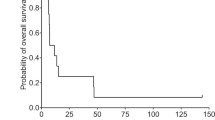
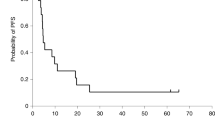
Summary
Purpose This open-labeled, phase I clinical trial was designed to determine the safety and tolerability of percutaneous intralesional administration of wild-type oncolytic revovirus type 3 Dearing (Reolysin®) in cancer patients with accessible and evaluable disease, who had otherwise failed to improve on standard cancer interventions. Experimental Design An escalating dose of Reolysin® starting from up to 1010 plague forming units (PFU) was administered to each cohort of three patients per dose level. Viral shedding, reovirus neutralizing antibody response, toxicity and clinical response were assessed. Results Nineteen patients with various advanced solid tumors were treated. The most common toxicities related to treatment were grade 2 (or less) local erythema and transient flu like symptoms. Viral shedding was not seen in cerebral spinal fluid (CSF), urine and stool samples in all patients. Rising viral antibody titres were seen in all patients. In addition, we observed some evidence of local target tumor response activity in 7/19 patients (37 %) at the end of six or more weeks follow-up, with one patient exhibiting a complete response (CR), two a partial response (PR), and four stable disease (SD) to the local injected lesion. Conclusions Reolysin® is well tolerated given intralesionaly, with DLT/MTD not reached at a dose of 1010 PFU. The favorable toxicity profile, lack of viral shedding and possible therapeutic activity has made this unattenuated oncolytic reovirus an attractive cancer therapeutic agent for ongoing clinical studies, including in the setting of locally advanced accessible disease for palliation of symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
First described at the turn of the last century [1], the idea of using viruses as cancer therapy has now been vividly testing in a large number of clinical trials [2–11]. Unlike classic gene therapy, which uses a virus as a vector to deliver therapeutic gene products, oncolytic viruses infect and kill tumor cells directly via a lytic infection; apoptosis may also occur [12]. Though the mechanisms are not entirely understood, the selectivity of at least some oncolytic viruses for cancer cells is because these viruses usurper a variety of similar cellular survival, proliferation, anti-apoptotic and anti-angiogenesis signalling pathway that are upregulated or constitutively active in cancer cells [12].
Reolysin® (Oncolytics Biotech Inc, Calgary, Canada) is a naturally occurring, unmodified, and replication competent strain of Type 3 Dearing reovirus. It is ubiquitous and non-pathological in humans [13], as witnessed by the fact that over half of adults have been exposed to reovirus, probably occur unnoticed in their young age [14]. It is well-known that reovirus is selectively oncolytic to cancer cells, but not to normal cells, in vitro, in vivo and ex vivo [15]. The exact mechanism of reovirus-mediated selective oncolysis still remains controversial. It is believed in part due to activation of Ras signalling pathway, that is frequently seen in cancer cells [16], either through aberrant activation mutation of Ras itself or the upstream or downstream elements, such as epidermal growth factor receptor (EGFR) or platelet-derived growth factor receptor (PDGFR) [15, 17–19]. Reovirus infection is restricted in normal cells because early viral transcripts activates double stranded protein kinase (PKR), which subsequently shuts down viral replication. In contrary, activation of Ras signalling in cancer cells inhibits PKR phosphorylation and activation, thus allows efficient viral propagation and eventually lysis of cancer cells [15, 19]. Reovirus in addition exploits other mechanisms such as reduction of protease inhibitors thus enhancing viral uncoating and viral particle infectivity, and promotion of apoptosis mediated through c-Jun N-terminal kinases (JNK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway [20–22].
Over the last decade or so, reovirus has been vigorously tested in a variety of preclinical animal models, in which reovirus therapy delivered either intratumorally or systemically yielded complete tumor regression [15, 23–27]. More recently, Reolysin® has been evaluated in a number of early phase clinical trials, mostly through systemic intravenous administration either as a monotherapy (three studies) [28–30] or a combination therapy with chemotherapy (three studies) [31–33]. Only two phase I trials have been completed to evaluate intratumoral administration of Reolysin®, one was used alone in recurrent malignant glioma [34], and the other one was used in combination with palliative radiotherapy in patients with advanced or cutaneous metastatic solid tumors [35]. These studies demonstrated that Reolysin® injection is a safe and well-tolerable cancer treatment, especially giving intratumorally. No significant toxicities (≥ grade 3) or adverse events related to the Reolysin® treatment observed in these intratumoral trials [34, 35]. Moreover, MTD has never been reached even at a maximum concentration of 1 × 1010 tissue culture infectious dose 50 (TCID50) given intratumorally up to six doses (two doses per week) [35].
