Summary
Gemcitabine is widely used for the treatment of advanced biliary tract cancer (BTC) as first-line chemotherapy. However, there is no standard chemotherapy for patient with advanced BTC refractory to gemcitabine. We conducted a multicenter phase II study of S-1 monotherapy as second-line chemotherapy for patients with advanced BTC that were refractory to gemcitabine. S-1 was administered orally at a dose of 80 mg/m2 for 28 days, followed by 14 days of rest. This regimen was repeated every 6 weeks. Tumor response was assessed every two cycles using the Response Evaluation Criteria in Solid Tumors version 1.0. Twenty-two patients were enrolled between March 2007 and January 2010, with 14 patients (64%) representing cases of recurrence after surgery. The overall response rate was 22.7%, and the overall disease control rate was 50.0%. The median overall survival time was 13.5 months (95% CI, 7.1–23.1 months) and the median time-to-progression was 5.4 months (95% CI, 2.6–17.2 months). Grade 3/4 toxicities included neutropenia (5%) and anemia (5%). The most common non-hematological toxicities were nausea (27%), anorexia (55%), and pigmentation (32%). In conclusion, S-1 monotherapy is feasible and moderately efficacious second-line chemotherapy for advanced BTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary tract cancer (BTC) is the sixth leading cause of death in Japan [1]. Surgery is still the only treatment that can cure this life-threatening disease. However, most patients treated with surgery experience recurrence. Therefore, chemotherapy is indispensable for the treatment of advanced BTC.
Gemcitabine is now the key drug for the treatment of advanced BTC, and several phase II studies of combination chemotherapies involving gemcitabine have been reported [2–5]. In 2009, the first large phase III study was reported, confirming the superiority of gemcitabine and cisplatin combination chemotherapy to gemcitabine monotherapy in patients with advanced BTC [6]. This effect was also observed in a randomized phase II study conducted in Japan [7]. Moreover, a phase III study is underway investigating gemcitabine and capecitabine combination chemotherapy versus gemcitabine monotherapy (NCT00658593). Thus, these studies are gradually accumulating a body of evidence on first-line chemotherapy for advanced BTC.
Only a few studies have been reported on second-line chemotherapy for BTC [8, 9], and no standard second-line chemotherapy has been established. One phase II study of second-line gemcitabine single chemotherapy showed a response rate of 6.9% [10]. Furthermore, we conducted a feasibility study of gemcitabine and cisplatin combination chemotherapy which enrolled the patient refractory to both gemcitabine and S-1, and we observed no tumor response [11].
S-1 is an oral fluoropyrimidine that has mainly been investigated in Asian countries. The efficacy and safety of S-1 monotherapy have previously been reported in the setting of first-line chemotherapy [12–14]. Only our single-center feasibility study has investigated S-1 for second-line chemotherapy of advanced BTC [15]. This feasibility study treated 16 patients and showed a response rate of 18.8%. The median overall survival and time-to-progression were 8.0 and 5.5 months, respectively. Because of the good anti-tumor activity, we conducted a multicenter phase II study to confirm the efficacy and safety of S-1 monotherapy as second-line chemotherapy in patients with advanced BTC refractory to gemcitabine.
Patients and methods
This multicenter phase II study was an open-label, single-arm study that was conducted in five institutions in Tokyo, Japan. The protocol was approved by each institutional review board. Informed consent was obtained from each participant, and the study was conducted according to the Declaration of Helsinki. The study was registered in the UMIN Clinical Trials Registry (UMIN000001614).
