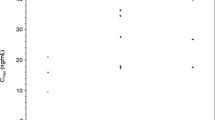Summary
Purpose Dasatinib has been shown preclinically to overcome resistance to gemcitabine. We evaluated the safety and biological activity of the combination of dasatinib and gemcitabine in patients with advanced solid tumors. Experimental Design In a phase 1 study (3 + 3 design), patients received daily dasatinib with weekly gemcitabine on days 1, 8 and 15 of a 28-day cycle (except cycle 1 which was 8 weeks). Dose escalation began with dasatinib 70 mg orally (PO) daily and gemcitabine 800 mg/m2 intravenously (IV) weekly. Results Forty-seven patients (15 men; median age = 55 years; median number of prior systemic treatments = 4) were enrolled. Dose-limiting toxicities were grade 3 fatigue and dehydration, with the maximum tolerated dose being dasatinib 100 mg PO qd and gemcitabine 600 mg/m2 IV weekly. The most common grade 3–4 toxicities were anemia (21.5 %), thrombocytopenia (26.2 %), leukopenia (26.2 %), and pleural effusion (10.7 %). Six of 47 patients attained stable disease (SD) ≥ 6 months or partial response including 2 of 8 patients with pancreatic cancer (SD ≥ 6 months; both gemcitabine-refractory), 2 of 3 patients with thymoma (SD for 9.8 and 15 months), 1 of 1 patient with anal squamous cancer (SD 15 months) and 1 of 5 patients with inflammatory breast cancer. No significant changes in circulating tumor cells or interleukin-8 levels were observed. Conclusions The combination was well tolerated at doses of dasatinib 100 mg PO daily and gemcitabine 600 mg/m2 IV weekly. SD ≥ 6 months/ PR was observed in gemcitabine-refractory pancreatic cancer, thymoma, anal cancer and inflammatory breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gemcitabine (2’,2’-difluorodeoxycytidine, LY188011, Gemzar), a nucleoside analog of deoxycytidine, alone and in combination with other chemotherapy, has been approved for treatment of pancreatic [1], non-small cell lung, breast, and ovarian [2] cancer. Gemcitabine’s primary function as a chemotherapeutic agent is inhibition of cellular DNA synthesis [3].
Increased Src expression has been associated with chemoresistance to different drugs [4, 5], including gemcitabine. Duxbury et al. [6] showed that constitutively active Src increased gemcitabine chemoresistance and that Src inhibitors, such as inhibitor PP2, increased gemcitabine-induced apoptosis and suppressed ribonucleotide reductase M2 (RRM2)-regulating the transcription factor E2F1 in gemcitabine-resistant cell lines [6].
Dasatinib competes with adenosine triphosphate (ATP) for the ATP-binding site in the kinase domain of selected protein tyrosine kinases (PTKs) and has been shown to inhibit at least five PTK families, including SRC family kinases. Dasatinib inhibited phosphorylation of tyrosine 418 of Src, the autophosphorylation site indicative of the activated form of Src, reducing Src autophosphorylation and downstream phosphorylation of critical Src substrates [7]. Trevino et al. [4] showed that dasatinib reduced tumor size and the incidence of metastasis in mice inoculated with L3.6 pl human pancreatic tumor cells. A murine study using human pancreatic cancer cell lines to promote tumor growth reported that a combined regimen of dasatinib and gemcitabine together with erlotinib significantly reduced tumor growth [8]. Based on preclinical evidence, we hypothesized that dasatinib, by inhibiting Src, can overcome chemoresistance to gemcitabine. Therefore, we conducted a phase 1 study of dasatinib and gemcitabine in patients with advanced cancer to determine the safety and biological activity of this regimen.
Patients and methods
Ethics
The study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Patients provided written, informed consent prior to participating in the clinical trial.
Patients
Eligible patients were at least 18 years of age with a histologic or cytologic diagnosis of a primary solid tumor, and evidence that the disease was metastatic or locally advanced, and measurable by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 [9]. Other inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and adequate bone marrow, hepatic and renal function, adequate serum levels of calcium, potassium and magnesium, and recovery from grade >1 toxicities related to prior chemotherapy, radiotherapy, hormone treatment or immune therapy.
