Summary
Purpose Angiogenesis plays a pivotal role in tumor growth and metastasis. Sorafenib, a tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR), combined with bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF-A), would vertically inhibit VEGF/VEGFR signaling. A phase I trial was performed to assess safety, maximum tolerated dose (MTD), and clinical correlates. Experimental design Patients with advanced solid tumors refractory to standard therapy were eligible. In cohorts of escalating doses, patients received sorafenib daily for 28 days and bevacizumab every two weeks. Clinical correlates included VEGF polymorphisms. Expansion cohorts of responding tumor types were enrolled. Results One hundred fifteen patients were treated, and the MTD was identified as 200 mg twice daily sorafenib and 5 mg/kg bevacizumab every two weeks. Median number of prior therapies was four. Twenty-nine patients (25 %) achieved stable disease ≥6 months; six patients (5 %) achieved a partial response (total SD ≥ 6 months/PR=35 (30 %)). 76 patients (66 %) experienced adverse events of grade 2 or higher, most commonly hand and foot syndrome (n = 27, 24 %) and hypertension (n = 24, 21 %). Dose-limiting toxicity occurred in eight patients (7 %), and 45 patients (39 %) required dose reduction for toxicity. Grade 3 and 4 hypertension was associated with longer time to treatment failure, overall survival, and higher response rate. Conclusions Combination sorafenib and bevacizumab was well-tolerated and demonstrated antitumor activity in heavily pretreated patients with advanced solid tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis plays a pivotal role in tumor growth and metastasis [1–3]. Because inhibiting neovascularization impedes the delivery of oxygen and necessary nutrients to the tumor, anti-angiogenesis has been pursued as an anti-cancer treatment strategy [2–4]. The vascular endothelial growth factor (VEGF) family of proteins is responsible for stimulating new vessel formation by binding to receptors on nearby blood vessels, i.e. vascular endothelial growth factor receptors (VEGFR), which activate the intracellular tyrosine kinase domain resulting in initiation of the cascade that ultimately stimulates new vessel production.
Vertical inhibition of both VEGF and VEGFR may be necessary to optimize pathway inhibition. An analogous study of epidermal growth factor receptor (EGFR) demonstrated both kinase-dependent and kinase-independent activity, suggesting the need for both kinase inhibition and inhibition of the receptor ligand [5].
Bevacizumab, a monoclonal antibody to VEGF-A (which serves as a ligand for VEGFR1 and VEGFR2), was the first US Food and Drug Administration (FDA) approved drug developed to target tumor angiogenesis [6]. While bevacizumab was first approved for metastatic colorectal cancer in February 2004 [6], further clinical trials have demonstrated activity in other malignancies, including lung, ovarian, renal cell, and glioblastoma [3, 7–14]. Sorafenib, a tyrosine kinase inhibitor of VEGFR1, VEGFR2, VEGFR3, Flt-3, Kit, Raf-1, and PDGFR-beta, is approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma, and has demonstrated activity in other cancer types [15–18].
We performed a phase I dose escalation and expansion trial administering sequential bevacizumab and sorafenib based on our hypothesis that this combination would dually inhibit VEGF/VEGFR signaling and have therapeutic effect. The primary objective of this study was to assess safety and tolerability as well as to define the maximum tolerated dose (MTD) of bevacizumab in combination with sorafenib. Secondary objectives were to establish a preliminary assessment of anti-tumor activity and correlation of surrogate anti-angiogenesis markers.
Materials and methods
Study design
The study was conducted at The University of Texas M. D. Anderson Cancer Center (MDACC) and patients were consented per Institutional Review Board guidelines. A modified 3 + 3 study design was used (Supplementary Methods). The treatment arm reported herein included all patients who started therapy between 10/22/2007 and 5/29/2012 as part of a dose escalation and expansion study conducted in patients with advanced cancer. The treatment cycle repeated once every 28 days until prohibitive toxicity, tumor progression, or patient withdrawal. Patients were treated at variable dose levels, depending on the time of study entry (Table 1). Once the maximum tolerated dose (MTD) was determined, the dose level was expanded to include up to 14 additional patients per specific tumor type that had complete response (CR), partial response (PR), or stable disease (SD) ≥4 cycles. These tumor types included adrenocortical, bladder, cholangiocarcinoma/gallbladder, colorectal, fallopian, hepatocellular, melanoma, ovarian, renal cell, and thyroid (papillary, follicular, medullary, hurthle cell) cancers.
