Summary
Purpose A two stage multi-institution Phase II study was undertaken by the Princess Margaret Hospital Consortium to evaluate the efficacy and toxicity of oral cediranib, an inhibitor of vascular endothelial growth factor receptors 1 and 2, in patients with previously untreated advanced malignant melanoma. Patients and Methods Between May 2006 and April 2008, 24 patients (median age 65 years) with advanced malignant melanoma were treated with oral cediranib. Cediranib was given on a continuous, oral once daily schedule of 45 mg, on a 28 day cycle. Results Of the 17 patients evaluable for response, there was stable disease in 8 patients, and progressive disease in 9 patients, with no objective responses seen. Only 2 patients had stable disease >/= 6 months, thus the study was terminated at the end of stage 1 accrual. The overall median survival was 9.9 months, and the median time to progression was 3.5 months. The most frequent non-hematologic adverse events were hypertension (78 %), fatigue (69 %), diarrhea (69 %) and anorexia and nausea (each 57 %). Conclusions Although 2 patients had stable disease at 6 months, the short median time to progression and lack of any objective responses indicate that single agent cediranib at this dose and schedule is not sufficiently active to warrant study continuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There were an estimated 80,670 new cases of malignant melanoma diagnosed in North America in 2015, with approximately 11,090 deaths. Melanoma has one of the most rapidly increasing rates of incidence, particularly in those less than 35 years of age. Median survival for patients with metastatic disease ranges from 4 to 15 months, depending on site of metastases. At the initiation of this study, there were few systemic treatment options, with surgical excision of resectable disease being a mainstay of management for eligible patients. Chemotherapy and cytokine therapy response rates were low, in the range of 10–20 %, with <5 % of patients obtaining a durable response. Clearly, there was a significant need to develop novel therapies for the treatment of this disease.
Cediranib (AZD2171), an orally available small molecule, is a potent inhibitor of receptor tyrosine kinases (RTKs) which influence the effects of a key angiogenic factor, vascular endothelial growth factor-A (VEGF). VEGF is implicated in tumor blood vessel formation and in disease progression in a wide range of solid tumor malignancies. VEGF also profoundly increases the permeability of the vasculature, potentially contributing to tumor progression. Cediranib is a potent inhibitor of both vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2 and VEGFR3 [1].
New blood vessel formation is a prominent feature of malignant melanoma [2]. In paraffin embedded biopsy specimens, invasive melanomas displayed strong VEGF expression, with benign nevi showing no immunoreactivity for VEGF [3]. In patients with malignant melanoma, increased serum concentration of VEGF correlates with poor overall and progression free survival [4].
Given this rationale for blocking the VEGF signaling pathway, the current study was undertaken to assess the objective tumor response rate of cediranib administered to patients with recurrent or metastatic malignant melanoma.
Patients and methods
Patients ≥18 years with histologically or cytologically confirmed unresectable recurrent or metastatic malignant melanoma (Stage IV acral lentiginous, lentigo maligna, superficial spreading or ocular malignant melanoma), were eligible for participation. The study protocol was approved by the institutional review boards at all participating sites, and patients were entered onto the trial after written informed consent was obtained. Other eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance score of ≤2, and a life expectancy of at least 12 weeks. Patients must have measurable disease according to Response Evaluation Criteria in Solid Tumors Committee (RECIST) criteria [5]. No prior chemotherapy or biological therapy for metastatic disease was permitted, though prior adjuvant immunotherapy was permissible if completed ≥3 months prior to study entry. Patients may have received prior radiation therapy or surgery, if completed ≥4 weeks prior to study entry. Patients also had to be able to tolerate oral medications.
Requirements for organ function included absolute granulocytes ≥1.5 x 109/L, leukocytes ≥3 x 109/L, platelets >100 x 109/L, serum creatinine within normal limits or creatinine clearance ≥50 mL/min, bilirubin ≤1.5 times normal limits and AST or ALT ≤ 3 times the upper limit of normal or ≤5 times the upper limit of normal if liver metastases were documented. Patients with documented brain metastases or any serious medical condition or illness that would not permit the patient to be managed according to the protocol were excluded. Other exclusion criteria included mean QTc >470 msec (with Bazett’s correction) in screening electrocardiogram; greater than +1 proteinuria on two consecutive dipsticks; HIV-positive patients on combination antiretroviral therapy due to the potential for pharmacokinetic interactions with cediranib and pregnant females, due to known abortifacient effects of the study drug.