Exclusion criteria included unwillingness or inability of patients of childbearing potential to use an acceptable method of birth control for study duration and at least 3 months after study completion, pregnant or breastfeeding; extensive prior radiation therapy to bone marrow; untreated or uncontrolled symptomatic brain metastasis; serious uncontrolled medical disorder or active infection; uncontrolled or significant cardiovascular disease; dementia or altered mental status that could hinder informed consent; history of a significant bleeding disorder or a documented major bleeding episode from the gastrointestinal tract within the previous 6 months; prior exposure to dasatinib; use of gastric pH modifying agents; history of allergic reactions to study drugs; or clinically significant pleural effusion.
Study design
This study was a phase 1 trial of dasatinib combined with gemcitabine in patients with advanced solid tumors. The trial began in March 2007, and a total of 47 patients were enrolled, with 32 enrolled into the dose-escalation portion and 15 enrolled into the dose-expansion portion of a standard 3 + 3 study design, with a 7-day run-in of dasatinib. The maximum tolerated dose (MTD) was defined as the highest dose level at which < 2/6 patients experienced treatment-related toxicity from the combination of dasatinib and gemcitabine. Once the MTD had been determined, an additional 15 patients were enrolled at the MTD to further assess tolerability and biological endpoints. All patients who received any study drug were considered evaluable for response and were included in the efficacy data set. All available pharmacodynamic data from patients who received dasatinib during the study were included in the pharmacodynamic data set, but only patients who had a baseline measurement and at least one post-dose assessment were included in the summary statistics and statistical analyses of pharmacodynamic parameters in blood.
Study treatment
Four weeks (28 days) was considered to be one cycle, except cycle 1 was 56 days (8 weeks). During the dose-escalation phase, there was an initial 7-day dasatinib “run-in”; patients were started on dasatinib alone on cycle 1 day 1, and dasatinib was given orally once daily for 7 days. If the patient experienced any ≥ grade 2 adverse event(s) from dasatinib, the patient was withdrawn from the study, and replaced with a new patient. Thus, only patients with < grade 2 events attributable to dasatinib continued on the combination of dasatinib and gemcitabine. Gemcitabine was started on cycle 1 day 8 and given once weekly for 7 of 8 weeks as a 30-minute infusion during cycle 1. For all other cycles, gemcitabine was given once weekly for 3 of 4 weeks as a 30-minute infusion with dasatinib given orally daily for 28 days. There was no dasatinib run-in in the expansion phase.
The dose escalation schedule was dasatinib 70 mg qd and gemcitabine 800 mg/m2 intravenously (IV) at dose level 1; dasatinib 90 mg qd and gemcitabine 800 mg/m2 at dose level 2; dasatinib 100 mg qd and gemcitabine 600 mg/m2 at dose level 3; and dasatinib 100 mg qd and gemcitabine 800 mg/m2 at dose level 4.
Patient monitoring
While on study therapy, patients were monitored continuously for adverse events and toxicity was graded using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0 [10]. Patients continued to receive therapy on the dose and schedule to which they were assigned in the absence of disease progression and if all non-hematologic toxicities (with the exceptions of alopecia, hematologic toxicity, and fatigue) considered related to dasatinib and that were CTCAE grade ≤1 had returned to baseline. In addition, platelet count and absolute neutrophil count (ANC) had to be adequate (criteria were ≥1,500 cells/mm3 for absolute neutrophil count and ≥100,000 cells/mm3 for platelet count) before the next dosing of dasatinib and gemcitabine therapy.
Patients were taken off study after grade 4 non-hematologic toxicities, grade ≥3 neuropathy, or second events of grade 3 nausea/vomiting or diarrhea. Subjects were immediately removed from the study if they withdrew informed consent, or if there was any clinical adverse event, treating physician’s decision that continuing on study was not in the best interests of the patient, or pregnancy, isolation due to illness (e.g., infectious disease), or legal reasons. Patients with a QTc ≥530 msec were also taken off study.
Outcome measures
Dose-limiting toxicity (DLT), defined as adverse events that were definitely, possibly or probably study drug-related and that occurred during the first 28 days of drug administration, included ≥ grade 3 nausea, vomiting, or diarrhea despite adequate/maximal medical intervention/ prophylaxis; delayed recovery of >14 days from toxicity related to treatment with dasatinib and gemcitabine; a QTc interval ≥530 msec seen on electrocardiogram (ECG) while on therapy; grade 4 neutropenia despite growth factor treatment; or grade 4 thrombocytopenia lasting ≥ 2 weeks.