Patients
Patients had metastatic or advanced cancer not amenable to standard therapy, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 [19], and adequate hematologic, hepatic, and renal function. Exclusion criteria included hemoptysis, unexplained bleeding, significant cardiovascular disease, intercurrent uncontrolled illness, significant gastrointestinal bleeding within 28 days, hemorrhagic brain metastases, prior abdominal surgery within 30 days, pregnancy, and a history of hypersensitivity to bevacizumab and/or sorafenib. Treatment with prior cytotoxic therapies must have ended at least three weeks prior to enrollment, and biologic therapy must have ended at least two weeks or five drug half-lives prior to enrollment (whichever was shorter).
Safety
Clinically significant adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 3.0 [20]. History, physical exam, hematology, blood chemistry, and urinalysis were performed at baseline and regular intervals while receiving treatment.
Evaluation of efficacy
Treatment efficacy was evaluated by diagnostic imaging per Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 [21]. Radiologic assessments were conducted at baseline and about every 8 weeks thereafter.
Molecular testing
Molecular testing was performed in the Clinical Laboratory Improvement Amendments (CLIA)-approved MDACC laboratory for patients with available archived tissue. BRAF, c-KIT, K/NRAS, c-MET, PIK3CA, and p53 mutation analysis were performed, as well as c-MET amplification by fluorescent in-situ hybridization and PTEN expression by standard immunohistochemistry.
For BRAF (codons 468–474 in exon 11 and codons 595–600 in exon 15), c-KIT (exons 9, 11, 13, and 17), K/NRAS (codons 12, 13 and 61), PIK3CA (codons 532–554 in exon 9 and codons 1011–1062 in exon 20), and p53 mutation (exons 4–9) testing, polymerase chain reaction (PCR)-based sequencing analysis was performed on DNA extracted from paraffin-embedded tumor tissue. The lower limit of detection was approximately one cell bearing the mutation per five to ten normal cells. PTEN expression was determined by immunohistochemistry using anti-PTEN monoclonal mouse antibody (Dako, Carpinteria, CA).
Single nucleotide polymorphism (SNP)
DNA was extracted from peripheral blood mononucleocytes (PBMCs) or paraffin-embedded tissue sections using the QIAamp® DNA Mini and Blood Mini Kit or QIAamp® DNA FFPE Tissue Kit (Qiagen, Valencia, CA) according to standard protocols recommended by the manufacturer. The region of interest was then amplified using custom PCR primers. Sanger sequencing was performed on a 3730xl DNA Analyzer (Applied Biosystems) using BigDye™ Terminator v3 chemistry (Applied Biosystems). Mutation analysis was performed using SeqScape® Software v2.5 (Applied Biosystems).
Statistical analysis
Analyses were descriptive and exploratory. Dichotomous variables were evaluated using Fisher’s exact test. Analysis of VEGF SNPs was performed using SPSS 19 computer software (SPSS Chicago, IL).
Results
Demographics
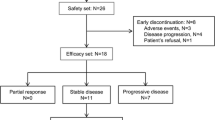
One hundred fifteen patients with advanced metastatic cancer were enrolled (Table 2), 70 of which were women (60.9 %). Most patients were pretreated, with a median of four prior systemic therapies (range 0–11). The median age was 61 years. Seventy-two patients (63 %) had archived tissue available for one or more molecular tests. Four of 49 patients (8 %) tested demonstrated BRAF mutation (V600E); two of 21 patients (10 %) demonstrated c-KIT mutation; nine of 57 patients (16 %) demonstrated K/NRAS mutation; two of 16 patients (13 %) demonstrated c-MET mutation; one of 47 patients (2 %) demonstrated PIK3CA mutation; four of 8 patients (50 %) demonstrated p53 mutation; one of 19 patients (5 %) demonstrated c-MET amplification; one of 16 patients (6 %) tested had loss of PTEN.
