms. In total, 22 patients (65%) received all six cycles of chemotherapy, of which 13/13 (100%) were in the experimental arm, 7/8 (87%) in the standard arm while 2/13 (15%) were second line. Reasons for stopping treatment earlier than 6 cycles were, disease progression (7), toxicity (3), concomitant illness (1) patient’s request in 1 patient.
Efficacy
All 34 patients received at least one cycle of chemotherapy and were therefore evaluated for efficacy. The overall response rate was 53% (18/34), with 13 (38%) partial responses and 5 (15%) complete responses. There was no statistically significant difference in ORRs according to the treatment arm (69% and 75% in the experimental and standard arms respectively, p = 1.000). Amongst the 13 s-line patients who progressed after previous one line of chemotherapy, 2 partial responses and one complete response were seen (ORR of 22%). The difference in response rates amongst first and second line patients was statistically significant (70% vs. 22%, p = 0.013). Three patients (two in experimental arm and 1 in second line cohort) experienced disease stabilisation. Response rates according to treatment type and line of therapy are shown in Table 2.
The cutoff date of overall survival update was 12 Dec 2016. At the time of last follow up 3 out of 34 patients were alive. There was no significant difference in PFS or OS among patients treated on the experimental or the standards arms (Fig. 2). The median progression free survival was 42 weeks for the experimental arm compared to 28 weeks for standard arm (Hazard ration [HR] 0.93, 95% CI 0.36–20.42, log rank P = 0.892). The median OS was 56.1 weeks for the experimental arm vs. 55.2 weeks for the standard arm (HR 0.99 95% CI 0.44–21.43, P = 0.943) (Fig. 2).
When compared according to the treatment line, patients with refractory disease (second line) had significantly worse survival compared to those treated as first line. Median PFS was 36.4 weeks in the first line cohort vs. 14.2 weeks in the second line cohort (P = 0.001). Median OS was 55.3 weeks in first line cohort compared to 28.5 weeks in second line (P = 0.003).
Safety and toxicities
Overall, the most common adverse events (AEs) were haematological (anaemia, neutropenia and thrombocytopenia) or gastrointestinal (diarrhea and vomiting). The majority of the toxicities were grade 1 or 2 (Table 3). In general, no major differences were observed in the incidences of common AEs across the three treatment arms, although the incidences of grade III/IV diarrhea were higher in the standard arm, while anaemia in the experimental arm. One patient died during treatment in the experimental arm, due to condition unrelated to chemotherapy.
Expression of CD44s and CD44v6 in SCLC
Of the entire population, tumour samples from 24 patients (70%) were evaluable for the expression of CD44s and for CD44v6 expression. CD44s staining were observed predominantly in the cytoplasmic membrane, while CD44v6 was expressed both on the membrane as well as in the cytoplasm. Six (6/24 = 25%) tumour samples were considered positive for CD44s, while 16/24 (66%) were positive for CD44v6 expression. Of the 6 CD44s positive cases, 5 were treated with HA-IR + C while only one was treated with IR + C. In the CD44s negative cohort (n = 18), 14 were treated with HA-IR + C while 4 were treated with IR + C. Similarly, in the CD44v6 positive cohort (n = 16), 13 were treated with HA-IR + C, in CD44v6 negative cohort (n = 8), 6 patients received HA-IR + C.
CD44 expression and the efficacy of HA-IR
There was no significant difference in the overall response rates according to the expression of CD44s and CD44v6 in the entire cohort. Response rates were 66% and 58% respectively for CD44s positive and negative populations respectively (P = 0.999), while 50% and 62% for CD44v6 positive and negative populations respectively (P = 0.679). When analysed according to the treatment arms, in the HA-IR + C group, the overall response rates were 50% in CD44s positive, while 36% in the CD44s negative group (P = 0.602). Similarly, the response rates were 54% and 50% in the CD44v6 positive and negative groups respectively (P = 0.999). There were only 5 patients in the IR + C cohort, with suitable tumour samples for CD44 analysis. Of those 5, only one was positive for CD44s and 3 were positive for CD44v6. All 5 patients had a confirmed response to the treatment, regardless of the CD44 status.