Here, we report the results of a conventional, single-institution, open-labeled, dose-escalation phase I clinical study. It was designed to determine the safety and tolerability of the percutaneous intralesional administration of Reolysin®, thereby to define the dose limiting toxicity (DLT) and maximum tolerated dose (MTD), in cancer patients with a variety of advanced solid tumors, who had otherwise failed to improve on standard cancer interventions. Secondary objectives included pharmacokinetic analysis of viral shedding in relate to dose and frequency of administration, characterisation of the immune response to Reolysin® challenge intratumuraly, and local antitumor activity in target lesions and if any, in synchronous lesions remote from the site of viral administration.
Patients and methods
Patients and eligibility criteria
Patients with at least one histological confirmed cutaneous lesion of any histological type who had exhausted standard cancer treatment were enrolled. The cutaneous lesions had to be accessible for measurement by palpation and percutaneous intralesional administration of Reolysin® measuring between 1 and 10 cm2. Fine needle aspiration with histological examination of the target lesion ≤ 14 days prior to intralesional administration of Reolysin® was performed in all patients and reviewed by a single pathologist (Dr L. DiFrancesco) to confirm that the palpable lesions were cancerous. Eligible patients had to have a life expectancy ≥ 12 weeks, performance status of Eastern Cooperative Oncology Group (ECOG PS) of ≤3, age ≥ 18 years and signed a written informed consent form. Eligible patients must have adequate organ function as defined by absolute granulocytes ≥ 2 × 109/L, absolute lymphocytes ≥ 75 % of the lower limit of normal, hemoglobin ≥100 g/L, plateletes ≥ 100 × 109/L; serum creatinine < 1.5 times the upper limit of normal; serum transminase levels < 3 times the upper limit of normal; quantitative immunoglobulins more than the lower limit of normal; and a left ventricular ejection fraction of more than the lower limit of normal as evaluated by Multi Gated Acquisition (MUGA) scan. A therapy-free period of ≥ 21 days (free of any active cancer treatment or other investigational drugs) and a corticosteroid dose equivalent or inferior to 10 mg of prednisone per day were also required as an inclusion criteria.
Female patients of childbearing potential not using medically approved contraceptive methods, pregnant or breastfeeding were excluded. Exclusion criteria included concurrent or prior radiation therapy to the lesion being injected unless new tumor growth within the radiation field which could be documented, and concurrent use of alternative, complementary or unproven systemic and/or local therapies.
The study was conducted in accordance with the principles of Good Clinical Practice, the Declaration of Helsinki, Health Canada and U.S Federal Drug Administration, the local Research Ethics Board approved this study and all patients were able to understand and signed the informed consent.
Study design and dose escalation
We used a standard phase 1, dose-escalation design in which both the dose and frequency of Reolysin® administrations increased (Table 1) depending on toxicities encountered. We selected a starting dose of 1 × 107 PFUs delivered once and escalated this to a maximum dose of 1 × 1010 PFUs given once weekly. In selecting an appropriate starting dose the usual approach of extrapolating from toxicity data in immunocompetent mice was not useful since they tolerate the highest dose which is impossible to manufacture without significant toxicities. Therefore, we selected 1 × 107 PFUs as a starting dose since others have done the same with other replication competent viruses [36–38]. A minimum of three patients were entered at each dose level until a patient experienced a dose-limiting toxicity (DLT) (see below toxicity assessment). When a DLT was encountered, three more subjected (total of six) were added to that dose level group. If two or more subjects (out of six) in a dose group experienced DLT, that dose level will be considered as maximum toxicity dose (MTD). Dose escalation was continued to the next level, provided it was well tolerated and MTD was not reached. Intrapatient dose escalations were not permitted.
Viral administration, patient evaluation and follow-up
Purified Reolysin® (tested by BioReliance Corporation (Rockville, Md) as per Good Laboratory Practice (GLP)/Good Manufacturing Practice (GMP) quality assurance) was provided in colour coded glass vials at approximately the concentration of virus to be used so that minimal dilution or mixing would have to be done at viral administration institution. Stock were stored at −70 °C, thawed rapidly, and the prescribed PFUs dose was prepared in a sterile syringe, fitted with a 25 gauge needle, and the volume was standardized to a total of 1 ml. In order to ensure adequate distribution of viral particles within the target lesion, the target lesion was palpated, overlain with a sterile grid marked in 1 cm2 areas and the total dose of Reolysin® was divided so that equal amounts would be administered per 1 cm2. For example, a 2 cm2 lesion would be treated with four intralesional injections of the total dose.
