Summary
The histone deacetylase inhibitor (HDACI) butyroyloxymethyl diethylphosphate (AN-7) has been shown to synergize doxorubicin (Dox) anticancer activity while attenuating its cardiotoxicity. In this study we further explored the selectivity of AN-7’s action in several cancer and normal cells treated with anticancer agents. The cells studied were murine mammary 4T1, human breast T47D and glioblastoma U251 cancer cell lines, neonatal rat cardiomyocytes, cardiofibroblasts and astrocytes, and immortalized cardiomyocyte H9C2 cells. Cell death, ROS production and changes in protein expression were measured and in vivo effects were evaluated in Balb-c mice. AN-7 synergized Dox and anti-HER2 cytotoxicity against mammary carcinoma cells with combination indices of 0.74 and 0.79, respectively, while it protected cardiomyocytes against their toxicity. Additionally AN-7 protected astrocytes from Dox-cytoxicity. Cell-type specific changes in the expression of proteins controlling survival, angiogenesis and inflammation by AN-7 or AN-7+Dox were observed. In mice, the protective effect of AN-7 against Dox cardiotoxicity was associated with a reduction in inflammatory factors. In summary, AN-7 augmented the anticancer activity of Dox and anti-HER2 and attenuated their toxicity against normal cells. AN-7 modulation of c-Myc, thrombospondin-1, lo-FGF-2 and other proteins were cell type specific. The effects of AN-7, Dox and their combination were preserved in vivo indicating the potential benefit of combining AN-7 and Dox for clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The steady state of histone acetyltransferases (HATs) and histone deacetylases (HDACs) activities serves as a key regulatory mechanism for gene expression and controls numerous developmental and diseases processes [1]. Pharmacological inhibition of HDAC activity mediated by HDAC inhibitors (HDACIs) provides a therapeutic benefit for a variety of diseases and disorders [2, 3]. In cancer, the recruitment of HDAC is associated with histone deacetylation and chromatin condensation, which can lead to the transcriptional repression of tumour suppressor genes and the proliferation of cancer cells. HDACIs are potential anticancer agents, some of which are under preclinical investigation. Others have recently gained approval for clinical use as anticancer agents [4].
We have developed and studied histone deacetylase inhibitory prodrugs of butyric acid (BA) that upon intracellular hydrolytic degradation release acids and aldehydes. These compounds have been shown to modulate gene expression, induce histone hyperacetylation, differentiation, and apoptosis of cancer cells in vitro, ex-vivo and in vivo [5]. Among these prodrugs, butyroyloxymethyl diethyl phosphate (AN-7) was shown to be the most efficacious anti-cancer agent. At doses of 25–50 mg/kg, it inhibited tumor growth, angiogenesis and metastasis in various mouse cancer models [6–8]. Importantly, while AN-7 interacted synergistically with doxorubicin (Dox) in killing cancer cells, as was demonstrated by Median Effect Analysis (MEA) [9], it also protected neonatal rat cardiomyocytes and adult mice against Dox toxicity [10, 11].
Herein we investigated the effects of AN-7 together with Dox or with the antibody against HER2 receptor on cancer cell viability. The protective effect of AN-7 on primary cultures of cardiomyocytes, cardiofibroblasts and astrocytes, as well as on mice receiving a single high dose of Dox, was examined.
Materials and methods
Compounds and reagents
AN-7 was synthesized as described [12]. Doxorubicin hydrochloride, 2 mg/mL was obtained from Teva (Petach-Tikva, Israel); bis-benzimide (Hoechst fluorescent probe), N-acetyl–L-cysteine (NAC, Sigma, USA) and 2′,7′-dichlorofluorescencin diacetate were purchased (DCF-DA, Sigma, USA).
Antibodies
Rabbit polyclonal primary antibodies were against: c-Myc (Cell Signaling, USA); lo-FGF-2 (Santa Cruz Biotechnology, USA); hypoxia-inducible factor-1 α (HIF-1α) (Chemicon, Hampshire, UK); HO-1 (Stressgen, Ann Arbor, MI, USA). Mouse monoclonal antibodies were against: phospho-H2AX (Ser 139) (BioLegend, San Diego, CA, USA); TSP-1 (Lab Vision, Fremont, CA, USA); β-actin (MP Biomedicals, Aurora, Ohio, USA); HER2 (Ab-4) (Calbiochem, Darmstadt, Germany). Secondary antibodies: Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and HRP-goat anti-mouse IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Cell cultures
Primary cultures of neonatal rat ventricular cardiomyocytes and cardiofibroblasts were prepared as described [13]. Second transfer cardiofibroblasts were used for the experiments. Astrocyte cultures were prepared from brains of the same neonatal rats as described [14]. The murine mammary breast carcinoma 4T1 (CRL-2539) and the human breast carcinoma T47D (HTB-133™) and embryonic rat heart H9C2 (CRL-1446) cell lines were obtained (ATCC, Rockville, MD, USA). U251 MG human glioma cell line was obtained as described (University of California, San Francisco brain tissue bank). Cells were grown in DMEM with 10% FCS and 2 mM L-glutamine. All cells were grown in the presence of 100 units/mL penicillin, 100 μg/mL streptomycin, 12.5 un/mL nystatin (Biological Industries, Beit-Haemek, Israel) and incubated in a humidified atmosphere of 5% CO2 95% air at 37°C.
Cell treatment
Cells were grown to 70–80% confluency prior to drug or vehicle treatment. AN-7 was dissolved in PBS followed by dilution with the medium. The antioxidants NAC was dissolved in PBS and neutralized to pH 7.45, and added to the growth medium 30 min prior to treatment with the anticancer agents. Doxorubicin was dissolved medium. DCF-DA was dissolved in 100% ethanol to 50 mM followed by dilution with the medium. The concentration of the agents used in the different assays were optimize to the specific assay, cell tested and the duration of exposure of the cells to the agent.