Further survival analysis was performed according to CD44 expression. In the entire cohort, there was no difference in median PFS and OS in the CD44s or D44v6 positive and negative groups. However, when analysed according to the treatment arms, first line patients with CD44s positive tumours had significantly longer median PFS and a trend towards improved OS when treated with HA-IR + C, compared to CD44s negative patients treated with the same treatment (PFS 94.7 vs 28 weeks, P = 0.013, HR (Fig. 3). In the same treatment group, there was no difference in survival according to CD44v6 status. Similarly, there was no difference in PFS or OS according to CD44s or CD44v6 expression in the second line patients treated with HA-IR + C. In the standard (IR + C) first line treatment arm, though there was numerically longer PFS in the CD44s positive group, but the number of patients were too small to get any significant results (data not shown).
A scatterplot of CD44 expression versus PFS and OS in the entire first line patients is shown in Fig. 4. There appears a positive correlation between CD44s expression and both PFS and OS (R2 0.65 and 0.61, respectively), but not with CD44v6.
Scatterplot showing correlation between CD44 expression and survival in the first line patients. Blue dots represent patients on experimental while red dots represent standard therapy. There is a significant correlation between CD44s expression and PFS and OS (A&B) but not between CD44v6 and survival (C&D)
CTCs and efficacy
Blood samples from 28 patients were analysed for CTCs. At baseline, two or more CTCs were detected in 86.6% of the patients (95% confidence interval [CI], 55.0–95.7). Nine (9) patients (32%) had a baseline CTC count of 1000/8 mls of blood or above. The median CTC count at baseline was 361 (range 0–19,000). There was no difference between first line and refractory patients in terms of median CTC count (338 and 359 respectively).
Baseline CTC levels and survival
To estimate the predictive value of baseline CTC level, a threshold of 8 cells per 8 mls of blood was used. The data showed that the favourable group (patients with CTC < 8/8 ml) had a trend towards better PFS compared to the unfavourable group (P = 0.186, HR 2.1 95% CI 0.69–6.13) (Fig. 5). Survival analysis according to the treatment arms was not performed, as the numbers in the standard arm were too small to get any meaningful result.
Changes in CTC count with treatment
To estimate the effect of treatment on CTC levels, the cohort was divided into three groups, responders (CR/PR), patients with stable disease (SD) and progressive disease (PD). Since the data for CTC after 6 cycles were available in only 35% of the patients, analysis only up to cycle 4 was performed. In the responders group (N = 18), patients had a low (below median) baseline CTC count, the SD group had almost similar while PD group had much higher (above median) CTC level at baseline. Serial monitoring of CTCs also revealed that patients in the PD group had the highest (2.1 folds) rise in median CTC numbers after 4 cycles of chemotherapy compared to baseline. In PR/CR and SD groups, same rise was 1.3 and 1.6 folds respectively (Table 4).
Discussion
This randomised study evaluated the safety and activity of CD44 targeting therapy (HA-IR) in small cell lung cancer, using irinotecan as a control arm. The regimen has been designed to deliver chemotherapy in the tumour microenvironment as well as to target CD44+ subpopulation within the tumour. Our results show that HA-IR is an active and tolerable option for patients with ES-SCLC. Although the previous phase III study in colorectal cancer did not show improvement in progression free survival despite positive phase II results [17], there was no bio-marker data reported in those studies. The current study on the other hand, was not adequately powered but the preliminary data suggest that CD44 may predict survival.
Various therapeutic strategies to target CD44 in solid cancers are being tested in preclinical models [6, 13, 15]. Formulation of irinotecan with HA was previously tested in a phase II study in colorectal cancer [17]. The study by Gibbs et al. showed that HA-IR was safe and resulted in the improvement in progression free survival in patients with colorectal cancer, compared to irinotecan [17], but the role of CD44 as a bio-marker was not reported. The data on expression and hence the prognostic role of CD44 molecule and its variants in lung cancer is conflicting. A large body of evidence suggests that the CD44 variant 6 may be a more specific marker of tumour progression and poor prognosis in lung cancer [22, 25, 54], but no predictive role of CD44 or any of its variants has been described. Moreover, CD44 is expressed in a large variety of normal tissues such as immune system and epithelia [35, 52]; the risk of toxicity with unselective CD44 targeting therapies could be a concern. In the current study, CD44v6 expression was observed in over 50% of tumour samples, compared to on 35% expression in case of CD44s. Since our regimen (HA-IR) appears to show improved survival in CD44s positive tumours, with a lack of excessive toxicities to normal tissues, it can be hypothesized that CD44s is the potential therapeutic target. Certainly these findings need to be validated in a large phase III clinical trial.