Response and progression were evaluated using RECIST 1.0 [9]. Best overall response, duration of overall response, and duration of stable disease (SD) were calculated based on RECIST measurements.
Pharmacodynamic and biomarker assessments
Pharmacodynamic markers, enumeration of circulating tumor cells (CTC) and interleukin-8 (IL-8) levels were evaluated following collection of blood samples at baseline prior to therapy, at day 8 after dasatinib alone, and at the end of week 4 following 3 weeks of therapy with combined dasatinib and gemcitabine. The number of CTC, an indicator of tumor activity, was determined using the Cell Search® assay (Veridex, LLC, Johnson & Johnson, Raritan, NJ).
Quantitation of IL-8 levels was accomplished using the Quantikine Human IL-8 immunoassay, a commercially available enzyme linked immunosorbent assay (ELISA) (R&D Systems, Mineapolis, USA). Absorbance of samples on the plate was read at 450 nm and IL-8 concentration was standardized by total protein or cell number. The rationale for the assessment of IL-8 levels was that Src regulates IL-8 expression, and IL-8 is a pro-angiogenic molecule that also promotes metastatic potential [11]. IL-8 levels were quantified in all patients at the MTD (dose level 3 comprised of dasatinib 100 mg qd and gemcitabine 600 mg/m2).
Statistical methods
Data were analyzed using SPSS 17.0 for Windows computer software (SPSS Inc, Chicago, IL). Frequency distributions of gender and race were tabulated by dose/cohort. Summary statistics for age, body weight, height, and body mass index (BMI) were also tabulated by dose/cohort.
To account for the minimum detectable dose or the lower limit of sensitivity of the CTC and IL-8 assays (lower limits of sensitivity were 1 CTC/ 7.5 mL of blood for the CTC Veridex assay, and 0–7.5 pg/mL for the IL-8 assay), CTC and IL-8 values below the lower limits of sensitivity of these assays were assigned a fixed value at the approximate mid-point of the range of the lower limit of sensitivity of the assay from zero. Thus, mean CTC values between 0 and 1.0 were assigned a value of 0.5, and mean IL-8 values between 0 and 7.5 were assigned a value of 3.5.
Descriptive statistics including means ± standard error (SE) were computed for CTC counts and IL-8 levels. The paired samples t-test was used to determine if overall, CTC or IL-8 levels changed between baseline and day 28 in all patients with available values. For further analyses involving CTC and IL-8 levels, patients were classified into two groups based on response outcome. The first group comprised patients with a best response of SD ≥6 months or partial response (PR); and the second group included all other patients. Differences in CTC and IL-8 between the patients who had SD ≥ 6 months/ PR and the other patients at baseline, day 28, and changes in CTC and IL-8 between day 28 and baseline were examined using the Mann–Whitney U Test. The level for statistical significance was set a priori at P < 0.05.
Results
Patients
Patient demographic and baseline clinical characteristics are shown in Table 1. A total of 47 patients were enrolled in the trial, including 32 in the escalation phase of the trial and 15 in the expansion phase. The most common cancers in the study population were colorectal (9 patients, 19 %), pancreatic (8 patients, 17 %), non-inflammatory (7 patients, 15 %) and inflammatory (6 patients, 13 %) breast cancers. Most patients were women (32 patients, 68 %), non-Hispanic white (35 patients, 74 %), had received prior radiotherapy (28 patients, 60 %) and/or ≥3 prior chemotherapeutic regimens (40 patients, 85 %).
Safety
The adverse event (AE) profile for dasatinib and gemcitabine treatment is shown in Table 2. Five patients received dasatinib alone, and 42 patients received the combination of dasatinib and gemcitabine and were evaluated for safety. Across all dose levels, most patients (n = 27, 64.3 %) reported only ≤ grade 2 toxicities, that were at least possibly drug-related. The most common grade 3–4 adverse events at least possibly drug-related were anemia (9/42 patients, 21.5 %), thrombocytopenia (11/42 patients, 26.2 %), leukopenia (11/42 patients, 26.2 %), and lymphopenia (9/42 patients, 21.5 %) Pleural effusion was noted in five patients (5/47 patients, 10.7 %), of which four were grade 2 and one was grade 3.