The intralesional administration was performed in an outpatient setting, under sterile conditions and with appropriate precautions (i.e. masks, gowns and gloves recommended at the time of the study). Patients were monitored under close observation (including blood pressure, temperature, heart rate and oxygen saturation monitoring) for at least 2 h following the procedure. Patients were given instructions regarding infectious precautions at home. Toxicities and adverse events were monitored after viral treatment weekly for 6 weeks and once every 4 weeks at week 10 and week 14 using physical examination, measurement of performance status, hematology and biochemistry. The pharmacokinetic analysis of virology including viral neutralizing antibodies and viral shedding in urine, sputum, stool and serum were analyzed at specified intervals (described as below). 2 and 4 weeks after viral treatment administration, cardiac function by electrocardiogram (ECG) and MUGA scan were evaluated. Patients had both a magnetic resonance image (MRI) of the brain and a lumbar puncture (LP) 2 weeks after the last corresponding viral administration.
Toxicity assessment
Toxicities and adverse events were graded according to the National Cancer Institute—Clinical Trials Group (NCI CTG) expanded Common Toxicity Criteria. DLT was defined as occurring whenever any one of the following occurred: 1) Grade ≥ 3 and was felt to be probably or definitely related to Reolysin®. 2) New or worse aggravation of existing peripheral vascular disease, or 3) Clinical evidence of myocarditis as demonstrated by MUGA scan ( ≥ 10 % decrease in left ventricular ejection fraction) or ST segment abnormalities on an ECG. These latter two criteria were used because severe combined immunodeficiency (SCID) (but not immunocompetent) mice developed hind limb necrosis, which was presumably vascular in nature, and myocarditis following subcutaneous intralesional administration of reovirus [15].
Response evaluation
Patients must have been followed for at least 6 weeks to be considered evaluable for response unless early progression occurs, in which case they were considered evaluable. Assessment of tumor response of the target lesion (which received intralesional Reolysin®) were performed at pretreatment and once weekly for 6 weeks and thereafter once every 4 weeks at week 10 and week 14 by manually measuring the palpated tumor using calipers (photograph were also taken). Up to a maximum of six synchronous lesions were also followed at the same interval to determine if there was evidence of a response at sites remote from the site of administration. Clinical response included local tumor response and systemic response was determined by RECIST criteria [39] for progressive disease (PD), stable disease (SD), partial response (PR) and complete response (CR).
Analysis of viral shedding
Samples of urine, sputum, stool, cerebral spinal fluid (CSF) and serum were tested for infectious particles using a semi-quantitative RT-PCR and PFU techniques at baseline and throughout the study. Specifically, these tests was done in serum at baseline and repeated weekly for 6 weeks after viral treatment and thereafter every 4 weeks at week 10 and week 14; two and 4 weeks after viral treatment in urine, sputum and stool; and 2 weeks after the last corresponding viral administration in CSF.
Detection of neutralizing antireoviral antibodies to Reolysin®
Serum was collected and stored at −70 °C for batch analysis of neutralizing antiretroviral antibodies at baseline (to determine previous exposure) and once weekly for 6 weeks after viral administration and thereafter once every 4 weeks at week 10 and week 14 to quantitate immune response to Reolysin®. It was performed by Alberta Provincial laboratory using a standard ELISA method. A neutralizing antibodies titre of <8 were considered indicative of prior exposure to reovirus when measured on baseline serum samples.