We also explored the prognostic value of circulating tumour cells (CTCs). Our results were compatible with previous reports, suggesting a worse survival in patients with high baseline levels of CTCs [30]. Moreover, serial measurements of CTCs showed some correlation with clinical response to the treatment; however, the number of patients was too small to make it a meaningful conclusion. If confirmed in a larger study, serial CTC measurement could serve as a useful surrogate to assess response to treatment. Moreover, the methodology used to isolate serial CTCs in our study appears to be efficient and reproducible. Isolation and propagation of CTCs from patients with ES-SCLC also allows for downstream analysis of potentially important molecular mechanisms of chemotherapy resistance and biomarkers of response to subsequent/novel therapies such as checkpoint inhibitors. It is a non-invasive technique and, therefore, has potential to replace the invasive biopsy of tissues from humans, which may be associated with complications.
In conclusion, this proof of concept study has provided preliminary evidence that HA can act as an effective novel excipient for irinotecan. HA-IR could potentially enhance the clinical benefit derived from irinotecan. Moreover, CD44s could be a potential predictive biomarker of response to HA-chemotherapy delivery system. An adequately powered, blinded and randomised phase III study incorporating HA with irinotecan or chemotherapy drugs would be needed for further validation of these early clinical data.
References
Alamgeer M, Ganju V, Kumar B et al. (2014) Changes in aldehyde dehydrogenase-1 expression during neoadjuvant chemotherapy predict outcome in locally advanced breast cancer breast cancer research : BCR 16:R44, doi:https://doi.org/10.1186/bcr3648
Alamgeer M, Ganju V, Szczepny A et al (2013) The prognostic significance of aldehyde dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage non-small cell lung cancer. Thorax 68:1095–1104. https://doi.org/10.1136/thoraxjnl-2012-203021
Allard WJ, Matera J, Miller MC et al (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical cancer research : an official journal of the American Association for Cancer Research 10:6897–6904. https://doi.org/10.1158/1078-0432.CCR-04-0378
Ando M, Kobayashi K, Yoshimura A et al (2004) Weekly administration of irinotecan (CPT-11) plus cisplatin for refractory or relapsed small cell lung cancer. Lung Cancer 44:121–127. https://doi.org/10.1016/j.lungcan.2003.10.003
Ardizzoni A, Manegold C, Debruyne C et al (2003) European organization for research and treatment of cancer (EORTC) 08957 phase II study of topotecan in combination with cisplatin as second-line treatment of refractory and sensitive small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 9:143–150
Auzenne E, Ghosh SC, Khodadadian M et al (2007) Hyaluronic acid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia 9:479–486
Brown MB, Jones SA (2005) Hyaluronic acid: a unique topical vehicle for the localized delivery of drugs to the skin. Journal of the European Academy of Dermatology and Venereology : JEADV 19:308–318. https://doi.org/10.1111/j.1468-3083.2004.01180.x
Burris HA 3rd (1998) Topotecan: incorporating it into the treatment of solid tumors. Oncologist 3:1–3
Calvert AH, Newell DR, Gumbrell LA et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 7:1748–1756
Chen G, Huynh M, Fehrenbacher L et al (2009) Phase II trial of irinotecan and carboplatin for extensive or relapsed small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27:1401–1404. https://doi.org/10.1200/JCO.2008.20.2127
Chua YJ, Steer C, Yip D (2004) Recent advances in management of small-cell lung cancer. Cancer Treat Rev 30:521–543. https://doi.org/10.1016/j.ctrv.2004.06.003
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Dalla Pozza E, Lerda C, Costanzo C et al (2013) Targeting gemcitabine containing liposomes to CD44 expressing pancreatic adenocarcinoma cells causes an increase in the antitumoral activity. Biochim Biophys Acta 1828:1396–1404. https://doi.org/10.1016/j.bbamem.2013.01.020