Eligibility criteria
Patient with advanced BTC that was not amenable to potentially curative surgery or that was refractory to surgery were eligible if they met the following criteria: 1) pathologically demonstrated BTC or graphically confirmed BTC; 2) the presence of measurable lesions defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 [16]; 3) refractory to gemcitabine monotherapy and confirmed as a progressive disease defined by RECIST version 1.0; 4) Eastern Cooperative Oncology Group (ECOG) performance status ranging from 0 to 2; and 5) adequate bone marrow function (white blood cell count > 3,000/mm3, hemoglobin > 9.0 g/dl, and platelet count > 100,000/mm3), liver function (total bilirubin < three times the upper limit of normal (ULN) and aspartate/alanine transaminases < five times ULN), and renal function (creatinine < 1.2 mg/dl or creatinine clearance > 50 ml/min). In patients with obstructive jaundice, serum total bilirubin was required to be within three times of the ULN after biliary drainage. Exclusion criteria included an age < 20 years, uncontrolled infection, uncontrolled massive pleural effusion or massive ascites, an active ulcer of the gastrointestinal tract, gastrointestinal obstruction compromising oral ingestion, pregnancy or lactation, a history of drug hypersensitivity, active concomitant malignancy, and concurrent severe medical conditions. All patients stopped the first-line chemotherapy at least 2 weeks before entry into this study.
Treatment
S-1 was administered orally twice daily for 28 days followed by 14 days of rest. Three doses of S-1 were established according to body surface area (BSA) as follows: BSA <1.25 m2, 80 mg/day; 1.25 m2 < BSA <1.5 m2, 100 mg/day; and BSA >1.5 m2, 120 mg/day. Dose reduction was based on adverse effects graded according to the National Cancer Institute Common Toxicity Criteria version 3.0. Treatment was temporarily suspended in the case of grade 3/4 hematological toxicity or grade 2 or higher non-hematological toxicity. After recovery to grade 1 toxicity level or lower, treatment was restarted at the following reduced doses: BSA <1.25 m2, 50 mg/day; 1.25 m2 < BSA and <1.5 m2, 80 mg/day; BSA >1.5 m2, 100 mg/day. No dose re-escalation was allowed following dose reduction. The study treatment was continued in cycles of 28 days of treatment followed by 14 days of rest until disease progression, unacceptable toxicity, or patient refusal occurred.
Response and toxicity assessment
Pretreatment evaluation included medical history and physical examination, complete blood counts, serum biochemical tests, urinalysis and echocardiogram. The ECOG performance status and laboratory tests, which included a complete blood count and serum biochemical tests, were checked every 2 weeks. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were measured at the beginning of the study and at day 1 of each treatment cycle. Pretreatment evaluation using contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was conducted within 4 weeks before the patient’s enrollment. Tumor response was assessed every two cycles. Toxicity was evaluated using the National Cancer Institute Common Toxicity Criteria version 3.0.
Statistical analysis
The primary endpoint was the objective response rate. The secondary endpoints were time-to-progression, overall survival, and toxicity. The sample size was calculated to reject a 5% response rate in favor of a target response rate of 20%, with a significance level of 0.05 and a power of 80%. The target sample size was thus 21 assessable patients. If four or more objective responses were observed among all 21 assessable patients, this study would consider the regimen to be effective [17].
The Mann-Whitney U test was used to compare quantitative variables. The objective response rate was evaluated according to RECIST version 1.0. Time-to-progression and overall survival were calculated using the Kaplan-Meier method. Time-to-progression was calculated from the start of treatment to the first date of documented disease progression. Overall survival was defined as the time from treatment initiation to final follow-up or until death from any cause. The final analysis was based on follow-up information, which was received until July 2010. All analyses were conducted based on the intention-to-treat principle. The JMP 8.0 statistical software program (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patient characteristics
Table 1 lists the characteristics of the 22 patients with advanced BTC enrolled between March 2007 and January 2010. All enrolled patients except three exhibited pathologically confirmed BTC. Fourteen patients (64%) had experienced recurrence after surgery. The median baseline sum of longest diameter (BSLD), which was evaluated as a measure of tumor volume according to RECIST version 1.0, was 6.5 cm (range 2.0–42.0 cm). As subset analyses, the median BSLDs of non-resectable (locally advanced and metastatic) and recurrent patients were 18.2 cm (range 5.9–42.0 cm) and 3.9 cm (range 2.0–15.2 cm), respectively (p < 0.01). The median duration of first-line chemotherapy was 5.9 months (95% CI, 4.5–8.1 months). As for third-line chemotherapy following the treatment for this study, seven patients (32%) received cisplatin-based combination chemotherapy (six patients received gemcitabine and cisplatin combination chemotherapy and one patient received S-1 and cisplatin combination chemotherapy). Three patients (14%) continued S-1 monotherapy despite tumor progression, and one patient (4%) was re-treated by gemcitabine monotherapy. All patients completed the study treatment, and 10 patients (45%) were still alive at the time of this analysis.