Serious adverse events (SAEs) at least possibly drug-related were reported in 14 patients (14/47 patients, 29.8 %), and included dehydration, nausea, vomiting, and fatigue, diarrhea, anemia, and fever without neutropenia, confusion, hemolytic-uremic syndrome (HUS), infection with normal neutrophil levels, abdominal pain, pleural effusion, pericardial effusion, dsypnea, hypokalemia, thrombocytopenia, and leukopenia. Drug-related death was reported in one patient who developed thrombotic thrombocytopenic purpura (TTP)-HUS attributed to gemcitabine, and two deaths were due to disease progression.
DLT and MTD
At dose level 2, one of six evaluable patients experienced a DLT of grade 3 fatigue and dehydration. At dose level 3, one of six evaluable patients experienced a DLT of grade 3 alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevation. At dose level 4, two of six evaluable patients experienced DLT: one patient had grade 3 fatigue and the other had grade 3 fatigue and dehydration. Therefore, dose level 3, dasatinib 100 mg PO qd and gemcitabine 600 mg/m2, was determined to be the MTD. In the dose expansion phase at dose level 3, two of 15 evaluable patients experienced DLT of grade 3 AST and ALT elevation, and grade 3 dehydration, diarrhea and vomiting.
Response
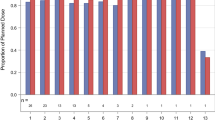
Figure 1 is a waterfall graph of each patient’s best response to treatment with gemcitabine + dasatinib by RECIST criteria. Of 47 patients, one patient achieved a PR, and 11 patients had SD. Ten patients had progressive disease by RECIST measurement criteria; eight patients came off due to new metastatic lesions or clinical progression, 11 patients withdrew consent, and six came off study due to toxicities.
Waterfall graph showing each patient’s best response to treatment by RECIST criteria. An arbitrary value of 21 % (indicated by † or *) was assigned for best response on the waterfall chart for those patients who failed early due to toxicity or other reasons (†) or for those with clinical progression or new metastatic lesions (*)
One of five patients with inflammatory breast cancer achieved a PR (duration = 2 months; number of prior systemic treatments = 4). Five patients (11 %) who received the combination (5/47) achieved SD ≥ 6 months. These included one patient with metastatic squamous cell carcinoma of the anal canal (15 months), two patients with metastatic thymoma (15 months and 9.8 months), two patients with pancreatic cancer (6.3 months and 7.5 months). Two patients exceeded 13 months on study: the patient with squamous cell carcinoma of the anal canal (dose level 3, best response 28 % decrease), and one patient with thymoma (dose level 3, best response 17 % decrease). Of the 18 patients who had received gemcitabine prior to the present combination regimen of dasatinib and gemcitabine, two (2/18, 11 %) stayed on the trial for more than 6 months; both of these patients had pancreatic cancer and stayed on study for 25 weeks (best response 3.7 % decrease) and 34 weeks (best response 2.9 % decrease), respectively. Thus, 2/8 (25 %) patients with pancreatic cancer who had received gemcitabine previously stayed on study for more than 6 months.
Pharmacodynamics
We hypothesized that a pharmacodynamic effect of dasatinib might correlate with the post-therapy reduction of CTC. IL-8 levels were assessed because we hypothesized that dasatinib, a multikinase inhibitor, could inhibit Src kinase, and thereby, alter levels of IL-8, a pro-angiogenic [11] and pro-metastatic cytokine. Mean [± standard error (SE)] CTC counts/ 7.5 mL of blood were 2.13 ± 0.48 at baseline versus 3.40 ± 1.38 at day 28 (N = 30 patients) (P = 0.38). Mean IL-8 level at baseline was 39.04 ± 15.0 pg/mL versus 55.6 ± 21.72 pg/mL at day 28 (N = 19 patients) (P = 0.076).