Viability assays
Cell viability was measured using Hoechst fluorescent reagent at 390–460 nm with a FluoStar fluorometer. The % of cells undergoing apoptosis or necrosis was evaluated by double-staining them with annexin V-FITC and propidium iodide (PI) and subjecting them to flow cytometry analysis (FACSCalibur cytometer, Becton Dickinson), as was described [11].
Reactive Oxygen Species (ROS)
ROS positive cells following treatment as specified were assessed by the ROS-specific probe DCF-DA and visualized under a fluorescence microscope (Olympus Tokyo, Japan) using excitation/emission filters of 485/540 nm. Photographs were captured with an Olympus DP50 digital camera system. The % of cells positive for ROS was determined using ImagePro Plus 5.1 software (Media Cybernetics’ Corporation, USA). Cells treated as specified were also analyzed for ROS positively stained cells by staining them with DCF-DA and analyzing (104 cells) by FACS at a 488 nm excitation beam. The % of cells producing ROS (% DCF (+)) was determined with CellQuest software (BD Biosciences, USA).
Western blot analysis
Cells were treated as was specified, washed in PBS and suspended in lysis buffer [7]. Hearts from mice were homogenized (Polytron; Kinematica, Lucerne, Switzerland) in Lysis buffer. Protein content was determined (BCA kit, Pierce, USA) and aliquots were taken for Western blot analysis loading 30–45 μg protein per lane, depending on the cell type. Separation of pH2AX (14 kDa) and lo-FGF-2 (19 kDa), c-Myc (57 kDa) and HO-1 (32 kDa), TSP-1 (170 kDa) and HIF-1α, (120 kDa) was performed on 15%, 12%, and 7.5% polyacrylamide gels, respectively. Reactive bands were detected by ECL, quantified by densitometry and normalized to that of actin.
In vivo studies
Animal experiments were conducted according to the NIH Laboratory Animal Care Guidelines approved by the Tel Aviv University Committee for Animal Experimentation.
For acute toxicity study, female Balb-c mice, 8–10 weeks old (Harlan, Israel) were given a single ip dose of 20 mg/kg of Dox, or the same dose of Dox together with 25 mg/kg of po AN-7 or vehicle. AN-7 or vehicle were given twice more during the following five days. On the sixth day, blood was drawn from the eyes, of slightly anesthetized mice (by isoflurane inhalation), collected in EDTA containing tubes and centrifuged for 10 min at 1000 g. Coagulation was allowed by 30 min incubation at room temperature. Lysates of hearts were homogenized as described and the plasma and heart samples were analyzed using mouse TNF-α (BD OptEIA, CA) and INF-γ (R&D Systems, Minneapolis, MN) ELISA kits [15].
Statistical analysis
A comparison of drug-treatment groups was conducted by t-test (Microsoft Excel 2000 or JMP 5.1). The Median Effect Analysis (MEA) was used for drugs interaction and the combination index (CI), was calculated as described [9, 11].
Results
AN-7 interacts synergistically with Dox in reducing the viability of 4T1 and U251 cancer cells
The effect of Dox or AN-7 on the viability of 4T1 murine mammary carcinoma and U251 human glioblastoma cell lines was evaluated after 72 h of treatment using Hoechst viability assay. The average concentrations causing 50% reduction in cell viability (IC50) were determined from the formula of the best fitted curve of the percent survival versus drug concentration (≥3 independent dose-response titrations). The combination studies were then conducted at the IC50 ratio of the drugs, according to Chou MEA, and the CI were calculated [9, 11]. The IC50 of Dox in the combination treatment was reduced by ~2-fold (from 18.7 ± 1.8 to 7.5 ± 0.9 nM in 4T1 and from 21.0 ± 1.6 to 11.0 ± 2.3 nM in U251cells) and that of AN-7 by ~3-fold (from 66.1 ± 6.6 to 22.6 ± 2.9 μM in 4T1 and from 71.3 ± 6.1 to 24.2 ± 6.8 μM in U251 cells). The calculated CI was 0.74 in 4T1 and 0.86 in U251 cells, demonstrating synergism between AN-7 and Dox in inducing cell death (Fig. 1).
Effect of AN-7, DOX and AN-7+Dox on the viability of 4T1 murine mammary and U251 MG human glioma cell lines. 4T1 (2 × 103/well) and U251 MG (3 × 103/well) cells were seeded in 96 well-plates, incubated overnight, and then treated with AN-7 (5–120 μM), Dox (10–100 nM) or their combination at the a 1:3000 molar ratio. After 72 h of treatment the viability of the cells was determined by the Hoechst assay and the IC50 values were calculated from the best fitted curve. Representatrive experiments of the % survival of the cells as a function of Dox concentrations (a) or AN-7 concentrations (b) either as a single agent or in combination with Dox are shown. The average IC50 values ± SEM of 3 independent experiments performed in triplicate wells of each drug as single agent and in the combination is shown in the inserts
Cell-type selective effects of AN-7 or AN-7+Dox
Primary cultures of cardiomyocytes, cardiofibroblasts and astrocytes, isolated from neonatal rats, and 4T1 and U251 cell lines, were treated as specified and analyzed to measure mortality. The distribution of annexin V-FITC and PI positively stained cells (Fig. 2a) and the average percentage of cell mortality (Fig. 2b) are shown. Dox treatment significantly reduced the viability of all cell types. In 4T1 and U251 cells Dox increased the percentage of early (annexin V-FITC positive, PI negative) and late (annexin V-FITC, PI positive) apoptotic cells. However, in primary cultures of cardiomyocytes, cardiofibroblasts and astrocytes, Dox increased to a large extent the percentage of the necrotic cells (PI positive, annexin V-FITC negative), and to a lesser extent the percentage of apoptotic cells. Treatment with AN-7, as a single agent, increased mortality of 4T1, U251 cells and cardiofibroblasts. Yet, it did not affect the viability of cardiomyocytes or astrocytes. Treatment with the combination of AN-7 and Dox (AN-7+Dox) resulted in a response that was cell type specific. In accordance with the viability test described in Fig. 1, AN-7+Dox treatment, compared to treatment with Dox or AN-7 alone, significantly augmented 4T1 and U251 cell death (p < 0.05). In cardiofibroblasts, Dox, and to a lesser degree AN-7, induced cell mortality that increased in an additive manner by AN-7+Dox treatment. AN-7 as a single agent did not affect the viability of cardiomyocytes and astrocytes, whereas Dox induced their mortality mostly by necrosis. Conversely, co-treatment of cardiomyocytes and astrocytes with AN-7+Dox resulted in a sharp decline in the mortality of cardiomyocytes (from 36% to 19%, p < 0.02) and astrocytes (from 31% to 14%, p < 0.05), indicating that AN-7 protected these cells against Dox toxicity.