Efficacy
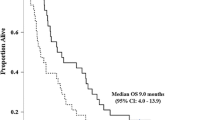
Five patients showed partial responses, for an overall objective response rate of 22.7%. The primary biliary sites of the patients who showed partial responses were three gallbladders, one intrahepatic bile duct and one ampulla of Vater (Fig. 1). Stable diseases were observed in six patients (27.3%), with an overall disease control rate of 50.0% (Table 2). One patient was moved to another hospital before tumor response could be assessed and was therefore designated “not evaluable”. The median overall survival time was 13.5 months (95% CI, 7.1–23.1 months), and the median time-to-progression was 5.4 months (95% CI, 2.6–17.2 months; Fig. 2). The 6-month progression-free survival and 1-year overall survival rates were 38.0% and 51.6%, respectively. The median overall survival time from first-line chemotherapy was 24.3 months (95% CI, 12.5–32.6 months).
Toxicity
A total of 82 cycles of S-1 monotherapy were delivered, with a median of two cycles per patient (range, 1–15 cycles). The dose intensity of S-1 was 92.1%. Table 3 presents the major adverse events that occurred during the study. No treatment-related deaths occurred. The major grade 3/4 adverse events included neutropenia (5%) and anemia (5%). The most common non-hematological toxicities were nausea (27%), anorexia (55%), and pigmentation (32%). There were two elderly patients (over 80 years old) enrolled in this study. These two patients could also take sufficient dose of S-1 (the dose intensities were 99.4% and 96.4%, respectively) and showed only grade 2 myelosuppressions and grade 1 anorexia.
Biliary events and drainages
Of the 22 patients, 14 (64%) had experienced recurrence after surgery, and eight patients (36%) had non-resectable (locally advanced and metastatic) disease. Among the patients with non-resectable BTC, two patients (25%) required drainage prior to treatment. These two patients were drained by uncovered self-expandable metallic stents. Two recurrent cases (14%) required drainage prior to treatment: one was drained using a covered, self-expandable metallic stent alone, and the other was drained by percutaneous transhepatic biliary drainage. After initiation of treatment, five patients (23%) experienced a total of eight biliary events. All patients except one recovered from biliary events and were able to restart the study treatment.
Discussion
This multicenter phase II study confirms that S-1 monotherapy is a feasible and moderately efficacious second-line treatment for advanced BTC. The response rate and disease control rate were 22.7% and 50.0%, respectively. The median overall survival and time-to-progression were 13.5 months and 5.4 months, respectively. Supportive to our study, another study on second-line S-1 monotherapy was recently reported at the 2010 annual meeting of the American Society of Clinical Oncology [18]. That study showed tumor response and disease control rates of 7.5% and 62.5%, respectively. The median overall survival and time-to-progression were 7.3 months and 2.5 months, respectively. That study was only reported at the annual meeting and full paper has not been published yet. Therefore the detail of that study was still unclear. However, these two studies indicate that second-line S-1 monotherapy could be a good treatment option in patients with advanced BTC refractory to gemcitabine.
The primary tumor site was different between these two studies. In the current study group, more than half of the patients were intra-hepatic cholangiocarcinoma patients, while there were more patients with gallbladder cancer and extra-hepatic cholangiocarcinoma in the study reported by Suzuki et al. [18]. Previous studies have reported a worse prognosis for gallbladder cancer and a better prognosis for extra-hepatic cholangiocarcinoma [2, 19]. On the other hand, we previously reported that the prognosis for patients receiving chemotherapy depends on tumor volume rather than on the primary tumor site [20]. Non-resectable gallbladder cancers are usually diagnosed at the advanced stage with an already large tumor volume, while extra-hepatic cholangiocarcinomas were diagnosed with small tumor volumes, even in non-resectable cases. Unfortunately, tumor volume information was lacking from the study reported by Suzuki et al., so it is difficult to compare these two studies in detail.