Adverse events
Twenty-one patients (18 %) did not experience a drug-related toxicity, and 39 patients (34 %) experienced no drug-related toxicity higher than grade 1. The most common treatment-related grade 2 or higher adverse events were hand and foot syndrome (n = 27, 24 %) and hypertension (n = 24, 21 %) (Table 1). Eight patients experienced dose-limiting toxicity (DLT) including elevated ALT/AST (n = 1, dose level 1), fatigue (n = 2, dose level 3), hand and foot syndrome (n = 4, dose levels 3, 4), hyperbilirubinemia (n = 1, dose level 1), hypertension (n = 5, dose levels 2, 3, 4), proteinuria (n = 1, dose level 3), skin rash (n = 1, dose level 3), and small bowel perforation (n = 3, dose level 3). Forty-five patients (39 %) required dose reduction for toxicity and 15 patients (13 %) withdrew due to toxicity. Grade 4 arterial ischemia resulting in below the knee amputation occurred in one patient who had clinically occult peripheral vascular disease. No deaths resulted from adverse events.
Dose reduction was required in 45 out of 115 patients (39 %) due to one or more toxicities. Toxicities requiring dose reduction included hand and foot syndrome (n = 18), hypertension (n = 11), fatigue (n = 7), rash (n = 5), mucositis (n = 5), proteinuria (n = 2), diarrhea (n = 2), drug interaction with voriconazole (n = 1), anorexia (n = 1), gastritis (n = 1), and sore throat (n = 1).
At dose level 1, one out of seven patients experienced grade 3 elevated AST and ALT and grade 3 hyperbilirubinemia. At dose level 2, one out of six patients experienced grade 3 hypertension. At dose level 3, one out of eight patients experienced grade 4 small bowel perforation. At dose level 4, one out of eight patients experienced grade 3 hand and foot syndrome and one out of eight patients experienced grade 3 hypertension. Therefore, the MTD was determined to be level 3 [22], which includes half of the recommended FDA-approved full dose of bevacizumab (5 mg/kg IV every 2 weeks) and half of the recommend FDA-approved full dose of sorafenib (200 mg BID). Once the MTD was determined, dose level 3 was further expanded by 86 patients to include up to 14 additional patients per specific tumor type that had CR, PR, or SD ≥4 cycles. These tumor types included adrenocortical, bladder, cholangiocarcinoma/gallbladder, colorectal, fallopian, hepatocellular, melanoma, ovarian, renal cell, and thyroid (papillary, follicular, medullary, hurthle cell) cancers. Among the expanded cohort of 86 patients, patients experienced DLTs including fatigue (n = 2), hand and foot syndrome (n = 2), hypertension (n = 3), proteinuria (n = 1), skin rash (n = 1), and small bowel perforation (n = 3).
Responses
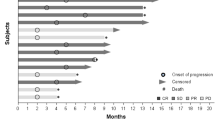
One hundred fifteen patients are included in the response data (Fig. 1). Seven patients withdrew before the first restaging assessment, five due to toxicity, one due to cost of travel, and one to pursue treatment closer to home. Any patients with clinical progression or new lesions are depicted as 21 % increase by RECIST (Fig. 1) and are considered treatment failures. Patients with non-measurable disease that achieved best response of stable disease are depicted on the figure as +0.5 %.
3D-Waterfall plot showing best response as determined by RECIST and time on study in 115 patients. The x-axis represents each patient. The y-axis indicates percent change in tumor size by RECIST. Patients who experienced at least 15 % tumor regression are color coded by tumor type. Patients with early clinical progression or new lesions and those that withdrew early due to toxicity are indicated arbitrarily as +21 % and are denoted with a star (*) and a “Ұ”, respectively. Additional information, including KIT mutations, early withdrawal due to other reasons (one for cost of travel and the other to pursue treatment closer to home), and non-measurable stable disease are also noted. The treatment duration (months) for each patient is depicted by the grey bars on the z-axis
Partial responses (PR) were observed in six patients (5 %), including patients with ovarian cancer (n = 3; 5+, 6, and 12 months), thyroid cancer (n = 1; 6 months), melanoma (n = 1; 8 months), and bladder cancer (n = 1; 9 months). The patient with a PR for 5+ months was still on study at the time of submission. 29 patients (25 %) achieved stable disease (SD) lasting at least 6 months.