Effect of AN-7 and Dox as single agents and in combination with Dox on cell death. Cell, 4T1, U251 MG, primary astrocytes, cardiofibroblasts and cardiomyocytes were treated as follows: AN-7 50 μM for 24 h; Dox 200 nM for 5 h; combination of AN-7+Dox where cells were exposed to AN-7 for 1 h, then 200 nM Dox was added for 5 h, Dox was removed and AN-7 was added back for additional 18 h. After treatments the cells were trypsinized, stained with annexin V-FITC and PI and subjected to FACS analysis. Representative dot-plots are shown (a) and the average percent of dead cells, Mean±SEM of three independent experiments were plotted (b). *p < 0.05 vs. Dox treatment
Dox induced cell death is associated with ROS production. The effects of treatments with Dox, AN-7 or their combination on the intracellular production of ROS were examined in 4T1 and U251 cells, cardiomyocytes, cardiofibroblasts and astrocytes. ROS-producing cells were detected using the cell-permeable probe DCF-DA that becomes fluorescent upon oxidation. ROS production was assessed microscopically or by FACS analysis. In 4T1 cells, treatment with AN-7 or Dox elevated the number of ROS positively stained cells, the combination of AN-7+Dox significantly increased their number compared to treatment with each agent separately (p ≤ 0.03) (Fig. 3a and b). In cardiofibroblasts, Dox increased the percent of ROS positive cells to a greater extent than AN-7, and treatment with AN-7+Dox resulted in a level of ROS that was similar to that observed with Dox alone. In cardiomyocytes, treatment with Dox dramatically increased ROS positively stained cells (from 0.2 ± 0.1 to 41.7 ± 4.9%), whereas AN-7 did not induce ROS production and treatment with AN-7+Dox abrogated Dox-induced ROS production.
Effect of AN-7, Dox and AN-7+Dox on ROS-production. The effect of AN-7, Dox and AN-7+Dox on the production of ROS in 4T1 cell line, neonatal rat cardiofibroblasts and cardiomyocytes, was examined microscopically. Cells were treated as follows: 100 μM AN-7 for 6 h; 2 μM Dox for 5 h; or, their combination. In the combination mode AN-7 was added 1 h before Dox. To detect ROS positive cells, the cultures were stained with 20 μM DCF-DA and images were capture at × 200 magnification under visible light (A) or fluorescence illumination (B). Representative images of cell cultures are shown (a). Average count of cells positively stained with DCF-DA (Mean±SEM) from five fields of each treatment performed in duplicates in two independent experiments (n = 20) was done using ImagePro Plus 5.1 (b). *p < 0.01 all indicated treatments vs. Dox
In another series of experiments we examined the effect of Dox, AN-7 and AN-7+Dox on ROS production and viability of U251, H9C2 and astrocytes in the absence or presence of the radical scavenger N-acetyl-cysteine (NAC). Dox increased the percent of ROS positive cells in all of the tested cell types, while AN-7 increased ROS production only in U251. The combination of AN-7+Dox significantly augmented ROS production in the cancer cells while in the non-cancer cells it significantly attenuated it. In the presence of NAC, ROS production was abrogated in all of the tested cells (Fig. 4a and b). The viability of the cells under the same experimental conditions was assessed by FACS analysis and showed that in the absence of NAC, the correlation between ROS production and cell mortality, was observed. However, while NAC decreased ROS levels in the cells, it did not always affect their viability. In the glioblastoma cell line, NAC did not diminish Dox-induced mortality. However, it partially reduced the mortality induced by AN-7 or AN-7+Dox (Fig. 4c). Therefore, it is likely that the reduction in cell mortality in the combination treatment resulted from the attenuation of AN-7 induced toxicity. In normal cells, NAC partially reduced Dox-toxicity and it did not affect the protection imparted by AN-7 in the combination treatment. The data indicates that compared to NAC, the protection imparted by AN-7 was greater, and selectively affected only normal cells.
Effect of AN-7, Dox and AN-7+Dox on ROS production and cell death in the absence or presence of NAC. U251 and H9C2 cell lines and primary rat astrocytes were treated in the absence or the presence of 5 mM NAC as follows: vehicle; 50 μM AN-7 for 6 h; 200 nM Dox for 5 h and their combination (where Dox was added 1 h after AN-7). At the end of the incubations DCF-DA was added (10 μM) and washed out after 30 min, the cells were suspended in PBS and subjected to FACS analysis. Representative histograms of cells distribution, where an increase in ROS production shifts the peak to the right, are shown (a). The average % of cells stained positively with DCF-DA from three independent experiments (for each experimental point 10,000 cells were analyzed) was calculated (b). The viability of the cells treated as described above and then incubated for a total of 24 h, stained with annexin V-FITC/PI and analyzed by FACS. Bar-graphs presentation of the average percent of total dead cells from three independent experiments is shown (c). *p < 0.05 indicates AN-7+Dox vs. Dox; # p < 0.05 indicates AN-7+Dox+NAC vs. Dox+NAC
Cell-selective regulation of protein expression by AN-7 or AN-7+Dox
Phosphorylation of histone H2AX (pH2AX) is a marker of double-strand breaks (DSBs) [16]. Treatment of 4T1 cells with AN-7 or Dox increased the level of pH2AX and treatment with AN-7+Dox resulted in an even higher level of pH2AX after 6 h, and after 24 h it further increased, indicating progressive DSB formation (Fig. 5a). In cardiofibroblasts, Dox or AN-7+Dox treatments were effective in the induction of pH2AX after 6 and 24 h, whereas only after 24 h of AN-7 treatment there was a small increase in pH2AX. In cardiomyocytes, Dox was the only agent that increased pH2AX; AN-7 alone had no effect, and when combined with Dox, the Dox-induced DNA damage was attenuated, providing further evidence for the protective effect of AN-7 on cardiomyocytes.
