Summary
Background: AT-101 is a BCL-2 Homolog domain 3 mimetic previously demonstrated to have tumoricidal effects in advanced solid organ malignancies. Given the evidence of activity in xenograft models, treatment with AT-101 in combination with docetaxel is a therapeutic doublet of interest in metastatic head and neck squamous cell carcinoma. Patients and Methods: Patients included in this trial had unresectable, recurrent, or distantly metastatic head and neck squamous cell carcinoma (R/M HNSCC) not amenable to curative radiation or surgery. This was an open label randomized, phase II trial in which patients were administered AT-101 in addition to docetaxel. The three treatment arms were docetaxel, docetaxel plus pulse dose AT-101, and docetaxel plus metronomic dose AT-101. The primary endpoint of this trial was overall response rate. Results: Thirty-five patients were registered and 32 were evaluable for treatment response. Doublet therapy with AT-101 and docetaxel was well tolerated with only 2 patients discontinuing therapy due to treatment related toxicities. The overall response rate was 11 % (4 partial responses) with a clinical benefit rate of 74 %. Median progression free survival was 4.3 months (range: 0.7–13.7) and overall survival was 5.5 months (range: 0.4–24). No significant differences were noted between dosing strategies. Conclusion: Although met with a favorable toxicity profile, the addition of AT-101 to docetaxel in R/M HNSCC does not appear to demonstrate evidence of efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unresectable recurrent and/or distant metastatic head and neck squamous cell carcinoma (R/M HNSCC) is a terminal diagnosis in which the goal of treatment remains palliative. It carries a median survival of approximately 10 months with aggressive first line therapy [1]. As traditional cytotoxic agents have demonstrated little improvement in survival there has been interest in molecularly targeted therapy. Multiple drugs have been studied with limited success with the exception of the epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab [1, 2] and afatinib [3]. Although a signal of efficacy has been noted with the multi-receptor tyrosine kinase inhibitor (TKI) axitinib [4], other TKIs and vascular endothelial growth factor (VEGF) directed therapies have demonstrated no evidence of clinical benefit. Therefore, advances in targeted therapy in R/M HNSCC are urgently needed.
The B-cell Lymphoma 2 (BCL-2) protein is a potent intracellular anti-apoptotic regulator [5, 6], and facilitates solid tumor progression via cross-talk between tumor cells and endothelial cells [7]. BCL-2 is up-regulated by signaling via VEGF [8]. Overexpression of BCL-2 also results in increased microvascular development and tumor growth with in vivo models, and up-regulates the pro-angiogenic chemokine C-X-C motif Ligand 8 (CXCL8 or IL-8). CXCL8 has been shown to increase endothelial cell proliferation and migration [9, 10].
The VEGF-BCL2-CXCL8 pathway presents an appealing opportunity for directed therapy in head and neck cancers. A significant proportion of HNSCC tumors have elevated BCL-2 protein expression [11, 12], with a 60,000-fold higher expression on endothelial cells of HNSCC samples compared with expression in normal oral mucosa endothelial cells [13]. An elevated BCL-2 level also portends a worse clinical outcome in HNSCC [14, 15], including resistance to typical antineoplastic chemotherapy with cisplatin [16].
A number of BCL-2 Homolog domain 3 (BH3) mimetic compounds have been formulated, including the molecule AT-101, derived from the cotton plant molecule (−)-gossypol [17]. AT-101 was shown to delay tumor progression and treatment failure in xenograft models with humanized endothelial cells [18]. AT-101 acted to provide additive anti-tumor and anti-tumor endothelial cell toxicity with docetaxel. Mathematical modeling of the BCL2 pathway suggested that metronomic dosing could provide optimal efficacy [19]. The model-based predictions were validated in a series of preclinical studies [18]. Metronomic dosing strategies have been evaluated with a number of traditional cytotoxic chemotherapy trials [20–22], but few have been performed utilizing molecularly targeted therapies.
