Summary
Background This multicenter, open-label, phase Ib study was designed to assess the safety, pharmacokinetics and preliminary efficacy of ME-344, a mitochondrial inhibitor, administered in combination with the topoisomerase I inhibitor, topotecan, in patients with previously treated, locally advanced or metastatic small cell lung (SCLC), ovarian and cervical cancers. Patients and methods In Part 1, patients received ME-344 10 mg/kg intravenously weekly on days 1, 8, 15 and 22 in combination with topotecan 4 mg/m2 on days 1, 8, and 15 of a 28 day cycle. Cycles were repeated until disease progression or unacceptable toxicity. Patients were evaluated for dose-limiting toxicity (DLT) in cycle 1 and ME-344 pharmacokinetic samples were obtained. In Part 2, patients with locally advanced or metastatic SCLC and ovarian cancer were enrolled in expansion cohorts treated at the recommended phase II dose (RP2D) determined in Part 1. Results Fourteen patients were enrolled in Part 1 and no DLTs were observed. The RP2D of ME-344 in combination with topotecan was established as 10 mg/kg. In Part 2, 32 patients were enrolled. The most common treatment-emergent all-grade and grade 3/4 toxicities included fatigue (65.2%, 6.5%), neutropenia (56.5%, 43.5%) and thrombocytopenia (50%, 23.9%). One patient with recurrent ovarian cancer experienced a partial response by RECIST 1.1 and 21 patients achieved stable disease as best response. Conclusions The combination of ME-344 10 mg/kg weekly and topotecan 4 mg/m2 was tolerable, however, the degree of anti-cancer activity does not support further investigation of the combination in unselected patients with SCLC, ovarian and cervical cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of resistance to chemotherapy remains a major obstacle in the development of cancer therapies [1]. Resistance to chemotherapy-induced caspase-dependent cell death or apoptosis, has been described in multiple cancers [2]. One strategy to circumvent resistance to chemotherapy-induced apoptosis is through the exploitation of caspase-independent cell death pathways. NV-128 and active metabolite ME-344 are novel isoflavone derivatives that induce caspase-independent cell death by promoting mitochondrial depolarization and mTOR inhibition [3–5]. These agents are promising combination partners with chemotherapy to enhance response.
ME-344 is a synthetic small molecule with an isoflavan ring structure, arising from metabolic demethylation of a first generation compound, NV-128 [1, 2]. ME-344 has overall similar biological and pharmacological properties to NV-128, however, exhibits more robust preclinical activity [4]. While the specific targets of NV-128 and ME-344 remain to be elucidated, exposure to these compounds in vitro results in a rapid decline in mitochondrial ATP production and the accumulation of mitochondrial superoxide (ROS) [3, 4]. These events in turn result in the activation of the MEK/ERK/Bax axis, the loss of mitochondrial membrane potential and mTOR pathway inhibition [3, 4]. The end result of NV-128 or ME-344 exposure in sensitive cancer cell line models is autophagic cell death and caspase-independent DNA fragmentation [3, 4]. NV-128 induces cell death in vitro in epithelial ovarian cancer cell lines, including chemotherapy-resistant models and ovarian cancer stem cells [3, 4]. ME-344 is cytotoxic to leukemia and lung cancer cell lines in vitro and demonstrates anti-tumor activity in leukemia xenograft models [6, 7].
A first-in-human phase I study of ME-344 was conducted in patients with advanced solid tumors and the recommended phase II dose (RP2D) was 10 mg/kg administered intravenously (IV) weekly [8]. The principal dose limiting toxicity (DLT) was grade 3 or higher peripheral neuropathy observed at an unacceptable frequency at doses >10 mg/kg [8]. Promising anti-tumor activity was observed in this study with 5 patients experiencing prolonged stable disease (31–52 weeks) and a single patient diagnosed with small cell lung cancer (SCLC) experienced a durable partial response lasting 52 weeks [8]. ME-344 is thought to be synergistic with chemotherapy, including the DNA-topoisomerase I inhibitor topotecan. Topotecan is an S-phase specific chemotherapy agent that inhibits DNA-topoisomerase I and is approved for the treatment of advanced ovarian cancer, cervical cancer and SCLC [9–11]. Traditionally, topotecan has been administered IV daily × 5 days every 21 days, however, in epithelial ovarian cancer similar efficacy has been reported with weekly IV administration days 1, 8 and 15 every 28 days [12, 13]. Additionally, weekly topotecan has a more favorable safety profile with a lower incidence of grade 3 or 4 toxicity and less myelosuppression, supporting the choice of this schedule for combination with ME-344 [14, 15].