Study design and treatment
This was an open label, phase II, multi-institutional study sponsored by the National Cancer Institute (NCI)-Cancer Therapy Evaluation Program. Study treatment consisted of cediranib 45 mg orally once daily, on a 28 day cycle. Cediranib was supplied by the National Cancer Institute (NCI), Division of Cancer Treatment and Diagnosis, Cancer Therapy Evaluation Program. Treatment was continued until disease progression, unacceptable toxicity, patient refusal, or physician’s decision to withdraw the patient. Hematology, biochemistry and urine dip for protein were evaluated on day 1 of each cycle, as well as on day 15 of cycle 1 only. Radiologic measurements, classified by RECIST, were performed every 8 weeks. Toxicity was assessed and graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3. For grade 3 or higher (non-hematologic) or grade 4 hematologic toxicity, cediranib was withheld until the toxicity was <grade 2, then resumed at 30 mg/d; a second dose reduction to 20 mg was allowed for further grade 3 or 4 toxicity. Need for a third dose reduction, persistent toxicity after 14 days off study drug or grade 4 hypertension required discontinuation of study therapy. For patients with. ≥ grade 2 renal toxicity, treatment was held until recovery ≤grade 1, then restarted without dose reduction; 24 h urine for creatinine clearance and protein was collected, and repeated weekly until grade 0–1.
Statistical methods
The primary endpoint of this study was to determine the response rate (objective tumor response, defined as a partial or complete response as per the RECIST criteria, or prolonged stable disease rate of at least 6 months). Secondary objectives include median survival time, 1-year survival rate, response or stable disease duration, time to disease progression, and toxicity of cediranib in this population.
A two stage phase II design was used as in Simon [6]. The null hypothesis assumed that the response rate was less than 15 % and the alternative hypothesis assumed that the response rate was at least 35 %. This design allowed enrollment of 19 evaluable patients in the first stage. If at least 1 patient had an objective tumor response, or 4 patients had stable disease of at least 6 months, an additional 14 evaluable patients were to be enrolled.
Results
Patients and treatment
Between May 2006 and April 2008, 24 patients with metastatic melanoma were enrolled at 8 centres in Canada and the United States. There were 7 females and 17 males, with a median age of 65 (range 28–82). Two thirds of the patients had a performance status of 0. Twenty-three of the 24 patients were evaluable for toxicity and survival (1 patient did not receive any study drug). Seventeen patients were evaluable for response (4 patients withdrew, and 2 patients had toxicities; one patient with grade 3 seizure plus grade 4 hypertension and the second patient with grade 3 hypertension). One additional patient was deemed inevaluable as the only measurable disease had been biopsied prior to starting treatment, and was no longer sufficiently measurable as per RECIST criteria. Patient characteristics are listed in Table 1.
Response
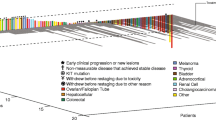
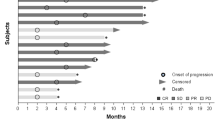
Of the 17 patients evaluable for response, only 2 patients had stable disease greater than 6 months in duration. An additional 6 patients had stable disease ranging from 3 to 5 months in duration; the overall median duration of stable disease was 4.6 months (range 3.1 to 9.1 months). The remainder of patients had progressive disease, with no complete or partial responders. The median number of cycles per patient was 2, with a range from 1 to 10.
For the 23 assessable patients, the median survival was 9.9 months (95 % CI 6.8 to 21.9 months). The median time to progression was 3.5 months (95 % CI 1.8 to not reached), and the one year overall survival was 38 % (95 % CI 21 %-68 %).
Toxicity
Cediranib was generally well tolerated, with the majority of adverse events grade 1 to 2 in intensity (Table 2). One patient had grade 4 hypertension, the only grade 4 event at least possibly related to treatment. An additional 6 patients had grade 3 hypertension, with 18 patients in total having any grade of hypertension (78 %). Thirty-five percent of patients developed proteinuria, none greater than grade 2 in intensity. No patients died on study. The most common nonhematologic toxicities were fatigue, diarrhea, anorexia and headache, occurring in greater than 50 % of patients at an intensity of grade 2–3. Lymphopenia and thrombocytopenia were the most common hematologic toxicities, occurring in 22 % of patients at a maximal intensity of grade 2.
Discussion
Despite the strong theoretical rationale for inhibiting signaling through the VEGF receptor in these hypervascular tumors, the results of this phase 2 trial of cediranib demonstrate that at this dose and schedule, there is only modest clinical benefit in metastatic melanoma. Drugs targeting the VEGF receptor, or its ligand, typically have more impact on disease stabilization, rather than objective responses. However, in the current study, while 8/17 response evaluable patients (47 %), had stable disease, only 12 % had a stable disease duration of ≥6 months. For the patients who had durable stable disease, one had metastases confined to the nodes, and one to lung only. Interestingly, 2 of the patients with stable disease of less than 6 months duration had nodal, lung and hepatic metastases, typically a group with very poor prognosis.
Mitchell et al. [7] assessed the effect of food on single dose pharmacokinetics of cediranib, noting that plasma area under the curve (AUC), and peak concentration (Cmax) were lower in the presence of food by 24 and 33 % respectively. However, in the present study, patients were instructed to take cediranib either 1 h before or 2 h after meals, making plasma concentration an unlikely contributor to the lack of antitumour effect.