Fukuoka M, Furuse K, Saijo N et al (1991) Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 83:855–861
Ganesh S, Iyer AK, Morrissey DV, Amiji MM (2013) Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials 34:3489–3502. https://doi.org/10.1016/j.biomaterials.2013.01.077
Gibbs P, Brown TJ, Ng R et al (2009) A pilot human evaluation of a formulation of irinotecan and hyaluronic acid in 5-fluorouracil-refractory metastatic colorectal cancer patients. Chemotherapy 55:49–59. https://doi.org/10.1159/000180339
Gibbs P, Clingan PR, Ganju V et al (2011) Hyaluronan-irinotecan improves progression-free survival in 5-fluorouracil refractory patients with metastatic colorectal cancer: a randomized phase II trial. Cancer Chemother Pharmacol 67:153–163. https://doi.org/10.1007/s00280-010-1303-3
Han D, Wang G, Sun L, Ren X, Shang W, Xu L, Li S (2017) Comparison of irinotecan/platinum versus etoposide/platinum chemotherapy for extensive-stage small cell lung cancer: a meta-analysis. Eur J Cancer Care (Engl) 26. https://doi.org/10.1111/ecc.12723
Hanna N, Bunn PA, Jr., Langer C et al. (2006) Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer J Clin Oncol 24:2038–2043 doi:https://doi.org/10.1200/JCO.2005.04.8595
Hermes A, Bergman B, Bremnes R et al (2008) Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase III trial. J Clin Oncol 26:4261–4267. https://doi.org/10.1200/JCO.2007.15.7545
Hiraga T, Ito S, Nakamura H (2013) Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res 73:4112–4122. https://doi.org/10.1158/0008-5472.CAN-12-3801
Hirata T, Fukuse T, Naiki H, Hitomi S, Wada H (1998) Expression of CD44 variant exon 6 in stage I non-small cell lung carcinoma as a prognostic factor. Cancer Res 58:1108–1110
Kurup A, Hanna NH (2004) Treatment of small cell lung cancer. Crit Rev Oncol Hematol 52:117–126. https://doi.org/10.1016/j.critrevonc.2004.08.005
Lara PN Jr, Natale R, Crowley J et al (2009) Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 27:2530–2535. https://doi.org/10.1200/JCO.2008.20.1061
Luo Z, RR W, Lv L, Li P, Zhang LY, Hao QL, Li W (2014) Prognostic value of CD44 expression in non-small cell lung cancer: a systematic review. Int J Clin Exp Pathol 7:3632–3646
Masuda N, Fukuoka M, Furuse K (1992a) CODE chemotherapy with or without recombinant human granulocyte colony-stimulating factor in extensive-stage small cell lung cancer. Oncology 49(Suppl 1):19–24
Masuda N, Fukuoka M, Kusunoki Y et al (1992b) CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 10:1225–1229
Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S (2011) Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J 278:1429–1443. https://doi.org/10.1111/j.1742-4658.2011.08071.x
Mizera-Nyczak E, Dyszkiewicz W, Heider KH, Zeromski J (2001) Isoform expression of CD44 adhesion molecules, Bcl-2, p53 and Ki-67 proteins in lung cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 22:45–53
Naito T, Tanaka F, Ono A et al (2012) Prognostic impact of circulating tumor cells in patients with small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 7:512–519. https://doi.org/10.1097/JTO.0b013e31823f125d
Noda K, Nishiwaki Y, Kawahara M et al (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91. https://doi.org/10.1056/NEJMoa003034
Orian-Rousseau V (2010) CD44, a therapeutic target for metastasising tumours. Eur J Cancer 46:1271–1277. https://doi.org/10.1016/j.ejca.2010.02.024
Pan D, Hou M, Li H, Yu P, Liu J (2006) Irinotecan plus cisplatin compared with etoposide plus cisplatin for small cell lung cancer: a randomized clinical trial. Zhongguo Fei Ai Za Zhi 9:443–446. https://doi.org/10.3779/j.issn.1009-3419.2006.05.11
Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4:33–45. https://doi.org/10.1038/nrm1004
Pure E, Cuff CA (2001) A crucial role for CD44 in inflammation. Trends Mol Med 7:213–221