Modulation of proteins expression by AN-7, Dox or their combination in 4T1 cells, neonatal rat cardiofibroblasts and cardiomyocytes. Cells were treated for 5 or 6 h as follows: vehicle (C); 100 μM AN-7 for 6 h (A); 2 μM Dox for 5 h (D); the combination of AN-7+Dox (A+D), where 100 μM of AN-7 was given for 6 h, 1 h prior to and further 5 h following the addition of 2 μM Dox. The treatments for 24 h were done as follows: 100 μM AN-7 for 24 h; 2 μM Dox for 5 h and then incubated for additional 19 h in medium without Dox; combination of AN-7+Dox, where 100 μM AN-7 was given for 24 h, 1 h prior to 2 μM Dox that was removed after 5 h and the cells were incubated for additional 18 h in the presence of 100 μM AN-7 only. Lysates of 4T1 cells were loaded at 30 μg protein/slot for the detection of pH2AX, c-Myc, HO-1 and TSP-1 and at 45 μg protein/slot for the detection of lo-FGF-2 on SDS gels. Lysates of cardiomyocytes or cardiofibroblasts were loaded at 40 μg protein/slot. Western-blot analyses using the appropriate antibodies are shown for: pH2AX (a); c-Myc (b); lo-FGF-2 (c); HO-1 (d) and TSP-1 (e). Actin immunolabeling is shown as loading control
The c-Myc protein has been shown to promote survival and angiogenesis [17]. In 4T1 cells, after 6 h of treatment with AN-7, Dox and their combination, c-Myc expression was reduced and treatment for 24 h further diminished its level. In cardiofibroblasts, AN-7 had a negligible effect on the c-Myc level, while treatment with Dox or AN-7+Dox, resulted in a notable reduction in its expression. In cardiomyocytes, compared to vehicle treated cells, Dox decreased the expression of c-Myc and AN-7 increased it. After 6 h of treatment with AN-7+Dox, c-Myc expression was similar to that of the control cells, but after 24 h it was substantially augmented (Fig. 5b).
Low molecular weight fibroblast growth factor-2 (18 kDa, lo-FGF-2) is a potent angiogenic stimulator [18]. In 4T1 cells, the downregulation of its expression was noted after 24 h of treatment with AN-7+Dox inducing the greatest and Dox the least, suppression. In cardiofibroblasts, only Dox decreased lo-FGF-2 expression after 6 h of treatment but at 24 h, Dox and AN-7+Dox substantially reduced lo-FGF-2 expression. At both time points, lo-FGF-2 expression was unaffected by AN-7. In cardiomyocytes, AN-7 had no effect on lo-FGF-2, Dox decreased its level in a time dependent manner, having the greatest effect after 24 h, while treatment with AN-7+Dox prevented the decrease (Fig. 5c).
In cancer cells, heme oxigenase-1 (HO-1) was shown to suppress growth, invasion and migration [19] while in the myocardium it conferred protection [20, 21]. In 4T1 cells, AN-7, Dox or AN-7+Dox increased the expression of HO-1 in a time-dependent manner. After 24 h, AN-7 and AN-7+Dox caused a considerably greater increase in its expression than Dox alone. In cardiofibroblasts, the treatments decreased HO-1 expression in a time dependent manner, where Dox was the most and AN-7 was the least effective. In cardiomyocytes AN-7 increased, whereas Dox, in contrast to its effect in 4T1 cells, decreased the expression of HO-1. AN-7+Dox treatment elicited an intermediate effect on HO-1 expression; it was less than that imparted by AN-7 and more than that found in vehicle treated cells (Fig. 5d).
Thrombospondin-1 (TSP-1) is an inhibitor of neo-vascularization and tumorigenesis [22]. In 4T1 cells the TSP-1 level was unchanged by Dox, whereas AN-7 and AN-7+Dox caused a substantial increase in its expression. In cardiofibroblasts, treatment with Dox enhanced TSP-1 level after 6 h and to a greater extent after 24 h and the combination treatment enhanced it only after 24 h. It should be noted that TSP-1 was undetected in cardiomyocytes (Fig. 5e).
AN-7 augmented the anticancer activity of antibody against HER2 (Ab-4) while it protected cardiomyocytes against its toxicity
The viability of T47D, a human breast carcinoma cell line expressing normal levels of the HER2 receptor was reduced in a dose-dependent manner by Ab-4, a monoclonal antibody against this receptor [23]. The IC50 for the inhibition determined by Hoechst assay after 72 h was 3.4 μg/ml. MEA of AN-7 and Ab-4 at IC50 ratio, revealed the synergistic interaction with CI of 0.79 (Fig. 6a).