The most common adverse event was hypertension, seen in 78 % of patients; this was of grade 3 or 4 intensity in 20/77 cycles. While hypertension is not an unanticipated adverse event in classes of drugs which affect VEGF and/or its receptor, this rate is much higher than that seen in the phase I study of cediranib in hormone refractory prostate cancer [8]. In that study, 31 % of patients developed hypertension (one patient grade 3), though a dose of 45 mg/d was not reached, as 20 mg/d was declared the MTD. Rates of all grade hypertension were 83 % in an ovarian cancer study [9] and 70 % in patients with mesothelioma treated with cediranib [10]. Goodwin et al. found that the development of hypertension was favorably prognostic, but not predictive of a differential treatment effect from cediranib [11].
Other investigators have targeted the VEGF signaling pathway with bevacizumab, a recombinant humanized murine anti-human VEGF monoclonal antibody. A randomized phase 2 trial reported by Varker et al. [12], looking at bevacizumab with or without daily low dose interferon alfa-2b (IFN-α2b), found prolonged disease stabilization (24–146 weeks) in this 32 patient trial. The addition of IFN-α2b did not augment the effects of bevacizumab. Perez et al. [13] conducted a phase II trial of carboplatin, paclitaxel and bevacizumab, reporting the combination to be well tolerated, with 57 % (30/53) of patients having stable disease for at least 8 weeks, and a median overall survival of 12 months. They concluded that the results warranted further study, given the efficacy of adding bevacizumab to chemotherapy in other disease sites such a colon.
Two large phase III, randomized, double blind, placebo controlled studies evaluated the efficacy of adding sorafenib, a multikinase inhibitor that targets the VEGFR, platelet derived growth factor receptors, and Raf/MEK/ERK pathways, to chemotherapy. One study evaluated the addition of sorafenib to carboplatin and paclitaxel in previously treated patients [14], while the second assessed this combination in chemo-naïve patients [15]. However, the combination of sorafenib, carboplatin and paclitaxel did not improve progression free survival (PFS), overall survival, or other clinical endpoints, in either patient population [14, 15].
In conclusion, the results of this phase II results do not warrant advancement of single agent cediranib, on this schedule, as first line therapy in advanced malignant melanoma. Similar to other agents which target the VEGF signaling pathway, there may be enhanced potential of this agent in combination with other agents [16].
References
Wedge SR, Kendrew J, Hennequin LF, et al. (2005) AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 65:4389–4400
Streit M, Detmar M (2003) Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene 22:3172–3179
Simonetti O, Lucarini G, Brancorsini D, et al. (2002) Immunohistochemical expression of vascular endothelial growth factor, matrix metalloproteinase 2, and matrix metalloproteinase 9 in cutaneous melanocytic lesions. Cancer 95:1963–1970
Ugurel S, Rappl G, Tilgen W, et al. (2001) Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol 19:577–583
Therasse P, Arbuck S, Eisenhauer EA, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors; European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Nat Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Controlled Clin Trials 10:1–10
Mitchell CL, O’Connor JP, Roberts C, et al. (2011) A two-part phase II study of cediranib in patients with advanced solid tumours: the effect of food on single-dose pharmacokinetics and an evaluation of safety, efficacy and imaging pharmacodynamics. Cancer Chemother Pharmacol 68:631–641
Ryan JR, Stadler WM, Roth B, et al. (2007) Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC). Investig New Drugs 25:445–451
Matulonis UA, Berlin S, Ivy P, et al. (2009) Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol 27:5601–5606
Garland LL, Chansky K, Wozniak AJ, et al. (2011) Phase II study of cediranib in patients with malignant pleural mesothelioma: SWOG S0509. J Thoracic Oncol 6:1938–1945
Goodwin R, Ding K, Seymour L, et al. (2010) Treatment-emergent hypertension and outcomes in patients with advanced non-small-cell lung cancer receiving chemotherapy with or without the vascular endothelial growth factor receptor inhibitor cediranib: NCIC clinical trials group study BR24. Ann Oncol 21:2220–2226
Varker KA, Biber JE, Kefauver C, et al. (2007) A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol 14:2367–2376
Perez DG, Suman VJ, Fitch TR, et al. (2009) Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central cancer treatment group study, N047A. Cancer 115:119–127
Hauschild A, Agarwala SS, Trefzer U, et al. (2009) Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 27:2823–2830
Flaherty KT, Lee SJ, Zhao F, et al. (2013) Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 31:373–379
Kendrew J, Odedra R, Logié A, et al. (2013) Anti-tumour and anti-vascular effects of cediranib (AZD2171) alone and in combination with other anti-tumour therapies. Cancer Chemother Pharmacol 71:1021–1032
Acknowledgments
This study (PHL-038/NCI 7137) is conducted by the Princess Margaret Hospital Phase II Consortium with support from US National Cancer Institute Grant #HHSN261201100032C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
McWhirter, E., Quirt, I., Gajewski, T. et al. A phase II study of cediranib, an oral VEGF inhibitor, in previously untreated patients with metastatic or recurrent malignant melanoma. Invest New Drugs 34, 231–235 (2016). https://doi.org/10.1007/s10637-016-0324-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0324-0