Recurrent cases usually show better prognoses [7], and they also have smaller tumor volumes. In fact, the tumor volume of our recurrent cases was significantly smaller than that of the non-resectable cases (18.2 cm versus 3.9 cm, p < 0.01). This study showed a fairly good anti-tumor effect compared with previous reports, which could have resulted from the fact that more recurrent patients were enrolled in this study (64%). Compared with our previous feasibility study of gemcitabine and cisplatin combination chemotherapy [11], the tumor volume of this study was quite small. The patients enrolled in this study were refractory only to gemcitabine and were administered S-1 as a second-line chemotherapy, while the patients enrolled in our feasibility study of gemcitabine and cisplatin combination chemotherapy were refractory to both gemcitabine and S-1 and were administered gemcitabine and cisplatin combination chemotherapy as a second-line or third-line chemotherapy. Based on the difference in patient characteristics, the BSLDs were quite different between this study of S-1 monotherapy and the feasibility study of gemcitabine and cisplatin combination chemotherapy (6.5 cm versus 17.0 cm, respectively) [11]. In consequence, the smaller tumor volume in this study could have affected the better tumor effect of S-1 monotherapy in this study. The limitation of this study was the small number of patients enrolled. However, it is still valuable as one of only a few studies to examine the effects of chemotherapy on refractory BTC (Table 4).
The results of the ABC-02 study led to the establishment of gemcitabine and cisplatin combination chemotherapy as the standard first-line treatment for advanced BTC [6]. However, no standard chemotherapy has yet been established for second-line treatment. The current study demonstrates that second-line S-1 monotherapy could be a good treatment option in patients with advanced BTC refractory to gemcitabine.
References
National Cancer Center. Cancer statistics in Japan 2009. http://www.fpcr.or.jp/publication/statistics.html. Accessed 1 August, 2010.
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96(6):896–902
Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E et al (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23(10):2332–2338
Andre T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O et al (2008) Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer 99(6):862–867
Sasaki T, Isayama H, Nakai Y, Ito Y, Kogure H, Togawa O et al (2010) Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 65(6):1101–1107
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–1281
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A et al (2010) Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103(4):469–474
Lee MA, Woo IS, Kang JH, Hong YS, Lee KS (2004) Gemcitabine and cisplatin combination chemotherapy in intrahepatic cholangiocarcinoma as second-line treatment: report of four cases. Jpn J Clin Oncol 34(9):547–550
Paule B, Herelle MO, Rage E, Ducreux M, Adam R, Guettier C et al (2007) Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 72(1–2):105–110
Oh SY, Jeong CY, Hong SC, Kim TH, Ha CY, Kim HJ, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 2010 (in press).
Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H et al (2010) Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs (in press).
Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C (2004) Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 91(10):1769–1774
Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T et al (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62(5):849–855
Park I, Lee JL, Ryu MH, Kim TW, Chang HM, Lee SS et al (2009) Efficacy and safety of S-1 monotherapy in patients with advanced biliary tract adenocarcinoma: retrospective analysis of 162 patients. Oncology 76(2):126–132
Sasaki T, Isayama H, Yashima Y, Yagioka H, Kogure H, Arizumi T et al (2009) S-1 monotherapy in patients with advanced biliary tract cancer. Oncology 77(1):71–74
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38(1):143–151
Suzuki E, Ikeda M, Okusaka T, Nakamori S, Ohkawa S, Nagakawa T et al (2010) A multicenter phase II of S-1 in gemcitabine-refractory biliary tract cancer. Proc Am Soc Clin Oncol 28:15s, abstract no: 4145
Yonemoto N, Furuse J, Okusaka T, Yamao Funakoshi A, Ohkawa S et al (2007) A multi-centerretrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 37(11):843–851
Sasaki T, Isayama H, Nakai Y, Togawa O, Kogure H, Ito Y et al (2010) Prognostic factors in patientswith advanced biliary tract cancer receiving chemotherapy. Cancer Chemother Pharmacol (in press)
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasaki, T., Isayama, H., Nakai, Y. et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 30, 708–713 (2012). https://doi.org/10.1007/s10637-010-9553-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9553-9