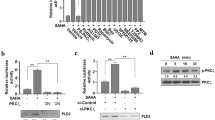
Effect of AN-7, Ab-4 or their combination on the viability of human breast cancer cells T47D and neonatal rat cardiomyocytes. T47D, 4 × 103/well, were seeded in 96 well-plates and after 24 h they were treated with AN-7 (5–200 μM), Ab-4 (0.2–4 μg/mL) or their combination at a ratio of 50:1 (μM: μg/mL). Cell viability after 72 h was determined by the Hoechst assay and the average IC50 values ± SEM of 3–4 independent experiments performed in triplicates were calculated (a). The primary neonatal rat cardiomyocytes were treated for 24 h as follows: vehicle (PBS); 50 μM AN-7; 4 μg/mL Ab-4, and their combination. At the end of treatment the cells were trypsinaized, stained with annexin V-FITC and PI and analyzed by FACS. Representative dot-plots and the average percent of dead cells ± SEM of 3-4 independent experiments are shown *p < 0.05 of all treatments compared to Ab-4 treatment (b). ROS formation in cardiomyocytes that were grown as in above and were treated for 24 h with 6 μg/ml Ab-4, 50 μM AN-7, and their combination. The cultures were then stained with 20 μM DCF-DA and ROS positive cells were quantified as in Fig. 3. Shown are representative images (left) and calculated averages (right) of two independent experiments (n = 10) (c). Mean±SEM, *p < 0.001 for AN-7+Ab-4 vs. Ab-4
In primary cultures of cardiomyocytes, Ab-4 increased the percentage of apoptotic cells from 7 ± 1.5% to 22 ± 3% (p = 0.006), while in combination with AN-7, the toxicity of Ab-4 was significantly attenuated (from 22 ± 3% to 11.3 ± 2.8%, p = 0.03) (Fig. 6b). Consistent with the viability data, Ab-4 dramatically increased the number of cardiomyocytes stained positively for DCF-DA, while the addition of AN-7 significantly reduced it (from 32.1 ± 5 to 13.7 ± 3.7, p = 0.001) (Fig. 6c). The data indicated that the selective enhancement of AN-7’s anticancer activity and protection against cardiotoxicity was not limited to Dox.
The in-vivo protective effect of AN-7 against cardiotoxicity induced by a single high dose of Dox
The protective effect of AN-7 against Dox cardiotoxicity was examined in a mouse model for acute cardiotoxicity induced by a single high-dose of Dox, which caused a sharp decline in body weight. In the first two days, mice treated with Dox or AN-7+Dox showed a similar decline in body weight, compared to vehicle-treated mice. From day three onward, the weight of the AN-7+Dox treated mice was significantly higher than that of the animals treated with Dox only (Fig. 7a). Compared to vehicle-treated mice, the levels of TNF-α in Dox-treated mice, were significantly higher in the heart (839.6 ± 66.4 pg/mg vs. 408.7 ± 66.4 pg/mg) and in the plasma (423.7 ± 111.2 pg/mL vs. 29.6 ± 5.4 pg/mL) (Fig. 7b). Co-treatment with Dox and AN-7 prevented the rise in TNF-α in mice. Comparable results were obtained for INF-γ levels in the hearts and plasma of these mice (Fig. 7c).
Effect of AN- 7 on the acute cardiotoxicity of Dox in vivo. Female Balb-c mice were treated as follows: a single ip dose of 20 mg/kg Dox (n = 13); the combination of the single 20 mg/kg ip Dox dose and 25 mg/kg po AN-7 given thrice during the 6 days of the experiment (n = 12) and vehicle (n = 9). Animal body weight was measured at the indicated days. *p < 0.05, AN-7+Dox vs. Dox (a). Detection of TNF-α and INF-γ levels in the plasma and the heart of mice was determined on the sixth day of the experiment. The levels of TNF-α (b) and INF-γ (c) in heart and plasma Mean±SEM, n = 5/group, *p < 0.02 treated vs. untreated and # p < 0.02 AN-7+Dox vs. Dox treated. Lysates (40 μg protein/well) from the hearts of untreated and treated animals were resolved on 7.5% (HIF-1α), 12% (c-Myc or HO-1) and 15% (pH2AX) SDS-polyacrylamide gels and were subjected to Western blot analyses (d). Expression of c-Myc, pH2AX, HO-1 and HIF-1α in hearts of untreated (control) and treated animals was quantified by densitometry and normalized to actin. Mean±SEM, n = 5/group, #p < 0.05 treated vs. untreated; *p < 0.01 AN-7+Dox treated vs. Dox treated (e)
Extracts of the hearts subjected to Western blot analyses revealed that Dox treatment decreased the expression of c-Myc compared to that seen in vehicle-treated mice, whereas AN-7+Dox treatment enhanced its expression level above that seen in vehicle-treated mice. Since c-Myc is a survival factor [24], these observations are consistent with the protective role of AN-7. Dox treatment increased the level of pH2AX in the hearts ~2.5-fold as compared to that in hearts of the untreated mice (Fig. 7d and e). Treatment with AN-7+Dox prevented the rise in pH2AX, indicating that AN-7 protected the heart from Dox-induced DNA damage. The levels of the antioxidant enzyme, HO-1, were 2.3-fold lower in the hearts of Dox treated mice compared to the control group, and were preserved in mice treated with AN-7+Dox. The expression of HIF-1α a transcription factor regulating many genes under hypoxic stress [25], was significantly higher in the hearts of mice treated with AN-7+Dox, as compared to the hearts of vehicle-treated or Dox-treated mice (Fig. 7e).
Discussion
The clinical efficacy of Dox and the antibody against HER2 receptor (anti-HER2), commonly used for the treatment of breast carcinoma is limited by cumulative, dose-dependent cardiotoxicity [26, 27]. Since we have shown that AN-7 protected cardiomyocytes against Dox toxicity [10], in this study we address the question of whether the combinations of AN-7 and Dox as well as AN-7 and anti-HER2, will display reduced toxicity in normal cells as well as increased anticancer activity against cancer cells. Moreover, we aimed to characterize some of the specific biological changes associated with the response to the treatments in the different cell types.
AN-7 induced apoptosis in mammary cancer and glioblastoma cell lines and to a lesser extent in cardiofibroblasts, yet it did not reduce the viability of cardiomyocytes or astrocytes. The combination of AN-7+Dox caused the following opposing effects: In cancer cells, it augmented cell death in a synergistic mode; in cardiofibroblasts it affected cell mortality in an additive manner; and in cardiomyocytes and astrocytes, it significantly attenuated cell death. Moreover, AN-7 interacted in synergy with anti-HER2 in inducing human breast carcinoma cell death. Concomitantly AN-7 also protected cardiomyocytes against anti-HER2 toxicity. These findings indicate that the protective effect of AN-7 is more general and it is not limited to a single injurious factor. The observation that AN-7 also protected astrocytes against Dox-toxicity, suggests that its protective effect is not restricted to a particular type of normal cell and it may protect CNS functions from symptoms associated with cognitive-dysfunction, frequently found among cancer survivors after chemotherapy.