This Phase Ib study enrolled patients with locally advanced or metastatic SCLC, ovarian cancer and cervical cancer. Part 1 of this study was designed to evaluate the safety and tolerability of ME-344 administered IV weekly in combination with weekly topotecan and to determine the RP2D of ME-344 in this combination. The pharmacokinetic (PK) profile of ME-344 in this combination was also investigated. In Part 2, enrollment was restricted to patients with locally advanced or metastatic, previously treated SCLC and ovarian cancer to assess efficacy of the combination.
Materials and methods
Patient selection
Eligible patients in Part 1 had locally advanced or metastatic SCLC, ovarian cancer or cervical cancer. Patients with SCLC or ovarian cancer must have failed initial therapy and received up to 4 prior regimens of therapy. Patients with cervical cancer had advanced disease not amenable to curative surgery and/or radiation therapy and received up to 4 prior regimens of therapy. In Part 2, enrollment was restricted to SCLC and ovarian cancer. Patients had evaluable or measurable disease by RECIST (Response Evaluation Criteria in Solid Tumors) [14], a minimum life expectancy of 12 weeks, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. Patients also had to be age 18 years or older with adequate hematopoietic, hepatic and kidney function. Exclusion criteria included: previous treatment with irinotecan, topotecan or other topoisomerase I inhibitors; women who were pregnant or nursing; treatment with radiotherapy or immunotherapy or major surgery within 3 weeks; chemotherapy regimens, biologic or targeted therapies within 2 weeks; known central nervous system metastasis except for SCLC patients with previously treated CNS lesions stable for at least 4 weeks; uncontrolled infection or systemic disease; clinically significant cardiac disease not well controlled with medication or myocardial infarction within the last 12 months; neuropathy > grade 1; known hypersensitivity to any components of ME-344 or topotecan; known human immunodeficiency virus (HIV) or hepatitis B or C; history of solid organ transplant; and psychiatric disorder or social or geographic situation that would preclude study participation.
Patients were recruited at 9 sites in the United States and the United Kingdom between April 2014 and May 2015. The institutional review boards of participating institutions approved the protocol and written informed consent was obtained for all patients prior to performing study-related procedures in accordance with federal and institutional guidelines.
Study design
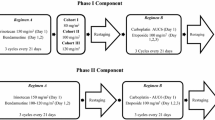
In part 1, ME-344 was administered at 10 mg/kg IV over 30 min on days 1, 8, 15, and 22 every 28 days in combination with topotecan 4 mg/m2 over 30 min on days 1, 8 and 15 every 28 days to a safety cohort of 12 patients. The starting dose of ME-344 in this combination was chosen based on the previously determined single-agent RP2D and non-overlapping toxicity with topotecan. The cohort initially enrolled 6 patients with a safety review occurring after the completion of the first 28-day cycle by all patients. A second safety review occurred after all 12 patients completed one cycle of treatment. The starting dose of ME-344 was considered not tolerated if the observed rate of DLT in at least 6 patients was >33%. Patients were considered evaluable if they received at least 2 doses of ME-344 and topotecan in the first cycle. An exception to this was patients requiring dose modification or discontinuation in cycle 1 due to treatment-related toxicity who were considered evaluable regardless of the number of doses received. In Part 2, expansion cohorts enrolled up to 20 patients each with SCLC and ovarian cancer at the RP2D of ME-344 in combination with topotecan as determined in Part 1.