(−)-Gossypol and AT-101 has been explored in numerous phase I and II clinical studies. Both have been found to be well tolerated with a low incidence of serious adverse effects [23–25]. However, phase II trials involving diverse malignancies have yielded conflicting results [25, 26] [24, 27]. No clinical trial has been performed examining efficacy in squamous cell carcinomas of the head and neck. Given the known molecular abnormalities of the VEGFR-BCL2-CXCL8 pathway in HNSCC, the preclinical data regarding efficacy of AT-101, and the suggestion of additive benefit of metronomic dosing, we conducted a single institution phase II trial to characterize the efficacy of AT-101 in patients with R/M HNSCC.
Methods
Patient eligibility
This was a phase 2, three-arm, randomized, open label trial approved by the Institutional Review Board (IRBMED) of the University of Michigan Comprehensive Cancer Center (UMCCC). All patients were provided written informed consent. Patients ≥18 years old with unresectable R/M HNSCC were eligible. All patients must have received no more than two prior lines of systemic chemotherapy for HNSCC in the locally advanced or metastatic setting and relapsed or become refractory to therapy. Systemic therapies given in the adjuvant setting or with chemoradiotherapy were counted if the patient relapsed a minimum of 6 months after the last cycle of chemotherapy or completion of radiation. All patients had histologically documented HNSCC, the presence of measurable disease by computed tomography (CT) scan, an ECOG performance status of 0–1, and a life expectancy of ≥12 weeks. Patients had to have adequate hematopoietic, hepatic, and renal function defined as: absolute neutrophil count ≥1.5 × 109 cell/ml, platelets ≥100,000 cells/mm3, hemoglobin ≥9.0 g/dL, concentrations of total serum bilirubin within 1 time the upper limit of normal (ULN), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) within 2.5× institutional upper limits of normal unless there were liver metastases in which case AST and ALT within 5.0 x ULN, serum creatinine clearance ≥60 ml/min). Women of childbearing potential must have had a negative serum or urine pregnancy test within 3 days prior to treatment initiation.
Eligible patients were required to have no prior treatment with docetaxel in the metastatic setting. Patients were excluded if they were unable to take oral medications, had grade 3 or 4 cardiac disease defined by the New York Heart Association, or symptomatic or greater than grade 2 hypercalcemia. Additional exclusion criteria included prior use of gossypol or AT-101, uncontrolled intercurrent illness, history of venous thromboembolism, or untreated central nervous system (CNS) metastases. Patients with previously known and treated CNS metastases were eligible if they were neurologically stable and not requiring steroids at enrollment.
Treatment plan
Patients were initially randomized to one of three arms; Arm A (docetaxel alone), Arm B (docetaxel plus pulse dose AT-101), or Arm C (docetaxel plus metronomic AT-101) (Fig. 1). In all three arms, cycle length was 21 days with docetaxel given on day 1 with a starting dose of 75 mg/m2. Arm B (pulse dose AT-101) consisted of AT-101 being administered at a dose of 40 mg B.I.D. on days 1–3 and in Arm C (metronomic AT-101) AT-101 was delivered as 20 mg daily days 1–14. After two cycles on the allocated arm specific therapy, all patients had a planned switch to Arm C (metronomic AT-101 + docetaxel). Therapy was continued for a total of 10, 21-day cycles of treatment or until unacceptable toxicity/disease progression occurred.
Pretreatment assessment of enrolled patients included a complete history and physical examination, baseline laboratory studies (CBC with differential, comprehensive metabolic profile, electrocardiogram (ECG), serum or urine pregnancy test as indicated), and radiographic staging studies (CT Neck/Chest and others as clinically warranted). All screening assessments were completed within 28 days prior to the start of treatment. A correlative analysis of serial tumor tissue specimens was also performed. When available, tumor tissue from the last applicable surgery or biopsy was collected and stored for correlative analyses. In addition, an optional tissue biopsy was collected after the second cycle of chemotherapy to assess the effect of AT-101 on tumor vascularization.