Prior antiangiogenic therapy and response
Of all 115 patients on study, 40 patients (35 %) had received prior bevacizumab but no prior sorafenib, ten patients (9 %) had received prior sorafenib but no prior bevacizumab, and four additional patients (3 %) had received prior sequential sorafenib and bevacizumab. Of the 40 patients who received prior bevacizumab but no prior sorafenib, 10 (25 %) had SD ≥6 months and two (5 %) achieved PR. Of the ten patients who received prior sorafenib but no prior bevacizumab, seven (70 %) had SD ≥6 months. Of the four patients who had received both prior sorafenib and bevacizumab, two (50 %) achieved SD ≥6 months.
Of the 6 patients with PR, two (33 %) had received prior bevacizumab alone. The remaining four patients with PR (66 %) had received neither prior sorafenib or bevacizumab. Therefore, prior antiangiogenic treatment did not preclude PR.
Dosing and response
Response rate and SD ≥6 months was the same for dose levels 1 and 2 versus dose levels 3 and 4 (23 % vs. 23 % respectively, p = 0.75).
Molecular aberrations and responses
Two of 21 patients tested were positive for c-KIT mutations (S476I and L576P), and one of these patients, with L576P c-KIT-mutant melanoma, had a PR lasting 8 months (Fig. 2). The other patient, with a S476I c-KIT-mutant gastrointestinal stromal tumor, also had a R988C MET mutation and had SD lasting 6 months.
Six of total 57 patients tested demonstrated KRAS mutations. One patient, with Q61H KRAS mutant ovarian carcinoma, demonstrated a 62 % regression and received treatment for 12 months. One patient, with G12D KRAS mutant colorectal cancer, had SD for ≥6 months.
Two patients had a c-MET mutation out of 16 tested, and both had SD for ≥6 months (one with concomitant c-KIT mutation mentioned above), including N375S MET-mutant ovarian carcinoma and R988C MET-mutant gastrointestinal stromal tumor. Only one patient out of the 46 tested had a PIK3CA mutation. This patient, with Q546R PIK3CA colorectal cancer, also had a KRAS mutation (mentioned above) and had SD for 10 months. Four of the eight patients tested had p53 mutation, and two patients, with A159D p53-mutant ovarian cancer and W91 p53-mutant fallopian tube cancer, achieved SD for ≥6 months (23 % and 29 % decrease per RECIST, respectively). Interestingly, the patient with fallopian tube cancer also had c-MET amplification and the patient with ovarian cancer had a concomitant N375S c-MET mutation.
Lastly, one patient out of 16 tested had PTEN loss and achieved SD for 11 months (17 % decrease per RECIST). Nineteen patients were tested for c-MET amplification, and only one patient (with concomitant w91 p53-mutatant fallopian tube cancer mentioned above) tested positive. This patient achieved stable disease lasting 16 months.
Toxicity and response
Hand and foot syndrome was the most frequently observed toxicity in patients (Table 1). The rate of SD ≥ 6 months/PR was the same for those patients with grade 0–1 hand and foot syndrome versus those with grade 2–3 (40 % vs. 48 % respectively, p = 0.72). We also measured if development of hypertension (according to NCI CTCAE version 4) was associated with outcome. Patients (n = 27) with grade 3 or 4 hypertension compared to 9 patients with grade 0–2 hypertension had longer OS (18.1 months, 95 % CI 11.7–24.6 vs. 2.2 months, 95 % CI 0.5–4.0, p < 0.001), longer TTF (3.6 months, 95 % CI 0.9–6.3 vs. 1.3 months, 95 % CI 0–3.6, p = 0.002) and higher rate of SD ≥ 6 months/PR (37 %, 10/27 vs. 0 %, 0/9; p = 0.039).