Patients were evaluated for efficacy approximately every 8 weeks for the first 6 cycles and then every 12 weeks. Patients with stable disease or better by RECIST 1.1 continued to receive ME-344 and topotecan for subsequent cycles until progressive disease, unacceptable toxicity or withdrawal of consent. Patients who could no longer tolerate topotecan were allowed to continue to receive ME-344 alone. One dose reduction was permitted for each agent due to unacceptable toxicity. Premedication with anti-emetics was allowed according to standard practice guidelines. Ondansetron was to be used with caution due to the possible risk of QT prolongation.
Safety monitoring
Before the initiation of study treatment, patients underwent a physical examination, ECOG performance status and 12-lead electrocardiogram (ECG). Laboratory assessments included: complete blood count; serum chemistry including glucose, blood urea nitrogen (BUN), creatinine, sodium, potassium, chloride, calcium, carbon dioxide (CO2), alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, total bilirubin, total protein, albumin, and lactate dehydrogenase; urinalysis; and serum pregnancy test for women of child-bearing potential. Safety assessments during study treatment in patients in both Parts 1 and 2 included physical examination, ECG and laboratory assessments. Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
DLT was defined as any of the following events assessed as related to study drug: ≥ grade 3 neutropenia lasting ≥5 days or accompanied by fever, febrile neutropenia, grade 4 thrombocytopenia, or grade 3 thrombocytopenia associated with bleeding; ≥ grade 3 abnormal lab values (except neutropenia and thrombocytopenia) assessed as clinically significant; ≥ grade 3 non-laboratory toxicity (excluding alopecia, rash, nausea, diarrhea, and vomiting if controlled with standard supportive therapy); any treatment related toxicity requiring study drug interruption or dose reduction during cycle 1.
Pharmacokinetic assessments
ME-344 drug levels were measured in patients enrolled in Part 1 during cycle 1 at the following time points: on day 1 pre-infusion, just prior to the end of infusion, 0.5, 1, 2, 4, 6 and 24 h post-ME-344 dosing; on day 15 pre-infusion and just prior to the end of infusion.
Statistical analysis
Descriptive statistics were used for baseline characteristics, safety assessments and tumor response. Descriptive statistics for ME-344 PK concentrations and PK parameters were generated using Phoenix® WinNonlin®, Version 6.3. Estimation of the PK parameters was performed by noncompartmental methods. All patients who received at least one dose of ME-344 were included in the safety analysis. The efficacy population included all patients with measureable disease at baseline and who completed at least 1 cycle of treatment and underwent at least one follow-up tumor evaluation or who discontinued treatment prior to the first tumor evaluation due to disease progression. The planned total trial sample size of approximately 50 patients (Part 1 and Part 2) was designed to evaluate the safety of the combination. With a sample size of 50 and an observed grade 3/4 hematologic toxicity rate of 20% for ME-344 and topotecan combination, the upper 95% confidence interval excludes the doubling of the grade 3/4 hematologic toxicity rate of 16.7% as reported for topotecan monotherapy [12].
Results
Patients
Overall, 46 patients were enrolled in the study (14 in Part 1 and 32 in Part 2). Patient demographics and baseline characteristics are described in Table 1. The average age was 58 years old and ECOG performance status was 1 in 29 patients (63%) and 0 in 17 patients (37%). Twenty-eight patients (60.9%) had ovarian cancer receiving on average 3.1 prior lines of therapy (range 1–4); 13 patients (28.3%) had SCLC receiving on average 1.5 prior lines of therapy (range 1–3); and 5 patients had cervical cancer (10.9%) receiving on average 2.8 prior lines of therapy (range 2–4). All patients received treatment with ME-344 and all but 1 patient received treatment with topotecan (97.8%). Reasons for discontinuation of ME-344 were disease progression in 35 patients (76.1%), an adverse event in 7 (15.2%), the subject’s decision to discontinue treatment in 2 (4.3%), non-compliance with study drug in 1 (2.2%), and other reasons in 1 (2.2%). Reasons for discontinuation of topotecan were disease progression in 33 (71.7%), an adverse event in 8 (17.4%), the subject’s decision to discontinue treatment in 2 (4.3%), non-compliance with study drug in 1 (2.2%), and other reasons in 1 (2.2%).).