Evaluation of response
Imaging studies for evaluation of response of target radiologic lesions were performed starting after three cycles after treatment initiation and continued at 6 week intervals. Target lesions were followed on each imaging study and analyzed primarily by following the sum of the largest diameter of all target lesions. Secondary radiologic evaluation data points included number of lesions, size of largest lesions, and location of target lesions. Radiologic response was assessed according to RECIST v1.0. During the first two cycles, if any patients were noted to progress in Arms A or B by clinical exam or radiologic imaging, the patients were switched to Arm C. Patients in Arm C were taken off trial at any point if there was radiographic evidence of tumor progression. When feasible, a repeat biopsy was obtained after the second cycle of chemotherapy for the purposes of correlative analyses.
Statistical considerations
The primary objective of this study was estimate the rate of clinical benefit (defined as complete response (CR), partial response (PR), or stable disease (SD)) associated with a regimen combining docetaxel and AT-101. Based on this endpoint, the trial was designed to detect an improvement in the rate of clinical benefit of 50 % versus 30 % with a target accrual of 48 patients. This trial design provided 85 % statistical power and 4.7 % overall type I error rate. Secondary objectives included survival, toxicity, and quality of life. Exploratory biological correlates were also performed as a secondary endpoint. Initial randomization to one of three arms was performed only to enable exploratory biologic analyses. Response endpoints from patients in all three groups were pooled for evaluation of the primary objective. On interim analysis after enrollment of 35 patients a lack of improvement in survival was noted hence the trial was stopped due to futility.
Treatment-related adverse events were graded according to the Common Terminology for Adverse Events version 4.0 (CTCAE v4). Treatment response was evaluated by Response Evaluation Criteria in Solid Tumors (RECIST version 1.0). Best overall response was defined as the best response achieved within 30 days of last treatment. 95 % confidence intervals for disease control rates (overall and by treatment arm) are presented using an exact binomial distribution for calculation of the standard error. Overall survival was defined as the time from study enrollment to death from any cause. Progression-free survival was defined as the time from study enrollment until disease progression or death. Data were censored at the last follow-up for patients who were progression-free or alive at the time of analysis. Median survival times were computed using the Kaplan-Meier method with standard error computed using Greenwood’s formula. Analysis of Variance (ANOVA) was used to evaluate changes of (log transformed) biomarkers before and after therapy across randomized treatment arms. All analyses were done using SAS 9.4 software.
Results
Patient characteristics
Thirty five patients were enrolled, 13 of whom were treated in Arm A, 11 each in Arms B and C. The patient characteristics are summarized in Table 1 and were well balanced between the three arms. The median age of enrolled patients was 57 years (range: 34.0–75.0). The majority of patients were male (n = 29, 88 %), Caucasian (n = 29, 97 %), and had isolated distant metastases (n = 14, 42 %). Human papillomavirus (HPV) status was available for 27 patients, which demonstrated a slight predominance of HPV negative versus positive tumors (16 vs. 11 patients, 47 % vs. 31 %, respectively). The oropharynx was the most common primary site of disease (n = 12, 36 %). Patients included in this trial were heavily pretreated (32 patients with >1 line of therapy in the R/M setting) and 91 % were refractory to platinum therapy (32 pts).
Toxicity
Treatment with AT-101 containing regimens was tolerated relatively well with only 6 % (2 patients) discontinuing treatment due to toxicity (Table 2). Twenty three percent (12 patients) of patients required dose modifications, most commonly for hematologic toxicities. A mean duration of therapy was 4 cycles (range: 1–8 cycles). Hematologic toxicities were the most common treatment related toxicities (Tables 3 and 4) of which 11 episodes of grade 3–4 lymphopenia and 5 episodes of grade 3–4 anemia were noted. Although no formal statistical analysis could be performed between dosing schedules (Arms A-C), no differences in treatment tolerability or toxicities were grossly apparent.