Ramalingam S, Belani CP, Day R, Zamboni BA, Jacobs SA, Jett JR (2004) Phase II study of topotecan and paclitaxel for patients with previously untreated extensive stage small-cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 15:247–251
Reeder JA, Gotley DC, Walsh MD, Fawcett J, Antalis TM (1998) Expression of antisense CD44 variant 6 inhibits colorectal tumor metastasis and tumor growth in a wound environment. Cancer Res 58:3719–3726
Rosenthal MA, Gibbs P, Brown TJ et al (2005) Phase I and pharmacokinetic evaluation of intravenous hyaluronic acid in combination with doxorubicin or 5-fluorouracil. Chemotherapy 51:132–141. https://doi.org/10.1159/000085621
Roth BJ, Johnson DH, Einhorn LH et al (1992) Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the southeastern cancer study group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 10:282–291
Roudi R, Madjd Z, Korourian A, Mehrazma M, Molanae S, Sabet MN, Shariftabrizi A (2014) Clinical significance of putative cancer stem cell marker CD44 in different histological subtypes of lung cancer. Cancer biomarkers : section A of Disease markers 14:457–467. https://doi.org/10.3233/CBM-140424
Sandler AB (2003) Chemotherapy for small cell lung cancer. Semin Oncol 30:9–25. https://doi.org/10.1053/sonc.2003.50012
Schmittel A, Sebastian M, Fischer von Weikersthal L et al (2011) A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposide-carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol 22:1798–1804. https://doi.org/10.1093/annonc/mdq652
Schuette W (2001) Chemotherapy as treatment of primary and recurrent small cell lung cancer. Lung Cancer 33(Suppl 1):S99–107
Seiter S, Arch R, Reber S et al (1993) Prevention of tumor metastasis formation by anti-variant CD44. J Exp Med 177:443–455
Shi Y, Hu Y, Hu X, Li X, Lin L, Han X (2015) Cisplatin combined with irinotecan or etoposide for untreated extensive-stage small cell lung cancer: a multicenter randomized controlled clinical trial. Thorac Cancer 6:785–791. https://doi.org/10.1111/1759-7714.12303
Simon GR, Wagner H, American College of Chest P (2003) Small Cell Lung Cancer Chest 123:259S–271S
Tran TA, Kallakury BV, Sheehan CE, Ross JS (1997) Expression of CD44 standard form and variant isoforms in non-small cell lung carcinomas. Hum Pathol 28:809–814
van Meerbeeck JP, Fennell DA, De Ruysscher DK (2011) Small-cell lung cancer. Lancet 378:1741–1755. https://doi.org/10.1016/S0140-6736(11)60165-7
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8:755–768. https://doi.org/10.1038/nrc2499
Wimmel A, Schilli M, Kaiser U et al (1997) Preferential histiotypic expression of CD44-isoforms in human lung cancer. Lung Cancer 16:151–172
Zatloukal P, Cardenal F, Szczesna A et al (2010) A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol 21:1810–1816. https://doi.org/10.1093/annonc/mdq036
Zeilstra J, Joosten SP, Vermeulen L et al (2013) CD44 expression in intestinal epithelium and colorectal cancer is independent of p53 status. PLoS One 8:e72849. https://doi.org/10.1371/journal.pone.0072849
Zhang MQ, Lin X, Li Y, Lu S (2015) Irinotecan as a Second-line Chemotherapy for Small Cell Lung Cancer: a Systemic Analysis Asian Pacific journal of cancer prevention : APJCP 16:1993–1995
Zhao S, He JL, Qiu ZX, Chen NY, Luo Z, Chen BJ, Li WM (2014) Prognostic value of CD44 variant exon 6 expression in non-small cell lung cancer: a meta-analysis. Asian Pacific journal of cancer prevention : APJCP 15:6761–6766
Zoller M (2011) CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 11:254–267. https://doi.org/10.1038/nrc3023
Acknowledgements
The authors thank Peter Midolo and Zdenka Prodanivc for their technical support.
Funding
The study was supported by a grant from Alchemia Oncology (Project ACO-003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Alamgeer, M., Neil Watkins, D., Banakh, I. et al. A phase IIa study of HA-irinotecan, formulation of hyaluronic acid and irinotecan targeting CD44 in extensive-stage small cell lung cancer. Invest New Drugs 36, 288–298 (2018). https://doi.org/10.1007/s10637-017-0555-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0555-8