Prognostic Scores and Outcome [23, 24]
Patients (n = 23) with MD Anderson scores 0 to 2 compared to 13 patients with scores 3 to 5 had longer median overall survival (OS) (18.1 months, 95 % CI 12.2–24.0 vs. 5.6 months, 95 % CI 1.0–10.2, p = 0.006), but not time to treatment failure (TTF) (2.5 months, 95 % CI 1.6–3.4 vs. 2.7 months, 95 % CI 0.1-5.3, p = 0.30) or rate of SD ≥ 6 months/PR/CR (22 %, 5/23 vs. 38 %, 5/13; p = 0.44). Patients (n = 22) with RMH scores 0 or 1 compared to 14 patients with scores 2 or 3 had longer median overall survival (OS) (14.9 months, 95 % CI 6.2–23.6 vs. 6.1 months, 95 % CI 0–17.8, p = 0.037), but not time to treatment failure (TTF) (2.7 months, 95 % CI 0.6–4.8 vs. 1.8 months, 95 % CI 1.6–2.0, p = 0.58) or rate of SD ≥ 6 months/PR/CR (23 %, 5/22 vs. 36 %, 5/14; p = 0.46).
VEGF selected genotypes analysis
Because of previous studies showing potential correlation with efficacy and toxicity, we analyzed whether VEGF SNPs (2578, 1154, 1498 and 634) were associated with treatment outcomes [25]. Of the 36 patients analyzed for VEGF-2578, 15 (42 %) had the AC genotype, 8 (22 %) had the AA genotype, and 13 (36 %) had the CC genotype. For VEGF-1498, 15 out of 33 patients (45 %) had the CT genotype, seven out of 33 (21 %) had the CC genotype, and 11 out of 33 (33 %) had the TT genotype. For VEGF-1154, 17 out of 33 (52 %) had the GG genotype, 12 out of 33 (36 %) had the GA genotype, and four out of 33 (12 %) had the AA genotype. For VEGF-634, 14 out of 31 (45 %) had the GG genotype, 12 out of 31 (39 %) had the GC genotype, and five out of 31 (16 %) had the CC genotype. We evaluated response rate and time to treatment failure by each VEGF polymorphism group and individual genotype. None of the tested VEGF SNPs predicted outcomes.
Multivariate analysis and outcome
Multicovariable analysis, which included MD Anderson score (0–2 vs. 3–5) and hypertension (grade 3–4 vs. 0–2), demonstrated longer OS for low MD Anderson score (HR 0.13, 95 % CI 0.05-0.36, p < 0.001) and grade 3 or 4 hypertension (HR 0.38, 95 % CI 0.17–0.85, p = 0.019). Similarly, multicovariable analysis, which included RMH score (0–1 vs. 2–3) and hypertension (grade 3–4 vs. 0–2), demonstrated longer OS for low RMH score (HR 0.38, 95 % CI 0.18–0.84, p = 0.016) and grade 3 or 4 hypertension (HR 0.10, 95 % CI 0.04–0.29, p < 0.001).
Discussion
Our study demonstrates that bevacizumab and sorafenib can be combined safely and that this regimen achieved antitumor activity in a large number of patients with varying tumor types. Toxicity with this combination, however, was greater than that of each drug used alone. The MTD was determined to be bevacizumab 5 mg/kg IV every 2 weeks and sorafenib 200 mg BID, which is about 50 % of the recommended dose of each drug.
Most patients (66 %) experienced an adverse event ≥ grade 2, the most common being hand and foot syndrome (24 %) and hypertension (21 %). These findings are similar to those reported in another study combining bevacizumab and sorafenib by Azad et al. in which hand and foot syndrome and hypertension were the most prevalent toxicities. Indeed, the same MTD was determined in both studies. A dose reduction was required in 74 % of patients in Azad’s study, whereas ours only required a dose reduction in 39 % [26], possibly because of earlier and more aggressive intervention in the current study. Careful attention should be paid to toxicity potential when using bevacizumab and sorafenib in combination in any future studies.
Special attention should be given to the potential vascular toxicity with this regimen. We observed one patient with clinically occult peripheral vascular disease who experienced grade 4 arterial ischemia that resulted in below the knee amputation six months after coming off-study. Furthermore, one case of grade 4 thrombosis and two cases of grade 3 thrombosis were noted in Azad’s study [26]. Both events are considered possibly related to the study treatment, and these findings suggest that caution should be used in the setting of cardiovascular or peripheral vascular disease. Indeed, similar unexpected serious toxicities have been observed in other trials combining VEGF and VEGFR inhibitors, including intracranial hemorrhage with combination cediranib (AZD2171) and bevacizumab [27], as well as microangiopathic hemolytic anemia with combination sunitinib and bevacizumab [28]. In contrast, other antiangiogenic combinations have not been problematic, such as trebananib (AMG386) and sorafenib or sunitinib [29].