Efficacy
The response rate of the entire study cohort was 11 % and an additional 66 % achieving stable disease (SD) with a rate of clinical benefit (CR, PR, or SD) of 74 %. The median progression free survival (PFS) was 4.3 months (range: 0.7–13.7) with a median overall survival (OS) of 5.5 months (range: 0.4–24). The 6 month PFS was 24 % (95 % Confidence Interval: 9–42). No statistical difference in OS and PFS were noted between patients based on HPV status although there was a trend towards improved OS in the HPV positive population.
Due to small sample sizes, formal statistical analysis was not performed between dosing schedules. However, patients in Arm A appeared to have a superior median OS and rate of clinical benefit when compared to the other arms although a relatively large range of patient survival outcomes were noted. Three patients stopped treatment prior to response evaluation after cycle 2; two in Arm A, one in Arm B. Of the patients who came off trial in Arm A, one was due to unacceptable treatment related toxicity whereas the other was due to intercurrent illness. Treatment was stopped in the patient in Arm B due to non-adherence with no associated treatment related toxicity.
Correlative studies
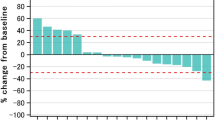
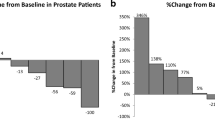
Serum correlative studies were performed on patient samples obtained at the time of enrollment and at the patient visit closest to 42 days. Samples were gathered for 35 patients total in the three treatment arms. Cytokines analyzed included CXCL1 and CXCL8. The log change in serum level from baseline was analyzed for CXCL1 and CXCL8. The differences between treatment arms were minor and not statistically significant (p = 0.30 and p = 0.15 for CXCL1 and CXCL8, respectively) (Figs. 2 and 3).
Discussion
This phase II trial is the first to evaluate the activity of the BH3 mimetic AT-101 in R/M HNSCC. This randomized, three arm, phase II trial demonstrates that although AT-101 was associated with a tolerable toxicity profile, the combination of AT-101 with docetaxel did not provide an incremental clinical benefit when compared to historic controls [28, 29].
Given the frequent dysregulation of BCL-2 and p53 in R/M HNSCC [30], therapy with BH3 mimetics has the potential to inhibit the hedgehog pathway, inhibit angiogenesis, augment chemotherapy induced apoptosis, and reverse chemoresistance [16, 18, 31, 32]. Previous in vitro studies have demonstrated this activity [32–34], including in HNSCC cell lines [18]. However, results regarding the clinical efficacy of AT-101 have been mixed with various trials showing both efficacy [25, 26] and futility [24, 27, 35] in varying malignancies. Although these lines of preclinical and early phase trials demonstrate evidence of activity, median overall survival in our study was not significantly different from previous trials examining activity of single agent cytotoxic compounds in platinum refractory R/M HNSCC (3.7–6.8 months) [28, 29].
Comparative toxicity assessments using historical controls are difficult since past studies with single agent docetaxel in R/M HNSCC have either incorporated different dosing schedules [29] or were used as different lines of therapy from what was administered in this trial. [36] However, when compared, [36] the addition of AT-101 to docetaxel does not seem to result in additional toxicities. The most notable adverse effects were hematologic in nature. Although 31 % of patients had grade 3–4 lymphopenia, only 2 patients discontinued therapy as a result of unacceptable toxicities, demonstrating the feasibility and tolerability of this novel doublet regimen.