Results
Patient characteristics
Nineteen patients (nine men and ten women), with a variety of advanced or metastatic solid tumors that were unresponsive to existing standard cancer therapies were enrolled into this study. Patient demographics are displayed in Table 2. Details of primary tumor diagnosis and prior treatments are shown in Table 3. Patients’ median age was 51 years (range 27–70). The median ECOG PS was 0 (range 0–1). The most common primary tumor types were soft tissue sarcoma (n = 5), melanoma (n = 4), head and neck (n = 4), breast (n = 3), and other tumors (n = 3). Ten (53 %) patients had prior radiotherapy. Fifteen (79 %) patients received a median of two prior chemotherapy regimens (range 0–5). (Tables 2 and 3)
Toxicities and adverse events
This treatment was overall well tolerated and all symptomatic toxicities encountered (either definitely or probably related to viral administration) were mild (≤ grade 2; Table 4). The most frequent reported events (summarized in Table 4), especially during the first few days after injection, were complaints such as nausea (n = 15; 79 %), vomiting (n = 11; 58 %), headache (n = 12; 63 %), local erythema of injection site (n = 8; 42 %), fever/chills (n = 7; 37 %), dizziness (n = 7; 37 %), transient flu-like symptoms (n = 6; 32 %), diarrhea (n = 6; 32 %), and arthralgia/myalgia (n = 5; 26 %). We observed a relatively frequent grade 1 to 3 headache in 12 patients (63 %) in this study. However, all headaches reported by patients were post lumber-punctures which is a well-known side effect from this procedure itself. Thus, we believe that headache is an unlikely toxicity to the intratumoral Reolysin® treatment. In regard to laboratory toxicities, majority of them were mild (≤ grade 2; Table 4) as well. There were some grade 1 to 2 transaminase level increase (n = 6; 32 %) and a few grade 1 total bilirubin level increase (n = 2; 22 %). These lab value abnormalities, although mild, seemed to overlap with some gastrointestinal (GI) symptoms such as nausea, vomiting and diarrhea. However, those liver tests of a significant amount of patients who had GI symptoms remained normal post intratumoral Reolysin® treatment. It was noted that there were nearly half of patients (n = 9; 47 %) had varied grade of lymphopenia with two in grade 3 (n = 2; 22 %) and one in grade 4 range (n = 1; 5 %). Lymphopenia is expected to associate with viral infection, however, these resulted in no clinical consequences such as serious infections. There were only two grade 1 asymptomatic thrombocytopenia (n = 2; 22 %) and no neutropenia were observed. Overall, a DLT was not encountered and therefore a MTD could not be determined.
During the conduction of the trial, a total of seven patients died (median time to death was 150 (range: 110–223) days) and in all cases these deaths occurred from disease progression or disease complications, none within 30 days of Reolysin® injection. No patient discontinued due to an adverse event, although one patient refused further involvement in the study. Five patients had one or more serious toxicities (grade ≥ 3) or adverse events during the study period, The most common were pain in five patients; pancreatitis in one patient; vena cava thrombosis in one patient; dysphagia in two patients; leg swelling in one patient; neuropathy in two patients. None of these adverse events was judged related to Reolysin® injection based on the NCI-CTG criteria for the relationship of the adverse event to the treatment.
Neutralizing antibodies response
All 19 patients were tested at baseline for neutralizing antibodies to Reolysin® and seven (37 %) were positive for neutralizing antibodies (Table 5). All patients became seropositive after treatment with Reolysin® a median of 1.4 weeks (range 1 to 3 weeks) from injection. Patients developed a median maximum antibody titre of >1364 (range 64 to > 4096). The median time until the maximum neutralizing antibody response was 3.8 weeks (range 1 to 10 weeks) following the first administration of Reolysin®.
Pharmacokinetic analysis of viral shedding
All patients’ samples of urine, stool and CSF (pre- and post Reolysin® treatment) were negative for viral shedding by viral culture and RT-PCR (Table 5). Unfortunately, no patient could expectorate sufficient sputum to be tested, thus sputum samples were not tested. Serum RT-PCR was positive for viral detection in the multiple injection 1 × 109 (n = 2; 67 %) and single injection 1 × 1010 (n = 1; 33 %) cohorts. This did not however correlate with flu-like symptoms.
Antitumor response assessment
The best target tumor response at 6 weeks (42 days) or more follow up was CR in one (5.3 %), PR in two (10.5 %), SD in four (21.1 %) and PD in ten (52.6 %) patients (Table 6). As a representative of a target tumour response to intralesional Reolysin® treatment, Figure 1 showed photograph pictures of a target injection lesion of a PR patient (05–02) pre- and 10 weeks post intralesional Reolysin® treatment. Synchronous lesions generally showed lower response rates, although one PR patient (05–02) had a best response of PR for one synchronous lesion and the other two patients (06–03, 03–03) had a SD for one synchronous lesion at 6 weeks or more follow-up (Table 6). In addition, best tumor responses at any time on study and maintained for ≥ 2 weeks were CR in two, PR in four, SD in 11 patients for the injection target lesion and three PR at their synchronous lesions (data not shown). For example, the target injection lesion of one patient 01–03 had a CR at week 2 and 3 follow-up, however the lesion grew back and was considered as a SD at the end of 14 weeks follow up.