Antitumor activity was demonstrated across a variety of tumor types using this regimen, and the responses in patients with ovarian cancer were especially notable. Of the 34 patients with ovarian or fallopian tube cancer, three (9 %) achieved a PR and seven (21 %) achieved SD ≥6 months. This antitumor activity was achieved in spite of most of these patients having received bevacizumab previously (21 of 34, 62 %) and in spite of patients receiving lower doses of bevacizumab in the combination compared to bevacizumab monotherapy (5 mg/kg vs. 15 mg/kg). In contrast, none of the patients in Azad’s study had received prior bevacizumab or sorafenib, and her trial observed a higher response rate of 43 % [26]. Considering the promising activity observed in our study, Azad’s study, and the phase 2 study of bevacizumab monotherapy in bevacizumab-naïve ovarian cancer patients, further study may be warranted with this combination in heavily pretreated ovarian cancer patients [7].
Another dramatic response seen was one patient with KIT-mutant melanoma who achieved a PR for 8 months (Fig. 2). Other studies using sorafenib monotherapy [30, 31] and sorafenib in combination with bevacizumab [26] only demonstrated SD in patients with melanoma. One sorafenib monotherapy study described a 27 % reduction at 4 weeks in a KIT-mutant melanoma patient, but further decrease was never achieved because treatment was interrupted due to toxicity [31]. In a study using sunitinib for KIT-mutant melanoma patients, one of four (25 %) had a CR for 15 months and two (50 %) had a PR for one and seven months, respectively [32]. Interestingly, this same study demonstrated only one of six (17 %) patients with KIT amplification or overexpression achieved a PR [32]. The use of sorafenib in combination with bevacizumab should be further explored in KIT-mutant melanoma patients.
Despite the antitumor activity observed, a promising biomarker for response could assist in further developing this regimen for this patient population. Of note, two out of six (33 %) patients with KRAS mutation had response, including one patient who had a 62 % regression. This observation is consistent with a previous report of sorafenib demonstrating clinical activity in patients with non-small cell lung cancer harboring KRAS mutations [33]. Sorafenib has activity against CRAF and BRAF, which are downstream of KRAS, and although the number of patients with KRAS mutation in our study was small, this observation may warrant exploration in future studies.
Maximum therapeutic effect of antiangiogenic therapy is limited by resistance [34]. In the present study, we demonstrated that combination sorafenib and bevacizumab may overcome resistance in patients previously treated with one or both of the study drugs. Of the 40 patients who received prior bevacizumab, ten (25 %) achieved SD ≥6 months and two (5 %) achieved a PR. Among the ten patients who received prior sorafenib, seven (70 %) achieved SD ≥6 months. Furthermore, among the four patients who received prior bevacizumab and sorafenib sequentially, two achieved SD ≥6 months on study. Vertical inhibition of both VEGF and VEGFR using this drug combination targets both kinase-dependent and –independent pathways, a strategy that has previously demonstrated increased activity, warranting dual inhibition [5, 35]. In addition, anti-HER2 monoclonal antibody trastuzumab has demonstrated clinical synergy when given in combination with anti-HER2 tyrosine kinase inhibitor lapatinib, although a subsequent phase 3 trial did not demonstrate survival advantage with the combination [36–38]. Future studies should consider exploring similar combination therapy strategies to overcome resistance.
A reliable biomarker for efficacy of antiangiogenic treatment remains elusive. None of the tested VEGF SNPs predicted outcomes in this study. However, grade 3 and 4 hypertension was associated with longer time to treatment failure, overall survival, and higher SD ≥ 6 months/PR. Although associations between hypertension and efficacy have been reported previously our observation in this study is notable because of the heterogeneity of the treated patient population, in terms of tumor type, as well as the heavily pretreated nature of the population.