Cardiofibroblasts are the main source of extracellular matrix in the myocardium, to which growth factors and cytokines attach and thereby control the neighboring cells [28]. Under pathological hypertrophy cardiofibroblasts contribute to heart failure by generating interstitial fibrosis [29]. The addition of AN-7 to Dox treatment of cardiomyocytes, not only attenuated cell death significantly, but also changed the mechanism of cell death from necrotic to apoptotic. The importance of this shift, rests in the propensity of necrosis (but not apoptosis) to promote inflammation which exacerbates heart damage by causing cardiomyocyte dysfunction and inducing fibroblast proliferation [30]. These results are consistent with the reduction of the inflammatory cytokine levels in the heart and in the plasma of mice treated with Dox and AN-7 compared to mice treated with Dox alone. Moreover, these data are also supported by our preliminary findings that AN-7 reduced heart-fibrosis in mice treated with Dox (preliminary unpublished data).
ROS play a role in the injury of cell membranes leading to the death of cancer and normal cells [31]. In correlation with the induction of cancer cell mortality, AN-7 or Dox increased ROS-production and AN-7+Dox further augmented it. AN-7, Dox or AN-7+Dox treatments increased ROS production in cardiofibroblasts. However, AN-7 abrogated ROS production by Dox in cardiomyocytes and in astrocytes contributing to the protection of the myocardium from ROS-induced inflammation and cardiomyocytes injury [31, 32]. The antioxidant NAC did not affect the outcome of Dox treatment but decreased AN-7 toxicity against cancer cells, indicating that the anticancer activity of Dox is independent of, and that of AN-7, depends on, ROS production. In either case, NAC did not contribute to the anticancer effects. In cardiomyocytes and astrocytes, the addition of NAC or AN-7 reduced Dox-induced cell mortality.. Compared to NAC, AN-7 imparted greater protection on normal cells, suggesting that an additional ROS-independent mechanism contributes to AN-7 protective activity. The advantage of AN-7 compared to NAC, rests in its ability to play a dual role, eliciting an anticancer activity against cancer cells, and a protective activity toward normal cells.
Characterization of changes in protein modification or expression illuminated some of the underlying cellular changes responsible for the cell-type selectivity of AN-7. Phosphorylation of H2AX is an early and a sensitive marker for DNA damage [16]. In 4T1 cells, the antineoplastic activity of AN-7, Dox or AN-7+Dox is manifest in an increase of pH2AX level. In cardiofibroblasts, while AN-7 exerted a minimal effect, Dox and AN-7+Dox induced substantial H2AX phosphorylation. In cardiomyocytes AN-7 inhibited H2AX phosphorylation, consistent with its protective effects against Dox-induced mortality.
The transcription modulator protein c-Myc plays an important role during embryogenesis of normal tissues and in pathological processes of tumorigenesis [17]. Multiple studies have demonstrated its oncogenic potential. Herein we demonstrate that the suppression of c-Myc expression in 4T1 cells by AN-7 and Dox, was associated with the upregulation of the angiogenic inhibitor TSP-1 and the downregulation of lo-FGF-2, indicating anti-angiogenic effects. The repression of TSP-1 expression by c-Myc, has been shown to involve the upregulation of miR17-92, a repressor of TSP-1 [33]. Therefore, the downregulation of c-Myc is expected to relieve TSP-1 of its repression, enabling it to act as a scavenger for matrix associated growth and angiogenic factors (e.g., lo-FGF-2, VFGF, SCF and HGF), thus limiting their bioavailability and activities [34]. Therefore, we propose that the anticancer effect of AN-7 was mediated, at least in part, by the suppression of c-Myc expression, which inhibits the growth of cancer cell and angiogenesis.
In cardiofibroblasts, AN-7 mildly enhanced c-Myc expression, while Dox and AN-7+Dox repressed it dramatically. Dox and AN-7+Dox treatments concomitantly increased TSP-1 and decreased lo-FGF-2 expression. Both changes indicated a decrease in the bioavailability and activity of pro-angiogenic factors, associated with increased mortality of cardiofibroblasts. These findings suggest that in the case of Dox induced damage to the heart, AN-7 attenuates Dox-induced fibrosis.
In cardiomyocytes, the opposite changes in protein expression were observed. The expression of c-Myc was augmented by AN-7 and abrogated by Dox, whereas treatment with AN-7+Dox resulted in the preservation of normal c-Myc expression. In the hearts of mice, c-Myc expression was downregulated by Dox and restored by AN-7+Dox treatment. Therefore, we propose that c-Myc expression plays an important role in protecting the heart, and that it does so by stimulating angiogenesis in the injured heart. This concept is supported by the vital role attributed to c-Myc in angiogenesis during embryogenesis [17], tumorigenesis [35] and the suppression of TSP-1, as discussed above. In addition, Dox repressed the expression of lo-FGF-2 in cardiomyocytes while AN-7 restored it. The protective effect of lo-FGF-2 following infarction injury has been described elsewhere [36]. This factor is a pro-survival contributor that protects the ischemic heart by functioning as a potent angiogenic agent promoting the proliferation of several cell types including cells with stem cell properties.
HO-1 has been shown to inhibit cancer cell proliferation and reduce their invasive capability [19, 37]. Herein, we demonstrate that AN-7 or AN-7+Dox treatments increased HO-1 expression in 4T1 cells, further contributing to the antineoplastic effect of these agents. In contrast to the enhancement of HO-1 expression in cancer cells, Dox and AN-7+Dox treatments repressed its expression in cardiofibroblasts. The meaning of this selective effect is unclear, yet it accentuates the specificity of the AN-7+Dox treatment.