RP2D determination
A total of 14 patients were enrolled in Part 1. No patients experienced DLT and ME-344 administered at 10 mg/kg IV over 30 min on days 1, 8, 15, and 22 every 28 days was determined to be the RP2D in combination with topotecan 4 mg/m2 over 30 min on days 1, 8 and 15 every 28 days.
Safety
Patients receiving at least one dose of study drug were evaluable for the safety analysis (N = 46). As the administered dose and schedule of ME-344 and topotecan was the same in Part 1 and Part 2, the safety analysis included all patients. All patients experienced at least 1 adverse event (AE) and 43 patients (93.5%) experienced an AE considered related to ME-344 by the study investigators. The most frequent all-grade and grade 3/4 treatment-emergent AEs regardless of attribution were fatigue (65.2%, 6.5%), neutropenia (56.5%, 43.5%), thrombocytopenia (50%, 23.9%), nausea (47.8%, 0%), diarrhea (45.7%, 4.3%), decreased appetite (41.3%, 0%) and hypertension (41.3%, 32.6%) (Table 2). Grade 3/4 treatment-emergent AEs reported in at least 3 patients regardless of attribution also included anemia (N = 9, 19.6%), hypokalemia (N = 4, 8.7%), decreased white blood cell count (N = 3, 6.5%), febrile neutropenia (N = 3, 6.5%) and small intestinal obstruction (N = 3, 6.5%). The incidence of peripheral neuropathy was very low in this patient cohort (N = 2, 4.3%) and no grade 3/4 peripheral neuropathy events were observed. Infusion reactions related to ME-344 occurred in 4 patients (8.7%), including 1 grade 3 event resulting in treatment discontinuation. Hypertension occurred in 21 patients (45.7%) including 15 patients (32.6%) with grade 3 hypertension. The mean duration of ME-344 treatment was 3.4 cycles. The maximum duration of therapy was 14 cycles (N = 1). The relative dose intensity of ME-344 as defined as the percentage of the actual dose delivered relative to the intended dose was 85.4%. ME-344 and topotecan were discontinued due to an AE in 3 patients who experienced ME-344-related infusion reaction, bacteremia and neutropenic sepsis (one patient each).
Seventeen patients (37%) experienced a total of 23 serious adverse events (SAEs) while on study. This included 1 patient enrolled in Part 2 with grade 3 diarrhea related to ME-344 and grade 4 thrombocytopenia and febrile neutropenia related to topotecan during the first treatment cycle. An additional 5 patients experienced 6 SAEs related to topotecan. This included one patient each with: grade 4 thrombocytopenia during cycle 1, grade 3 fatigue during cycle 2, grade 3 febrile neutropenia during cycle 1 and 1 patient with both grade 3 bloodstream infection caused by an extended spectrum beta lactamase (ESBL)-producing bacteria and bacteremia during cycle 5. One patient with SCLC had a fatal event of neutropenic sepsis during cycle 4 of therapy assessed by the investigator as related to topotecan. An additional 5 patients (10.9%) in the safety population died due to progressive disease within 30 days of treatment discontinuation.
Pharmacokinetics
Samples from 13 patients enrolled in Part 1 were available for PK analysis during cycle 1 on days 1 and 15. Samples were analyzed for plasma ME-344 with a lower limit of quantification of 1.00 ng/mL and PK parameters are summarized in Table 3. Maximal ME-344 plasma concentrations were observed at the end of the 30-min infusion. The peak and extent of exposure parameter values were: Cmax of 20,880 ng/mL and AUC0-t and AUC0-inf of 21,830 and 22,040 ng*hr./mL, respectively. Mean terminal half-life was 5.3 h. Upon visual inspection of the mean AUC profile, ME-344 plasma levels declined in a multi-exponential manner on Day 1, and the end-of-infusion concentration on Day 15 was approximately 27% lower than on Day 1, suggesting no accumulation of the drug (Fig. 1). Mean total body clearance and total volume of distribution adjusted for patients’ body weight were 0.505 L/h and 1.2 L, respectively.