Various dosing regimens were evaluated in an exploratory analysis for signal of efficacy including initial pulse (Arm B) versus metronomic dosing (Arm C). The concept of the superiority of metronomic dosing versus pulse dosing is based on the fact that as malignant cells may have varying rates of replication, slow dividing cells may be less affected by high dose episodic chemotherapy, whereas the addition of a continuous agent may lead to tumoricidal synergy. In addition, this dosing schema could limit the time for malignant cells to repair between chemotherapy doses and lead to greater cytotoxic effect [37, 38]. Mixed data have emerged regarding metronomic dosing which is complicated by its evaluation in different tumor types with various chemotherapeutic regimens [21, 39, 40]. Previous in vitro evidence from head and neck cell lines demonstrated more potent activity when AT-101 was administered in a metronomic fashion with docetaxel [18] validating mathematical models [19]. Although numbers were too small to draw formal comparisons, our data suggests that despite preclinical evidence, there is no difference in efficacy between dosing regimens. Arm A was noted to have a higher rate of clinical benefit than Arms B and C. Similarly, median OS was longer in Arm A but the wide range of survival outcome in each arm rendered it difficult to identify the true meaning of this finding. Metronomic dosing has been proposed to be a method to lower the rate of side effects compared to pulse dosing; however, in our study, no gross differences in toxicity profiles were noted between the three dosing regimens.
In conclusion, combined therapy with AT-101 and docetaxel does not provide an incremental clinical benefit in R/M HNSCC. Although analysis of dosing regimens was limited due to sample size, no apparent benefit was seen with metronomic dosing. The lack of response to AT-101 may reflect the dysregulation of multiple cellular pathways and tumor heterogeneity in heavily pretreated R/M HNSCC. Future studies regarding the use of AT-101 could evaluate the incorporation of this targeted therapy in the management of loco-regionally advanced HNSCC as primary therapy.
References
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359(11):1116–1127. doi:10.1056/NEJMoa0802656
Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A, Baselga J (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol Off J Am Soc Clin Oncol 25(16):2171–2177. doi:10.1200/JCO.2006.06.7447
Machiels JP, Haddad RI, Fayette J, Licitra LF, Tahara M, Vermorken JB, Clement PM, Gauler T, Cupissol D, Grau JJ, Guigay J, Caponigro F, de Castro G Jr, de Souza Viana L, Keilholz U, JM DC, XJ C, Ehrnrooth E, EE C, Lux H, investigators N (2015) Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. The Lancet Oncology 16(5):583–594. doi:10.1016/S1470-2045(15)70124-5
Swiecicki PL, Zhao L, Belile E, Sacco AG, Chepeha DB, Dobrosotskaya I, Spector M, Shuman A, Malloy K, Moyer J, McKean E, McLean S, Wolf GT, Eisbruch A, Prince M, Bradford C, Carey T, Worden FP (2015) A phase II study evaluating axitinib in patients with unresectable, recurrent or metastatic head and neck cancer. Investig New Drugs. doi:10.1007/s10637-015-0293-8
Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM (1984) Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science (New York, NY) 226(4678):1097–1099
Cleary ML, Smith SD, Sklar J (1986) Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell 47(1):19–28
Ashimori N, Zeitlin BD, Zhang Z, Warner K, Turkienicz IM, Spalding AC, Teknos TN, Wang S, Nor JE (2009) TW-37, a small-molecule inhibitor of bcl-2, mediates S-phase cell cycle arrest and suppresses head and neck tumor angiogenesis. Mol Cancer Ther 8(4):893–903. doi:10.1158/1535-7163.mct-08-1078
Nor JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, Polverini PJ (2001) Up-regulation of bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res 61(5):2183–2188