Discussions
Over the last decade or two, there is a growing interest in the development of oncolytic viruses in clinical studies, particularly a wild-type reovirus type 3 Dearing (Reolysin®) for use as a targeted cancer therapeutics either alone [28–30, 34] or in combination with conventional modalities [31–33, 35]. Here, we reported the results of a first-in-world phase 1 clinical trial of Reolysin® by giving percutaneous intralesionaly as a monotherapy to a variety of oncology patients with advanced solid cancers. The major finding of this study is that percutaneous intralesional administration of Reolysin® into metastatic accessible tumors of a variety of oncology patients is safe and well tolerated. Nausea, vomiting, diarrhea, injection site erythema, fever/chills, flu-like illness and arthralgias/myalgias were the main toxicities but all these toxicities were mild requires no treatment. No DLT was found even at the maximum used dose of 1 × 1010 PFUs during the study, and therefore, MTD was not defined. To date, there were two phase I trials of intratumural injection of Reolysin®. One was used alone in recurrent malignant glioma [34], and the other one was used in combination with palliative radiotherapy in patients with advanced or cutaneous metastatic solid tumors [35]. Neither of these two trials observe a DLT at their maximum used dose, one was at 1 × 109 TCID50 [34], the other was at 1 × 1010 TCID50 given intratumorally up to six doses (two doses per week) [35]. One should note that TCID50 and PFUs are not equivalent viral quantification units because they use two distinct infectivity assays. TCID50 quantifies the amount of virus required to kill 50 % of inoculated tissue culture cells, whereas PFUs is representative of infective virus particles. It was generally believed that about two thirds of TCID50 equals to PFUs. Therefore, on the basis of these data, we postulate that it may be possible to increase the dose level beyond 1 × 1010 PFUs or TCID50 (this was the maximum concentration that could be manufactured at the time of the trial), and give multiple injections at an interval of 2 or 3 days in the future trials in order to increase the efficacy of intratumoral injection of Reolysin® either alone or in combination with other modalities such as radiotherapy in locally advanced tumors.
Pharmacokinetic viral analyses in our study using both RT-PCR and viral culture techniques detected no viral shedding in urine, stool and CSF samples in any patient at 2 and 4 weeks post Reolysin® intralesional injections. Serum RT-PCR, but not viral culture, was only positive in two patients (05–02 and 06–01) in the multiple injection 1 × 109 and 1 × 1010 cohorts, respectively. As for patient 06–01, only serum RT-PCR at baseline was positive, became and remained negative repeatedly in the subsequent follow-up studies done post Reolysin® injections. As for patient 05–02, serum RT-PCR was only positive at week 6 post injection but did not persist afterwards. In addition, this finding did not however correlate with any flu-like symptoms. Our study as well as others [33, 35] confirm the biosafety of this agent used both intravenously or intratumorally. However, other studies [28, 34] occasionally observed a positive viral detection in body fluids (such as feces and saliva) post Reolysin® treatment, although this was very infrequent and short-lived. The discrepancy might reflect an earlier detection time interval (2 versus 10 days) post treatment and a more extensive RT-PCR cycles (35 versus 25) used in viral detection in latter studies. Based on these studies, we recommend that this treatment can be safely giving in an out-patient setting.
Rising Reolysin® neutralizing antibody titres measured by an ELISA assay were seen in all patients post Reolysin® intralesional injection treatment regardless of baseline viral titre in this study. Increment of antibody titres seemed to correlate with injection dose given. It was noted that the higher the injection dose was, the higher level the maximum antibody titres increased to, and the less time the antibody titres needed to reach the peak level. However, there was discrepancy in some patients to this observation. This might simply reflect the heterogeneity of individual patient’s baseline immune function, which might also be altered by previous cancer treatments, thus distinct immune response to Reolysin® treatment. The term “NARA” (Neutralizing AntiRetroviral Antibody) was frequently noted to be used in recent Reolysin® trials [28–33]. This NARA titre was determined by a modified neutralizing antibody assay which was used to detect Reolysin® neutralizing antibody titres by measuring effect of patient serum samples on the ability of a reovirus to kill a monolayer of the target mouse L929 cells [40]. Despite a different assay used in this study, we did observe a similar trend of fold increase of antibody titre although the actual values of antibody titres in this study were not comparable to other trials. It was generally believed that rising Reolysin® neutralizing antibody titres (or a NARA response) plays an important role in preventing spread of viral progeny, thus protecting against virus-mediated systemic toxicity. The minimal systemic toxicities in all patients observed in this study were consistent with this theory. On the contrary, this rising neutralizing antibody response seemed to act as an obstacle for efficient viral delivery to tumors especially after intravenous treatment. Therefore, more recent intravenous Reolysin® trials explored if concomitant use with chemotherapy (eg. cyclophsphamide [41] and gemcitabine [33]) or immunotherapy (eg. rituximab) would attenuate the NARA response thus enhance the antitumor effect of Reolysin® therapy. In light of this theory, intratumural approach has a significant advantage over intravenous administration because direct injection of Reolysin® into tumors effectively kills the target tumor cells which might not be or less affected by a systemic neutralizing antibody response, while not losing the protection against systemic toxicities.