Efficacy
Forty-one patients were evaluable for efficacy (Table 4). The median time on study was 3.4 cycles. One patient with recurrent ovarian cancer achieved a partial response (PR) and remained on study for 14 cycles. In addition, 21 patients experienced stable disease (SD) as their best response, of which 9 patients (22%) had SD lasting for at least 12 weeks. The overall response rate was 2.4% and the clinical benefit rate (CR + PR + SD) was 53.7%. Study enrollment was stopped in Part 2 due to lack of efficacy for the combination of ME-344 and topotecan, as compared to historical controls with topotecan alone.
Discussion
The combination of ME-344 and topotecan administered once weekly was well tolerated in patients with locally advanced or metastatic SCLC, ovarian cancer and cervical cancer. The toxicity profile of the combination was consistent with that of topotecan monotherapy when administered on a weekly schedule [12, 13]. The most common treatment-emergent adverse events were fatigue, neutropenia, thrombocytopenia, nausea and diarrhea. No DLTs were observed with the combination of ME-344 and weekly topotecan at the doses evaluated in this study and the RP2D of ME-344 in combination with weekly topotecan was determined to be 10 mg/kg IV weekly. A modest degree of efficacy for the combination was observed with an overall response rate of 2.4% and a clinical benefit rate of 53.7%. While this study is somewhat limited due to sample size and a mixed patient population, the degree of efficacy was similar to historical controls of topotecan weekly as a single agent [13].
Although the toxicity profile of the combination was driven mostly by known topotecan-related adverse events, we observed a higher than expected rate of hypertension. In fact, hypertension occurred in 45.7% of patients, including 32.6% with grade 3 hypertension. The timing of onset of hypertension was during the ME-344 infusion or shortly thereafter for many patients in this study. One similar event was observed in the first-in-human Phase I trial of ME-344 where a patient experienced grade 3 hypertension and bradycardia during the infusion [8]. The mechanism of this is unclear, however, can by hypothesized to occur as a result of ME-344 treatment-induced accumulation of mitochondrial superoxide (ROS). Increased ROS production has been associated with hypertension and evidence supports increased ROS as a mechanism of angiotensin II mediated hypertension [15]. The potential for treatment-induced hypertension should be considered when designing future clinical trials of ME-344. Of note, neurotoxicity was not observed with ME-344 at a dose of 10 mg/kg IV weekly, validating the conclusions of the Phase I trial of ME-344 where peripheral neuropathy was a DLT only observed at doses of 15–20 mg/kg IV weekly [8].
The combination of ME-344 and topotecan failed to reach the threshold of promising efficacy, however, other rational combination strategies with ME-344 should be explored. ME-344 inhibits in vivo mitochondrial respiration, however, this therapeutic intervention may not result in meaningful tumor growth inhibition if tumors are primarily using aerobic glycolysis for macromolecule biosynthesis [16]. Emerging data support the hypothesis that normalization of the tumor vasculature with anti-angiogenic therapies leads to a decrease in aerobic glycolysis and an increase in mitochondrial respiration [16]. The addition of ME-344 to anti-angiogenic tyrosine kinase inhibitors results in synergistic tumor growth inhibition in xenograft models of breast and lung cancer and this strategy is currently being investigated in a Phase Ib trial of ME-344 in combination with antiangiogenic therapy (NCT02806817). Additional studies should incorporate pharmacodynamic biomarker testing to investigate mTOR and mitochondrial metabolism pathway inhibition with combination therapies to further elucidate the mechanism of ME-344 in cancer.