Figure 2 shows the mean number of CTC over time (days 0, 8, and 28) by response outcome. The baseline CTC in patients with ≥6 months/PR was 1.42 ± 0.55 CTC / 7.5 mL of blood (N = 4) vs. 2.24 ± 0.55 CTC/ 7.5 mL of blood in all other patients (N = 26) (P = 0.93). The mean change in CTC between day 28 and baseline in the patients with SD ≥6 months/PR was −0.92 ± 0.55 counts (N = 4) vs. 1.61 ± 1.65 (N = 26) (P = 0.123) in all other patients. At day 28, the difference in mean CTC counts between patients with SD ≥ 6 months/ PR and others approached significance (0.5 ± 0.0 vs. 3.85 ± 1.57, P = 0.052).
Mean number of circulating tumor cells (CTC) at baseline (prior to start of treatment with any study drug), day 8 (following a 7-day run-in of dasatinib only), and day 28 (following 3 weeks of the combination treatment of dasatinib and gemcitabine). The lower level of sensitivity of the assay was 1 CTC/ 7.5 mL of blood. a Difference in mean change in CTC from baseline to day 28 between patients with PR or SD ≥ 6 months versus other patients. b Difference in CTC at day 28 between patients with PR or SD ≥ 6 months versus other patients
Figure 3 shows IL-8 levels over time (days 0, 8, and 28) by response outcome in all patients evaluated at the MTD. The mean ± SE IL-8 levels at baseline for patients with SD ≥ 6 months/PR was 6.27 ± 2.77 pg/ mL (N = 2) versus 42.9 ± 16.56 pg/mL for the others (N = 17) (P = 0.234). The mean change in IL-8 levels between day 28 and baseline was 6.88 ± 2.57 pg/mL for patients with SD ≥ 6 months/PR (N = 2) vs. 14.96 ± 8.37 pg/mL in the others (N = 15) (P = 1.00). At day 28, IL-8 levels were 13.15 ± 5.34 in patients with SD ≥ 6 months vs. 61.26 ± 24.31 in the others (P = 0.368).
Mean levels of interleukin-8 on days 0, 8, and 28 of cycle 1 in the dasatinib plus gemcitabine study of the patients in the dose expansion phase. The lower level of sensitivity of the assay was 0–7.5 pg/ mL. a Difference in mean change in IL-8 from baseline to day 28 between patients with PR or SD ≥ 6 months versus other patients. b Difference in IL-8 at day 28 between patients with PR or SD ≥ 6 months versus other patients
Discussion
This phase 1 study of gemcitabine combined with dasatinib showed that the combination was tolerable, with the main associated toxicities being hematologic. This may be related to gemcitabine, or possibly a combined treatment effect of dasatinib plus gemcitabine, on the bone marrow. Observed DLTs were similar to those in other solid tumor studies with dasatinib or gemcitabine alone, i.e., grade 3 fatigue and grade 3 elevated liver function tests [12–15]. The rate of pleural effusion (14 %) was similar to other studies with dasatinib in solid tumors [13, 14, 16, 17]. One significant SAE was the development of hemolytic uremic syndrome in a patient with inflammatory breast cancer who had initiallyachieved a PR. Hemolytic uremic syndrome is a rare side effect that has been reported with gemcitabine and other chemotherapies [18, 19]. The rate of hemolytic uremic syndrome with gemcitabine alone has been reported to be 0.4 %, but has not been reported with dasatinib [19].
CTC levels have previously been shown to predict progression-free (PFS) and overall survival (OS) in patients with metastatic breast cancer [20]. CTC might be tumor cells that have become resistant to anoikis [21], a natural physiological process of detachment-induced apoptosis in normal cells [22]. Indeed, it has been shown that activation of Src and closely related kinases (for example, FAK) confers resistance to anoikis in tumor cells that metastasize [22, 23]. Thus, we hypothesized that the pharmacodynamic effect of dasatinib might correlate with the post-therapy reduction of CTC. However, in our study, despite the high patient tumor burden, the median and mean number of CTC even prior to study enrollment was low (median = 0.5/7.5 ml, mean = 2.13 ± 0.48/7.5 ml). There was no change in CTC number after single agent dasatinib or in combination with gemcitabine, which could be an artifact of the low number of CTC at baseline. The reason for the low number of CTC in this population with highly metastatic and heavily pretreated disease is unclear. However, Mego et al. [24] described the absence of detectable CTC in approximately one-third of patients with metastatic breast cancer [24]. Of possible interest, the difference in day 28 CTC between patients with SD > 6 months or PR vs. other patients approached significance. Therefore, CTC may merit further study in trials of this combination.