In cardiomyocytes AN-7 elevated and Dox repressed HO-1 expression, while in the combination treatment, AN-7 attenuated Dox-repression of HO-1. The importance of HO-1 in the heart is apparent under stress conditions where it rapidly degrades pro-oxidant heme to carbon monoxide (CO) and biliverdin/bilirubin. CO inhibits inflammation [38] and biliverdin/bilirubin possesses anti-oxidative activity [39]. Consequently their combined action has been shown to prevent left ventricular hypertrophy [21].
Higher levels of TNF-α and INF-γ were detected in the heart and plasma of Dox-treated mice compared to that detected in mice treated with AN-7+Dox. As mentioned above, the attenuation of the inflammation can be attributed to the shift from necrotic to apoptotic death induced by AN-7+Dox in cardiomyocytes and in cardiofibroblasts. Also, the elevation of HO-1 expression by AN-7 may protect the heart by attenuating pro-inflammatory chemokine production [40].
The injury to the heart caused by Dox led to DSB formation as was reflected by the increase of pH2AX expression, whereas AN-7+Dox treatment imparted a protective effect, as was evident by the diminished pH2AX level. A significant increase in c-Myc, HO-1 and HIF-1-α expression in the hearts of mice treated with AN-7+Dox, compared to those treated with Dox, also indicated cardioprotection against Dox cardiotoxicity. Elevation of HIF-1-α enhances the transcription of important pro-angiogenic and protective factors including VFGF, VFGF-receptor, erythropoietin and HO-1 [41]. These changes may further contribute to the observed cardioprotective effect of AN-7
In conclusion, the biological effects exerted by AN-7+Dox and AN-7+ monoclonal antibody against HER2 in cancer cells compared to those imparted on cardiomyocytes, were cell type specific. The protective effect of AN-7 against Dox cardiotoxicity was associated with a reduction in inflammation, increased survival and angiogenesis. The effects of AN-7+Dox on cancer cells were inverse, i.e. inhibition of survival and angiogenesis. The association of AN-7+Dox treatment with selective changes in the expression of key proteins, participating in the control of viability, inflammation and angiogenic processes, contributes to the elucidation of the specific detrimental or protective cell-type processes involved.
Abbreviations
- HDAC:
-
histone deacetylase
- HDACI:
-
histone deacetylase inhibitor
- HAT:
-
histone acetyltransferase
- BA:
-
butyric acid
- Dox:
-
doxorubicin
- NAC:
-
N-acetyl–L-cysteine
- DCF-DA:
-
2′,7′-dichlorofluorescencin diacetate
- PI:
-
propidium iodide
- MEA:
-
Median Effect Analysis
- CI:
-
combination index
- pH2AX:
-
phosphorylation of histone H2AX
- DSBs:
-
double-strand breaks
- lo-FGF-2:
-
fibroblast growth factor-2
- TSP-1:
-
thrombospondin-1
- HO-1:
-
heme oxigenase-1
- SCF:
-
stem cell factor
- CO:
-
carbon monoxide
- TNF-α:
-
tumor necrosis factor-alpha
- INF-γ:
-
interferon-gamma
- HER2:
-
Human Epidermal growth factor Receptor 2
References
Rosato RR, Grant S (2003) Histone deacetylase inhibitors in cancer therapy. Cancer Biol Ther 2:30–37
Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ (2008) Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J 22:3549–3560
Haberland M, Montgomery RL, Olson EN (2009) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10:32–42
Prince HM, Bishton MJ, Harrison SJ (2009) Clinical studies of histone deacetylase inhibitors. Clin Cancer Res 15:3958–3969
Rephaeli A, Zhuk R, Nudelman A (2000) Prodrugs of butyric acid from bench to bedside: synthetic design, mechanisms of action, and clinical applications. Drug Dev Res 50:379–391
Blank-Porat D, Gruss-Fischer T, Tarasenko N, Malik Z, Nudelman A, Rephaeli A (2007) The anticancer prodrugs of butyric acid AN-7 and AN-9, possess antiangiogenic properties. Cancer Lett 256:39–48
Tarasenko N, Nudelman A, Tarasenko I, Entin-Meer M, Hass-Kogan D, Inbal A, Rephaeli A (2008) Histone deacetylase inhibitors: the anticancer, antimetastatic and antiangiogenic activities of AN-7 are superior to those of the clinically tested AN-9 (Pivanex). Clin Exp Metastasis 25:703–716
Rephaeli A, Entin-Meer M, Angel D, Tarasenko N, Gruss-Fischer T, Bruachman I, Phillips DR, Cutts SM, Haas-Kogan D, Nudelman A (2006) The selectivity and anti-metastatic activity of oral bioavailable butyric acid prodrugs. Investig New Drugs 24:383–392
Chou TC (1991) The median-effect principle and the combination index for quantitation of synergism and antagonism. In: Chou TC, Rideeout DC (eds) Synergism and antagonism in chemotherapy. Academic, New York, pp 66–102
Rephaeli A, Waks-Yona S, Nudelman A, Tarasenko I, Tarasenko N, Phillips DR, Cutts S, Kessler-Icekson G (2007) Anticancer prodrugs of butyric acid and formaldehyde protect against doxorubicin induced cardiotoxicity. Br J Cancer 96:1667–1674
Engel D, Nudelman A, Levovich I, Gruss-Fischer T, Rephaeli A (2006) Mode of interaction between the HDAC inhibitor AN-7 and doxorubicin in MCF-7 and resistant MCF-7/Dx cell lines. J Cancer Res Clin Oncol 132:673–683
Nudelman A, Gnizi E, Katz Y, Azulai R, Cohen-Ohana M, Zhuk R, Sampson SR, Langzam L, Fibach E, Prus E, Pugach V, Rephaeli A (2001) Prodrugs of butyric acid (lll) novel derivatives possessing increased aqueous solubility and potential for treating cancer and blood diseases. Eur J Med Chem 36:63–74
Shalitin N, Friedman M, Schlesinger H, Barhum Y, Levy MJ, Schaper W, Kessler-Icekson G (1996) The effect of angiotensin II on myosin heavy chain expression in cultured myocardial cells. In Vitro Cell Dev Biol Anim 32:573–578