This study was not primarily designed to evaluate the anti-tumor activity of Reolysin® intralesional injection. Patients recruited in this study were heterogeneous in all aspects in terms of tumor histological type, aggressiveness of disease, and previous treatment regimes. We showed a significant treatment efficacy of local tumor response in 7/19 patients (37 %) (who had been heavily pretreated) at the end of six or more weeks follow-up, with one patient exhibiting a complete response (CR), two a partial response (PR), and four stable disease (SD) to the local injected lesion based on the RECIST criteria [39]. However, we did not observe a significant anti-tumour activity in synchronous lesions remote from the site of viral administration. This could be explained by above theory that the rising neutralizing antibody response serves as a significant obstacle in preventing efficient viral delivery elsewhere. In addition, we showed some evidence of significant local antitumor activity in a variety of specific tumor types including head and neck, melanoma and Kaposi’s sarcoma, consistent with the observation seen in other intratumoral trial [35], strongly supporting the future exploration of clinical use of Reolysin® intralesional injection in these locally advanced tumor types that had exhausted standard treatments.
Patients were not selected for this study based on the Ras status of their tumors. Unfortunately, we did not have sufficient molecular data to show a possible preferential Ras activation in these particular tumor types that responded significantly to Reolysin® intralesional treatment. In future clinical studies, a detailed molecular analysis of an oncogenic Ras mutation status as well as an activated Ras pathway either through upstream or downstream signalling effectors would be particularly useful for us to better elucidate the underlying mechanisms of reovirus selective oncolysis, and better select patient population who would benefit this treatment the most. Another enlightening thought is that Reolysin® treatment may synergize better with targeted therapies specifically targeting activated Ras pathway such as small molecular tyrosine kinase inhibitors rather than chemotherapy and radiotherapy. We are excitingly anticipating the future trials to investigate this possibility further.
A post-trial long-term follow up of available patients indicated that several patients in our study had a relative long-term survival following treatment with reovirus (5 survived more than 1 year), and one patient (03–03, non-HIV Kaposi’s sarcoma) still remains alive 10 years after completion of this clinical trial. Amazingly, his injected target lesion remains to be a SD after 10 years. However, this might reflect patient selection rather than a treatment effect. Nonetheless, this is a promising finding, further suggesting the safety of this treatment over time.
In conclusion, this study confirms the safety of percutaneous intralesional injection of Reolysin® in a variety of oncology patients with advanced cancer. Furthermore, the data reported here serves as an essential background information for the ongoing phase II and III studies of this agent worldwide.
References
Dock G (1904) Rabies virus vaccintion in a patient with cervical carcinoma. Amer J Med Sci 127:563
Deweese TL, Van Der Poel H, Li SD, Mikhak B, Drew R, Goemann M, Hamper U, Dejong R, Detorie N, Rodriguez R, Haulk T, Demarzo AM, Piantadosi S, Yu DC, Chen Y, Henderson DR, Carducci MA, Nelson WG, Simons JW (2001) A phase I trial of Cv706, a replication-competent, Psa selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation Therapy. Cancer Res 61:7464–7472
Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, Goldberg S, Gross P, O’neil JD, Groene WS, Roberts MS, Rabin H, Bamat MK, Lorence RM (2002) Phase I trial of intravenous administration of Pv701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol 20:2251–2266
Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S, Kirn DH, O’neill V, Siddiqui N, Seiden MV, Kaye SB (2002) Phase I trial of intraperitoneal injection of the E1b-55-Kd-Gene—deleted adenovirus Onyx-015 (Dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol 20:1562–1569
Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, Kracher J, Grossman SA, Fisher JD, Carson K, Rosenblum M, Mikkelsen T, Olson J, Markert J, Rosenfeld S, Nabors LB, Brem S, Phuphanich S, Freeman S, Kaplan R, Zwiebel J (2004) A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1b-attenuated adenovirus, Onyx-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther 10:958–966
Reid TR, Freeman S, Post L, Mccormick F, Sze DY (2005) Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-Fu/Leucovorin. Cancer Gene Therapy 12:673–681
Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E, Rozenman-Yair S, Panet A, Libson E, Irving CS, Galun E, Siegal T (2006) Phase I/Ii trial of intravenous Ndv-huj oncolytic virus in recurrent glioblastoma multiforme. Mol Ther 13:221–228
Laurie SA, Bell JC, Atkins HL, Roach J, Bamat MK, O’neil JD, Roberts MS, Groene WS, Lorence RM (2006) A phase I clinical study of intravenous administration of Pv701, an oncolytic virus, using two-step desensitization. Clin Cancer Res 12:2555–2562
Sonpavde G, Thompson TC, Jain RK, Ayala GE, Kurosaka S, Edamura K, Tabata KI, Ren CZ, Goltsov AA, Mims MP, Hayes TG, Ittmann MM, Wheeler TM, Gee A, Miles BJ, Kadmon D (2011) Glipr1 tumor suppressor gene expressed by adenoviral vector as Neoadjuvant Intraprostatic injection for localized intermediate or high-risk prostate cancer preceding radical prostatectomy. Clin Cancer Res 17:7174–7182
Hwang TH, Moon A, Burke J, Ribas A, Stephenson J, Breitbach CJ, Daneshmand M, De Silva N, Parato K, Diallo JS, Lee YS, Liu TC, Bell JC, Kirn DH (2011) A mechanistic proof-of-concept clinical trial with Jx-594, a targeted multi-mechanistic Oncolytic Poxvirus, in patients with metastatic melanoma. Mol Ther 19:1913–1922
Nakao A, Kasuya H, Sahin TT, Nomura N, Kanzaki A, Misawa M, Shirota T, Yamada S, Fujii T, Sugimoto H, Shikano T, Nomoto S, Takeda S, Kodera Y, Nishiyama Y (2011) A phase I dose-escalation clinical trial of Intraoperative direct Intratumoral injection of Hf10 Oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Therapy 18:167–175
Parato KA, Senger D, Forsyth PAJ, Bell JC (2005) Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer 5:965–976
Rosen L, Spickard A, Evans HE (1963) Reovirus infections in human volunteers. American Journal Hygiene 77:29
Jackson GG, Muldoon RL (1973) Viruses causing common respiratory-infection in Man.4. Reoviruses and adenoviruses. J Infect Dis 128:811–866
Coffey MC, Strong JE, Forsyth PA, Lee PWK (1998) Reovirus therapy of tumors with activated ras pathway. Science 282:1332–1334
Bos JL (1989) Ras oncogenes in human cancer—a review. Cancer Res 49:4682–4689
Kelly K, Nawrocki S, Mita A, Coffey M, Giles FJ, Mita M (2009) Reovirus-based therapy for cancer. Expert Opin Biol Ther 9:817–830
Strong JE, Lee PWK (1996) The V-Erbb Oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol 70:612–616
Strong JE, Coffey MC, Tang D, Sabinin P, Lee PWK (1998) The molecular basis of viral oncolysis: Usurpation of the ras signaling pathway by reovirus. EMBO J 17:3351–3362
Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PWK (2007) Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther 15:1522–1530
Clarke P, Meintzer SM, Wang YB, Moffitt LA, Richardson-Burns SM, Johnson GL, Tyler KL (2004) Jnk regulates the release of proapoptotic mitochondrial factors in reovirus-infected cells. J Virol 78:13132–13138
Connolly JL, Rodgers SE, Clarke P, Ballard DW, Kerr LD, Tyler KL, Dermody TS (2000) Reovirus-induced apoptosis requires activation of transcription factor Nf-Kappa B. J Virol 74:2981–2989
Alain T, Hirasawa K, Pon KJ, Nishikawa SG, Urbanski SJ, Auer Y, Luider J, Martin A, Johnston RN, Janowska-Wieczorek A, Lee PWK, Kossakowska AE (2002) Reovirus therapy of lymphoid malignancies. Blood 100:4146–4153
Hanel EG, Xiao ZW, Wong KK, Lee PWK, Britten RA, Moore RB (2004) A novel intravesical therapy for superficial bladder cancer in an orthotopic model: Oncolytic reovirus therapy. J Urol 172:2018–2022
Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PWK (2002) Oncolytic reovirus against ovarian and colon cancer. Cancer Res 62:1696–1701
Norman KL, Coffey MC, Hirasawa K, Demetrick DJ, Nishikawa SG, Difrancesco LM, Strong JE, Lee PWK (2002) Reovirus oncolysis of human breast cancer. Human Gene Therapy 13:641–652