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science (New York, NY) 258(5089):1798–1801
Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM (1994) Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 179(5):1409–1415
Wang S, Yang D, Lippman ME (2003) Targeting bcl-2 and bcl-XL with nonpeptidic small-molecule antagonists. Semin Oncol 30(5 Suppl 16):133–142
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219
Kaneko T, Zhang Z, Mantellini MG, Karl E, Zeitlin B, Verhaegen M, Soengas MS, Lingen M, Strieter RM, Nunez G, Nor JE (2007) Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res 67(20):9685–9693. doi:10.1158/0008-5472.can-07-1497
Xie X, Clausen OP, De Angelis P, Boysen M (1999) The prognostic value of spontaneous apoptosis, Bax, bcl-2, and p53 in oral squamous cell carcinoma of the tongue. Cancer 86(6):913–920
Yu Y, Dong W, Li X, Yu E, Zhou X, Li S (2003) Significance of c-Myc and bcl-2 protein expression in nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 129(12):1322–1326. doi:10.1001/archotol.129.12.1322
Andrews GA, Xi S, Pomerantz RG, Lin CJ, Gooding WE, Wentzel AL, Wu L, Sidransky D, Grandis JR (2004) Mutation of p53 in head and neck squamous cell carcinoma correlates with bcl-2 expression and increased susceptibility to cisplatin-induced apoptosis. Head Neck 26(10):870–877. doi:10.1002/hed.20029
Adams R, Geissman TA, Edwards JD (1960) Gossypol, a pigment of cottonseed. Chem Rev 60(6):555–574. doi:10.1021/cr60208a002
Imai A, Zeitlin BD, Visioli F, Dong Z, Zhang Z, Krishnamurthy S, Light E, Worden F, Wang S, Nor JE (2012) Metronomic dosing of BH3 mimetic small molecule yields robust antiangiogenic and antitumor effects. Cancer Res 72(3):716–725. doi:10.1158/0008-5472.CAN-10-2873
Jain HV, Nor JE, Jackson TL (2009) Quantification of endothelial cell-targeted anti-bcl-2 therapy and its suppression of tumor growth and vascularization. Mol Cancer Ther 8(10):2926–2936. doi:10.1158/1535-7163.MCT-08-1223
Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, Folkman J (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60(7):1878–1886
Bello L, Carrabba G, Giussani C, Lucini V, Cerutti F, Scaglione F, Landre J, Pluderi M, Tomei G, Villani R, Carroll RS, Black PM, Bikfalvi A (2001) Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res 61(20):7501–7506
Pietras K, Hanahan D (2005) A multitargeted, metronomic, and maximum-tolerated dose "chemo-switch" regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol Off J Am Soc Clin Oncol 23(5):939–952. doi:10.1200/JCO.2005.07.093
Stein RC, Joseph AE, Matlin SA, Cunningham DC, Ford HT, Coombes RC (1992) A preliminary clinical study of gossypol in advanced human cancer. Cancer Chemother Pharmacol 30(6):480–482
Van Poznak C, Seidman AD, Reidenberg MM, Moasser MM, Sklarin N, Van Zee K, Borgen P, Gollub M, Bacotti D, Yao TJ, Bloch R, Ligueros M, Sonenberg M, Norton L, Hudis C (2001) Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat 66(3):239–248
Schelman WR, Mohammed TA, Traynor AM, Kolesar JM, Marnocha RM, Eickhoff J, Keppen M, Alberti DB, Wilding G, Takebe N, Liu G (2014) A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Investig New Drugs 32(2):295–302. doi:10.1007/s10637-013-9999-7
Flack MR, Pyle RG, Mullen NM, Lorenzo B, Wu YW, Knazek RA, Nisula BC, Reidenberg MM (1993) Oral gossypol in the treatment of metastatic adrenal cancer. J Clin Endocrinol Metab 76(4):1019–1024. doi:10.1210/jcem.76.4.8473376
Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, Galsky MD, Berry WR, Karlov P, Holmlund JT, Wood BA, Brookes M, Leopold L (2012) Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 23(7):1803–1808. doi:10.1093/annonc/mdr555
Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, Vodvarka P, Rischin D, Garin AM, Hirsch FR, Varella-Garcia M, Ghiorghiu S, Hargreaves L, Armour A, Speake G, Swaisland A, Vokes EE (2009) Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol Off J Am Soc Clin Oncol 27(11):1864–1871. doi:10.1200/JCO.2008.17.0530