The assessment of IL-8 in this study was based on the knowledge that Src regulates IL-8, a pro-angiogenic [11] and pro-metastatic molecule. Trevino et al. [11] showed that activation of Src results in increased IL-8 production, and that small interfering RNA mediated inhibition of c-Src expression significantly reduced IL-8 synthesis. Mean IL-8 levels did not change with time in our study. Indeed, there was a trend towards an increase in levels. This may be because IL-8 is produced by both tumor cells and host cells in response to tumor [25] and most of our patients had progressive disease.
One PR was seen in this study in a patient with ER, PR, HER-2 negative inflammatory breast cancer. In some tumor cell line screens, triple negative breast cancers have been shown to be possibly amenable to Src kinase inhibition [26–29].
A patient with squamous cell cancer of the anus had tumor regression of 28 %, which lasted 15 months. A recent paper published by Hammerman et al. [30] identified mutations in the discoidin domain receptor 2 (DDR2) tyrosine kinase gene as markers for response in squamous cell lung cancer patients who respond to dasatinib. Future studies should include tissue biopsies to explore this aberration in responders.
The hypothesis that prompted this study was that Src inhibition could overcome gemcitabine resistance, particularly in pancreatic cancer. Two of eight patients with pancreatic cancer stayed on study for at least 6 months (25 and 34 weeks, respectively) despite having failed prior gemcitabine. Of interest, dasatinib may also be particularly effective in inhibiting metastasis [31]. Two phase 2 studies of dasatinib and gemcitabine are –ongoing, one in patients with locally advanced pancreatic cancer (NCT 01395017) and another in pancreatic cancer patients who have been treated previously with surgery (NCT01234935).
In conclusion, the combination of dasatanib and gemcitabine was well tolerated at doses of dasatinib 100 mg/m2 PO daily and gemcitabine 600 mg/m2 weekly. Although CTC counts did not differ at baseline between patients with SD ≥6 months / PR versus other patients, at day 28, there was a strong trend (p = 0.052) towards significantly lower CTCs in the former group. Prolonged SD in gemcitabine refractory pancreatic cancer, thymoma and squamous cancer of the anus and a PR in inflammatory breast cancer suggests that Phase II trials in these diseases are warranted.
References
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Bruning A, Mylonas I (2011) New emerging drugs targeting the genomic integrity and replication machinery in ovarian cancer. Arch Gynecol Obstet 283:1087–1096
Jordheim LPSP, Tredan O, Dumontet C (2011) The ribonucleotide reductase large subunit (RRM1) as a predictive factor in patients with cancer. Lancet Oncol 12:693–702
Trevino JG, Summy JM, Lesslie DP, Parikh NU, Hong DS, Lee FY, Donato NJ, Abbruzzese JL, Baker CH, Gallick GE (2006) Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am J Pathol 168:962–972
Chen T, Pengetnze Y, Taylor CC (2005) Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol Cancer Ther 4:217–224
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE (2004) Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res 10:2307–2318
Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim S, Wang Z, Gallick GE (2008) Targeting Src family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res 68:3323–3333
Nagaraj NS, Washington MK, Merchant NB (2011) Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res 17:483–493
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Trevino JG, Summy JM, Gray MJ, Nilsson MB, Lesslie DP, Baker CH, Gallick GE (2005) Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res 65:7214–7222
Demetri GD, Lo Russo P, MacPherson IR, Wang D, Morgan JA, Brunton VG, Paliwal P, Agrawal S, Voi M, Evans TR (2009) Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res 15:6232–6240
Johnson FM, Bekele BN, Feng L, Wistuba I, Tang XM, Tran HT, Erasmus JJ, Hwang L-L, Takebe N, Blumenschein GR, Lippman SM, Stewart DJ (2010) Phase II study of dasatinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 28:4609–4615
Araujo J, Logothetis C (2010) Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev 36:492–500