References
Lopez JS, Banerji U (2017) Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol. 14(1):57–66
Igney FH, Krammer PH (2002) Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2:277–288
Alvero AB, Montagna MK, Chen R et al (2009) NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway. Cancer 115:3204–3216
Alvero AB, Montagna MK, Holmberg JC et al (2011) Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol Cancer Ther 10:1385–1393
Lim SC, Carey KT, Mckenzie M (2015) Anti-cancer analogues ME-143 and ME-344 exert toxicity by directly inhibiting mitochondrial NADH: ubiquinone oxidoreductase (complex I). Am J Cancer Res 5:689–701
Jeyaraju DV, Hurren R, Wang X et al (2016) A novel isoflavone, ME-344, targets the cytoskeleton in acute myeloid leukemia. Oncotarget 7:49777–49785
Manevich Y, Reyes L, Britten CD et al (2016) Redox signaling and bioenergetics influence lung cancer cell line sensitivity to the isoflavone ME-344. J Pharmacol Exp Ther 358:199–208
Bendell JC, Patel MR, Infante JR et al (2015) Phase 1, open-label, dose escalation, safety, and pharmacokinetics study of ME-344 as a single agent in patients with refractory solid tumors. Cancer 121:1056–1063
Hirte H, Kennedy EB, Elit L et al (2015) Systemic therapy for recurrent, persistent, or metastatic cervical cancer: a clinical practice guideline. Curr Oncol 22:211–219
Riemsma R, Simons JP, Bashir Z et al (2010) Systematic review of topotecan (Hycamtin) in relapsed small cell lung cancer. BMC Cancer 10:436
Hertzberg RP, Caranfa MJ, Hecht SM (1989) On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry 28:4629–4638
Safra T, Berman T, Yachnin A et al (2013) Weekly topotecan for recurrent ovarian, fallopian tube and primary peritoneal carcinoma: tolerability and efficacy study--the Israeli experience. Int J Gynecol Cancer 23:475–480
Sehouli J, Stengel D, Harter P et al (2011) Topotecan weekly versus conventional 5-day schedule in patients with platinum-resistant ovarian cancer: a randomized multicenter phase II trial of the north-eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol 29:242–248
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Dikalova AE, Bikineyeva AT, Budzyn K et al (2010) Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107:106–116
Navarro P, Bueno MJ, Zagorac I et al (2016) Targeting tumor mitochondrial metabolism overcomes resistance to Antiangiogenics. Cell Rep 15:2705–2718
Acknowledgements
The authors would like to thank the patients and their families for their participation in this study, as well as the study staff, and the Sarah Cannon Research Institute for managing the study. Martin D. Forster is supported by the UCL/UCLH NIHR Biomedical Research Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was conducted according to ICH guidelines and all sites had IRB/EC approval prior to enrolling patients.
Funding
This study was funded by MEI Pharma, Inc.
Conflict of interest
Jennifer R. Diamond, Barbara Goff, Carolyn D. Britten, Michael S. Gordon, Hani Gabra, David M. Waterhouse, D. Ross Camidge, and Erika P. Hamilton report no potential conflict of interest. Mark Poole is an employee of MEI Pharma. Kathleen M. Moore reports advisory boards for: Astra Zeneca, Advaxis, Clovis, Immunogen, Genentech/Roche and VBL Therapeutics, and Steering Committees for: Advaxis and Astra Zeneca. Johanna C. Bendell reports research funding from Gilead, Genentech, BMS, Five Prime, Lilly, Merck, Medimmune, Celgene, EMD Serono, Taiho, Macrogenetics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, Astrazeneca, BI, Baiichi Sankyo, Bayer, Incyte, Apexigen, Roche, Koltan, SynDevRx, Forty Seven, Abbvie, StemCentrix, Array, Agios, ARMO, and CytoMx.
Informed consent
All participants gave their informed consent in writing prior to inclusion in the study.
Rights and permissions
About this article
Cite this article
Diamond, J.R., Goff, B., Forster, M.D. et al. Phase Ib study of the mitochondrial inhibitor ME-344 plus topotecan in patients with previously treated, locally advanced or metastatic small cell lung, ovarian and cervical cancers. Invest New Drugs 35, 627–633 (2017). https://doi.org/10.1007/s10637-017-0444-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0444-1