Guardiola E, Peyrade F, Chaigneau L, Cupissol D, Tchiknavorian X, Bompas E, Madroszyk A, Ronchin P, Schneider M, Bleuze JP, Blay JY, Pivot X (2004) Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer 40(14):2071–2076. doi:10.1016/j.ejca.2004.05.019
Trask DK, Wolf GT, Bradford CR, Fisher SG, Devaney K, Johnson M, Singleton T, Wicha M (2002) Expression of bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope 112(4):638–644. doi:10.1097/00005537-200204000-00009
Cragg MS, Harris C, Strasser A, Scott CL (2009) Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer 9(5):321–326. doi:10.1038/nrc2615
Wang J, Peng Y, Liu Y, Yang J, Huang M, Tan W (2015) AT-101 inhibits hedgehog pathway activity and cancer growth. Cancer Chemother Pharmacol 76(3):461–469. doi:10.1007/s00280-015-2812-x
Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee JS, Chen J, Wang S, Bradford CR, Carey TE (2005) Reversal of cisplatin resistance with a BH3 mimetic, (−)-gossypol, in head and neck cancer cells: role of wild-type p53 and bcl-xL. Mol Cancer Ther 4(7):1096–1104. doi:10.1158/1535-7163.MCT-05-0081
Wolter KG, Wang SJ, Henson BS, Wang S, Griffith KA, Kumar B, Chen J, Carey TE, Bradford CR, D'Silva NJ (2006) (−)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia 8(3):163–172. doi:10.1593/neo.05691
Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, Wood BA, Leopold L (2011) Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 6(4):781–785. doi:10.1097/JTO.0b013e31820a0ea6
Catimel G, Verweij J, Mattijssen V, Hanauske A, Piccart M, Wanders J, Franklin H, Le Bail N, Clavel M, Kaye SB (1994) Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC early clinical trials group. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 5(6):533–537
Hanahan D, Bergers G, Bergsland E (2000) Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 105(8):1045–1047. doi:10.1172/JCI9872
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS (2000) Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 105(8):R15–R24. doi:10.1172/JCI8829
Correale P, Cerretani D, Remondo C, Martellucci I, Marsili S, La Placa M, Sciandivasci A, Paolelli L, Pascucci A, Rossi M, Di Bisceglie M, Giorgi G, Gotti G, Francini G (2006) A novel metronomic chemotherapy regimen of weekly platinum and daily oral etoposide in high-risk non-small cell lung cancer patients. Oncol Rep 16(1):133–140
Krzyzanowska MK, Tannock IF, Lockwood G, Knox J, Moore M, Bjarnason GA (2007) A phase II trial of continuous low-dose oral cyclophosphamide and celecoxib in patients with renal cell carcinoma. Cancer Chemother Pharmacol 60(1):135–141. doi:10.1007/s00280-006-0347-x
Acknowledgments
The authors thank the many investigators in the University of Michigan Head and Neck Specialized Program of Research Excellence for their contributions to patient recruitment, assistance in data collection and encouragement including Lisa Peterson, MPH, Sonia Duffy, PhD, Joseph Helman, DDS, Jonathan McHugh, MD, Tamara H. Miller, RN, Nancy Rogers, RN, Nancy E. Wallace, RN, Heather Walline, PhD, Brent Ward, DDS, Mary Beth DeRubeis, NP-C, M.S.N., Heidi Mason, NP-C, M.S.N., Leah Shults, RN, and Terri Jobkar, RN.Contract grant sponsor.
This work was supported by the University of Michigan Head and Neck Specialized Program of Research Excellence NIH/NCI P50CA097248 and NIH/NIDCD T32 DC005356, NIH/NIDCR R01-DE023220 (JEN), as well as University of Michigan Cancer Center Core Grant NIH/NCI P3O CA046592.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Swiecicki, P.L., Bellile, E., Sacco, A.G. et al. A phase II trial of the BCL-2 homolog domain 3 mimetic AT-101 in combination with docetaxel for recurrent, locally advanced, or metastatic head and neck cancer. Invest New Drugs 34, 481–489 (2016). https://doi.org/10.1007/s10637-016-0364-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0364-5