There are several limitations to this study. First, as noted, we treated a heterogeneous population, which could have attenuated our ability to find associations. On the other hand, the fact that hypertension correlated with better response in our patients might suggest that these results are generalizable. Of course, this is a retrospective and uncontrolled study, and therefore differentiating prognostic from predictive factors is challenging. Finally, molecular analysis was available in only a small number of patients, so correlations could not be examined. For instance, we have previously reported that patients with p53 mutations have better progression-free survival after bevacizumab than patients with wild-type p53 [39]. However, in our current study only eight patients were tested for p53 mutations, and four had mutations. These numbers were too small for analysis.
In conclusion, the combination of bevacizumab and sorafenib was well-tolerated and demonstrated antitumor activity in heavily pretreated patients with advanced malignancy. Prior antiangiogenic treatment did not preclude clinical response, suggesting potential ability to overcome resistance. This regimen merits further investigation, especially in selected tumor types. Hypertension as a surrogate biomarker for efficacy may also merit further exploration.
References
Beaudry P, Force J, Naumov GN, Wang A, Baker CH, Ryan A, Soker S, Johnson BE, Folkman J, Heymach JV (2005) Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res 11(9):3514–3522. doi:10.1158/1078-0432.CCR-04-2271
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23(5):1011–1027. doi:10.1200/JCO.2005.06.081
Fidler IJ, Ellis LM (1994) The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 79(2):185–188
Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, Hung MC (2008) Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 13(5):385–393. doi:10.1016/j.ccr.2008.03.015
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400. doi:10.1038/nrd1381
Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI (2007) Phase II trial of Bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a gynecologic oncology group study. J Clin Oncol 25(33):5165–5171. doi:10.1200/JCO.2007.11.5345
Ferrara N (2005) VEGF as a therapeutic target in cancer. Oncology 69(Suppl 3):11–16. doi:10.1159/000088479
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22(11):2184–2191. doi:10.1200/JCO.2004.11.022
Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23(4):792–799. doi:10.1200/JCO.2005.05.098
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349(5):427–434. doi:10.1056/NEJMoa021491
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740. doi:10.1200/JCO.2008.19.8721
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745. doi:10.1200/JCO.2008.16.3055
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5(10):835–844. doi:10.1038/nrd2130
Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O′Dwyer PJ (2006) Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24(16):2505–2512. doi:10.1200/JCO.2005.03.6723
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134. doi:10.1056/NEJMoa060655
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. doi:10.1056/NEJMoa0708857
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 5(6):649–655
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(3):176–181. doi:10.1016/S1053-4296(03)00031-6
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of Canada. J Nat Cancer Instit 92(3):205–216
Falchook GS, Wheler JJ, Naing A, Hong DS, Moulder SL, Piha-Paul SA, Ng CS, Jackson E, Kurzrock R (2010) A phase I study of bevacizumab in combination with sunitinib, sorafenib, and erlotinib plus cetuximab, and trastuzumab plus lapatinib. Paper presented at the American Society of Clinical Oncology Annual Meeting. IL, Chicago
Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S (2008) Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the royal Marsden hospital experience. Br J Cancer 98(6):1029–1033. doi:10.1038/sj.bjc.6604218
Wheler J, Tsimberidou AM, Hong D, Naing A, Falchook G, Piha-Paul S, Fu S, Moulder S, Stephen B, Wen S, Kurzrock R (2012) Survival of 1,181 patients in a phase I clinic: the MD Anderson clinical center for targeted therapy experience. Clin Cancer Res 18(10):2922–2929. doi:10.1158/1078-0432.CCR-11-2217
Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD (2008) Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 26(28):4672–4678. doi:10.1200/JCO.2008.16.1612
Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A, Cao L, McNally D, Chow C, Chen HX, Wright JJ, Figg WD, Kohn EC (2008) Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol 26(22):3709–3714. doi:10.1200/JCO.2007.10.8332
Hong DS, Garrido-Laguna I, Ekmekcioglu S, Falchook GS, Naing A, Wheler JJ, Fu S, Moulder SL, Piha-Paul S, Tsimberidou AM, Wen Y, Culotta KS, Anderes K, Davis DW, Liu W, George GC, Camacho LH, Percy Ivy S, Kurzrock R (2014) Dual inhibition of the vascular endothelial growth factor pathway: a phase 1 trial evaluating Bevacizumab and AZD2171 (cediranib) in patients with advanced solid tumors. Cancer. doi:10.1002/cncr.28701
Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, Fischer P, Ronnen E, Ishill N, Patil S, Motzer RJ (2009) Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol 27(9):1432–1439. doi:10.1200/JCO.2008.19.0108
Hong DS, Gordon MS, Samlowski WE, Kurzrock R, Tannir N, Friedland D, Mendelson DS, Vogelzang NJ, Rasmussen E, Wu BM, Bass MB, Zhong ZD, Friberg G, Appleman LJ (2014) A phase I, open-label study of trebananib combined with sorafenib or sunitinib in patients with advanced renal cell carcinoma. Clin Genitourin Cance 12(3):167–177. doi:10.1016/j.clgc.2013.11.007, e162
Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, Gibbens I, Hackett S, James M, Schuchter LM, Nathanson KL, Xia C, Simantov R, Schwartz B, Poulin-Costello M, O′Dwyer PJ, Ratain MJ (2006) Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer 95(5):581–586. doi:10.1038/sj.bjc.6603291
Handolias D, Hamilton AL, Salemi R, Tan A, Moodie K, Kerr L, Dobrovic A, McArthur GA (2010) Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Br J Cancer 102(8):1219–1223. doi:10.1038/sj.bjc.6605635
Minor DR, Kashani-Sabet M, Garrido M, O′Day SJ, Hamid O, Bastian BC (2012) Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res 18(5):1457–1463. doi:10.1158/1078-0432.CCR-11-1987
Dingemans AM, Mellema WW, Groen HJ, van Wijk A, Burgers SA, Kunst PW, Thunnissen E, Heideman DA, Smit EF (2013) A phase II study of sorafenib in patients with platinum-pretreated, advanced (Stage IIIb or IV) non-small cell lung cancer with a KRAS mutation. Clin Cancer Res 19(3):743–751. doi:10.1158/1078-0432.CCR-12-1779
Bottsford-Miller JN, Coleman RL, Sood AK (2012) Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol 30(32):4026–4034. doi:10.1200/JCO.2012.41.9242
Janku F, Huang HJ, Angelo LS, Kurzrock R (2013) A kinase-independent biological activity for insulin growth factor-1 receptor (IGF-1R): implications for inhibition of the IGF-1R signal. Oncotarget 4(3):463–473
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O′Shaughnessy J (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28(7):1124–1130. doi:10.1200/JCO.2008.21.4437
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gomez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horvath Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379(9816):633–640. doi:10.1016/S0140-6736(11)61847-3
de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, Untch M, Jackisch C, Lang I, Smith I, Boyle F, Xu B, Barrios CH, Perez EA, Azim HA Jr, Kim SB, Kuemmel S, Huang CS, Vuylsteke P, Hsieh RK, Gorbunova V, Eniu A, Dreosti L, Tavartkiladze N, Gelber RD, Eidtmann H, Baselga J (2014) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 15(10):1137–1146. doi:10.1016/S1470-2045(14)70320-1
Said R, Hong DS, Warneke CL, Lee JJ, Wheler JJ, Janku F, Naing A, Falchook GS, Fu S, Piha-Paul S, Tsimberidou AM, Kurzrock R (2013) P53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget 4(5):705–714
Acknowledgments
We would like to thank the patients and their families for their participation in this clinical trial. We would also like to thank Goran Cabrilo for assistance with lab sample collection. This work was supported by the Paul Calabresi Career Development Award for Clinical Oncology K12 CA088084.
Ethical standards
The experiments performed in this study comply with the current laws of the country in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support
Paul Calabresi Career Development Award for Clinical Oncology K12 CA088084
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falchook, G.S., Wheler, J.J., Naing, A. et al. Dual antiangiogenic inhibition: a phase I dose escalation and expansion trial targeting VEGF-A and VEGFR in patients with advanced solid tumors. Invest New Drugs 33, 215–224 (2015). https://doi.org/10.1007/s10637-014-0176-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0176-4