Wilcox ME, Yang WQ, Senger D, Rewcastle NB, Morris DG, Brasher PMA, Shi ZQ, Johnston RN, Nishikawa S, Lee PWK, Forsyth PA (2001) Reovirus as an oncolytic agent against experimental human malignant gliomas. Journal National Cancer Institute 93:903–912
Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, Melcher A, Coffey M, Harrington KJ, Debono JS (2008) A phase I study of intravenous oncolytic reovirus type 3 dearing in patients with advanced cancer. Clin Cancer Res 14:7127–7137
Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, Coffey M, Gill GM, Mettinger K, Mariadason JM, Mani S, Goel S (2010) Intravenous administration of reolysina (R), A live replication competent Rna virus is safe in patients with advanced solid tumors. Investigational New Drugs 28:641–649
Galanis E, Markovic SN, Suman V, Nuovo G, Vile R, Coffey M, Linette G, Maples W, Zwiebel J, Kendra K (2011) Phase II trial of intravenous administration of Reolysin (Reovirus Serotype-3-Dearing Strain) in patients with metastatic melanoma. Mol Ther 19:1375
Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, Vile R, Melcher A, Coffey MC, Mettinger KL, Nuovo G, Cohn DE, Phelps M, Harrington KJ, Pandha HS (2010) Reo-10: A Phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res 16:5564–5572
Karapanagiotou E, Pandha HS, Hall G, Chester J, Melcher A, Coffey M, De Bono J, Gore Me, Nutting CM, Harrington KJ (2009) Phase I/Ii trial of oncolytic reovirus (Reolysin) in combination with Carboplatin/Paclitaxel in patients (Pts) with advanced solid cancers. J Clinical Oncology 27
Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V, Twigger K, Coffey M, Mettinger K, Gill G, Evans TRJ, De Bono JS (2011) A phase I study of the combination of intravenous reovirus type 3 dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res 17:581–588
Forsyth P, Roldan G, George D, Wallace C, Palmer CA, Morris D, Cairncross G, Matthews MV, Markert J, Gillespie Y, Coffey M, Thompson B, Hamilton M (2008) A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther 16:627–632
Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, Beirne D, Prestwich R, Newbold K, Ahmed M, Thway K, Nutting CM, Coffey M, Harris D, Vile RG, Pandha HS, Debono JS, Melcher AA (2010) Two-stage phase I Dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res 16:3067–3077
Clayman GL, El Naggar AK, Lippman SM, Henderson YC, Frederick M, Merritt JA, Zumstein LA, Timmons TM, Liu TJ, Ginsberg L, Roth JA, Hong WK, Bruso P, Goepfert H (1998) Adenovirus-mediated P53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J Clin Oncol 16:2221–2232
Fujiwara T, Grimm EA, Mukhopadhyay T, Zhang WW, Owenschaub LB, Roth JA (1994) Induction of chemosensitivity in human lung-cancer cells in-vivo by adenovirus-mediated transfer of the wild-type P53 gene. Cancer Res 54:2287–2291
Nguyen DM, Spitz FR, Yen N, Cristiano RJ, Roth JA (1996) Gene therapy for lung cancer: enhancement of tumor suppression by a combination of sequential systemic cisplatin and adenovirus-mediated P53 gene transfer. J Thorac Cardiovasc Surg 112:1372–1376
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. Journal National Cancer Institute 92:205–216
White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, Morgan R, Merrick A, Errington F, Vile RG, Melcher AA, Pandha HS, Harrington KJ (2008) Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing Type 3) during a phase i clinical trial. Gene Therapy 15:911–920
Qiao J, Wang HX, Kottke T, White C, Twigger K, Diaz RM, Thompson J, Selby P, De Bono J, Melcher A, Pandha H, Coffey M, Vile R, Harrington K (2008) Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res 14:259–269
Acknowledgements
We thank all our participating patients, our research nurses and coordinators in TBCC. This work is funded by Oncolytics Biotech Inc. and Alberta Cancer Board.
Disclosure of potential conflicts of interest
M. Coffey and B. Thompson are employed by Oncolytics Biotech Inc and they both have an ownership interest in that company; D. Morris and P. Forsyth has received research grants from Oncolytics Biotech Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, D.G., Feng, X., DiFrancesco, L.M. et al. REO-001: A phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs 31, 696–706 (2013). https://doi.org/10.1007/s10637-012-9865-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9865-z