Murphy S (1990) Generation of astrocytes cultures from normal and neoplastic central nervous system. In: Conn P (ed) Methods in neurosciences, vol 2. Academic Press Inc, San Diego, pp 33–47
Rephaeli A, Gil-Ad I, Aharoni A, Tarasenko I, Tarasenko N, Geffen Y, Halbfinger E, Nisemblat Y, Weizman A, Nudelman A (2009) Gamma-aminobutyric acid amides of nortriptyline and fluoxetine display improved pain suppressing activity. J Med Chem 52:3010–3017
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868
Shchors K, Evan G (2007) Tumor angiogenesis: cause or consequence of cancer? Cancer Res 67:7059–7061
Bikfalvi A, Klein S, Pintucci G (1997) Biological roles fibroblast growth factor-2. Endocr Rev 18:26–45
Lin CW, Shen SC, Hou WC, Yang LY, Chen YC (2008) Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol Cancer Ther 7:1195–1206
Koneru S, Penumathsa SV, Thirunavukkarasu M, Vidavalur R, Zhan L, Singal PK, Engelman RM, Das DK, Maulik N (2008) Sidenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: role of VEGF/angiopoietin-1. J Cell Mol Med 12:265–2664
Mito S, Ozono R, Oshima T, Yano Y, Watari Y, Yamamoto Y, Brydun A, Igarashi K, Yoshizumi M (2008) Myocardial protection against pressure overload in mice lacking Bach1, a transcriptional repressor of heme-oxygenase-1. Hypertension 51:1570–1577
Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP (1990) A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 87:6624–6628
Klapper LN, Vaisman N, Hurwitz E, Pinkas-Kramarski R, Yarden Y, Sela M (1997) A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER-2 blocks crosstalk with growth factor receptors. Oncogene 14:2099–2109
Dubois NC, Adolphe C, Ehninger A, Wang RA, Robertson EJ, Trumpp A (2008) Placental rescue reveals a sole requirement for c-Myc in embryonic erythroblast survival and hematopoietic stem cell function. Development 135:2455–2465
Kim J-W, Gao P, Liu Y-C, Semenza GL, Dang CV (2007) Hypoxia-Inducible Factor 1 and dysregulated c-Myc cooperatively induce Vascular Endothelial Growth Factor and metabolic switches hexokinase 2 and pyruvate dehydrogenese kinase 1. Mol Cell Biol 27:7381–7393
Ements LA, Davidson NE (2003) The follow-up of breast cancer. Semin Oncol 30:338–348
Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, Plummer CJ, Wardley AM, Verrill MW (2009) Management of cardiac health in trastuzumab- treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer 100:684–692
Zhao L, Eghbali-Webb M (2001) Release pro- and anti-angiogenic factors by human cardiac fibroblasts: effects on DNA synthesis and protection under hypoxia in human endothelial cells. Biochim Biophys Acta 1538:273–282
Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D (2005) NAD(P)H Oxidase 4 mediates Transforming growth factor-β1-indued differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97:900–907
Diwan A, Tran T, Misra A, Mann DL (2003) Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med 3:161–182
Vanlangenakker N, Berghe TV, Krysko DV, Festjens N, Vandenabeele P (2008) Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med 8:207–220
Hori M, Nishida K (2009) Oxidative stress and left ventricular remodeling after myocardial infarction. Cardiovasc Res 81:457–464
O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT (2005) c-Myc-regulated microRNAs modulate E2F1 expression. Nature 35:839–843
Margosio B, Rusnati M, Bonezzi K, Cordes BA, Annis D, Urbanati C, Givazzi R, Presta M, Ribatti D, Mosher DF, Taraboletti G (2008) Fibroblast growth factor-2 binding to the trombospondin-1 type III repeats, a novel antiangiogenic domain. Int J Biochem Cell Biol 40:700–709
Vita M, Henriksson M (2006) The Myc oncoprotein as a therapeutic target for human cancer. Semin Canc Biol 16:318–330
Virag JAI, Rolle ML, Reece J, Hardouin S, Feigl EO, Murry CE (2007) Fibroblast growth factor-2 regulates myocardial infarct repair. Effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol 171:1431–1440
Gueron G, Siervi AD, Ferrando M, Salerno M, Luca PD, Elguero B, Meiss R, Navone N, Vazquez ES (2009) Critical role of endogenous heme oxygenase 1 as a tuner of the invasive potential of prostate cancer cells. Mol Cancer Res 7:1745–1755
Otterbein LE, Bach FH, Alam J, Soares M, Lu HT, Wysk M, Davis RJ, Flavell RA, Choi AM (2000) Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6:422–428
Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN (1987) Bilirubin is an antioxidant of possible physiological importance. Science 235:1043–1046
Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, AA FIII, Kukreja RC (2005) HIF-1 activation postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol 289:H542–H548
Losordo DW, Demmeler S (2004) Therapeutic angiogenesis and vasculogenesis for ischemic disease: Part 1: angiogenic cytokines. Circulation 109:2487–2491
Acknowledgments
We thank Ms. S. Dominitz for editorial assistance. This work was supported in part by grants from The Israel Cancer Association (Grant No 20092001, Israel); Teva Special Funds (Dr. A. Swartz) and The Marcus Center for Pharmaceutical and Medicinal Chemistry at Bar Ilan University, Ramat Gan, Israel. Nataly Tarasenko carried out this work as part of the requirements for a PhD degree from the Department of Hematology, Sackler School of Medicine, Tel-Aviv University, Israel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarasenko, N., Kessler-Icekson, G., Boer, P. et al. The histone deacetylase inhibitor butyroyloxymethyl diethylphosphate (AN-7) protects normal cells against toxicity of anticancer agents while augmenting their anticancer activity. Invest New Drugs 30, 130–143 (2012). https://doi.org/10.1007/s10637-010-9542-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9542-z