Sessa C, Aamdal S, Wolff I, Eppelbaum R, Smyth JF, Sulkes A, Ten Bokkel HW, Vermorken J, Wanders J, Franklin H et al (1994) Gemcitabine in patients with advanced malignant melanoma or gastric cancer: Phase II studies of the EORTC Early Clinical Trials Group. Ann Oncol 5:471–472
Kluger HM, Dudek AZ, McCann C, Ritacco J, Southard N, Jilaveanu LB, Molinaro A, Sznol M (2011) A phase 2 trial of dasatinib in advanced melanoma. Cancer 117:2202–2208
Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, Morris MJ, Hudes G, Calabro F, Cheng S, Trudel GC, Paliwal P, Sternberg CN (2009) Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res 15:7421–7428
Walter RB, Joerger M, Pestalozzi BC (2002) Gemcitabine-associated hemolytic-uremic syndrome. Am J Kidney Dis 40:E16
Izzedine H, Isnard-Bagnis C, Launay-Vacher V, Mercadal L, Tostivint I, Rixe O, Brocheriou I, Bourry E, Karie S, Saeb S, Casimir N, Billemont B, Deray G (2006) Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant 21:3038–3045
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781–791
Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, Meng S, Morrison L, Tucker T, Lane N, Ghadimi BM, Heselmeyer-Haddad K, Ried T, Rao C, Uhr J (2002) Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res 8:2073–2084
Coates JM, Galante JM, Bold RJ (2010) Cancer therapy beyond apoptosis: autophagy and anoikis as mechanisms of cell death. J Surg Res 164:301–308
Loza-Coll MA, Perera S, Shi W, Filmus J (2005) A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene 24:1727–1737
Mego M, De Giorgi U, Dawood S, Wang X, Valero V, Andreopoulou E, Handy B, Ueno NT, Reuben JM, Cristofanilli M (2011) Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer 129:417–423
Saylor PJ, Kozak KR, Smith MR, Ancukiewicz MA, Efstathiou JA, Zietman AL, Jain RK, Duda DG (2012) Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer. The Oncologist 17:212–219
Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E (2007) Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res 67:2226–2238
Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ (2007) Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/"triple-negative" breast cancer cell lines growing in vitro. Breast Cancer Res Treat 105:319–326
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767
Tryfonopoulos D, Walsh S, Collins DM, Flanagan L, Quinn C, Corkery B, McDermott EW, Evoy D, Pierce A, O'Donovan N, Crown J, Duffy MJ (2011) Src: a potential target for the treatment of triple-negative breast cancer. Ann Oncol 22:2234–2240
Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, Brace LE, Woods BA, Lin W, Zhang J, Deng X, Lim SM, Heynck S, Peifer M, Simard JR, Lawrence MS, Onofrio RC, Salvesen HB, Seidel D, Zander T, Heuckmann JM, Soltermann A, Moch H, Koker M, Leenders F, Gabler F, Querings S, Ansén S, Brambilla E, Brambilla C, Lorimier P, Brustugun OT, Helland A, Petersen I, Clement JH, Groen H, Timens W, Sietsma H, Stoelben E, Wolf J, Beer DG, Tsao MS, Hanna M, Hatton C, Eck MJ, Janne PA, Johnson BE, Winckler W, Greulich H, Bass AJ, Cho J, Rauh D, Gray NS, Wong KK, Haura EB, Thomas RK, Meyerson M (2011) Mutations in the DDR2 Kinase Gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discovery 1:1
Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, Doyle B, McKay C, Heung M, Oien KA, Frame MC, Evans TRJ, Sansom OJ, Brunton VG (2010) Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterol 139:292–303
Acknowledgements
We would like to thank Joann Aaron, MA, Scientific Editor in the Department of Investigational Cancer Therapeutics at MD Anderson, for her editorial assistance.
Research Support
This study was funded in part by Bristol-Myers Squibb, Inc.
Conflict of interest statement
Dr. Lewis C. Strauss is employed with Bristol-Myers Squibb. Dr. Razelle Kurzrock has received a commercial research grant from Genentech and honoraria from Speakers Bureau of Genentech. The other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, D.S., Choe, J.H., Naing, A. et al. A phase 1 study of gemcitabine combined with dasatinib in patients with advanced solid tumors. Invest New Drugs 31, 918–926 (2013). https://doi.org/10.1007/s10637-012-9898-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9898-3