Summary
Background: To analyze the feasibility of capecitabine with weekly irinotecan and concurrent radiotherapy followed by laparoscopic-total mesorectal excision (LTME) in rectal cancer patients. Methods: Eligible criteria included adenocarcinoma of the rectum staged by endoscopic ultrasonography (u), spiral abdominal and pelvic CT and chest X-ray. Patients received weekly irinotecan 50 mg/m2 (days 1, 8, 15, 22, 29) and capecitabine (days 1 through 5 for 5 weeks); dose level; (DL) I 250 mg/m2/bid; DL II 375 mg/m2/bid; DL III 500 mg/m2/bid, according to phase I methodology. External beam radiotherapy was delivered up to a total dose of 45 Gy in daily fractions of 1.8 Gy, 5 days a week. LTME was planned 5–7 weeks after CRT. Results: From February 2003 to February 2006, 22 patients were included. Median age was 62 (range 48 to 78). Seven pts were uT3N0 and 15 pts uT3N1. Seven patients were treated at DL I, six at DL II and nine at DL III. Grade 3 adverse events were observed in all levels. The maximum tolerated dose was reached at 375 mg/m2 (DL II). Conversion rate to open surgery was 5%. Median hospital stay was 6.6 days. One month post-surgical complications were noted in five patients (23%). Median excised nodes were 11 (range 4–21). Pathological complete response was observed in two patients (9%). Conclusions: LTME after preoperative CRT with CAPIRI is feasible but severe adverse events were found in all levels despite the use of lower dose of capecitabine than previously published.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preoperative chemoradiotherapy (CRT) with fluorouracil (FU) and open-total mesorectal excision (OTME) could be considered the standard therapy for patients with rectal cancer [1–3]. Laparoscopic-total mesorectal excision (LTME) is feasible and safe [4, 5], but large randomized controlled trials are needed to confirm that oncologic results are comparable to OTME.
FU in continuous endovenous infusion is probably the most common schedule used in preoperative CRT with pathological complete responses up to 30% [6]. However, it requires portable pump systems, which are associated with a number of complications.
Capecitabine an oral fluoropyrimidine, has demonstrated its safety and effectiveness preoperatively in rectal cancer [7]. Irinotecan a topoisomerase-I inhibitor with radiosensitizing properties [8], has shown activity, in combination with continuous infusion FU in rectal cancer [9, 10].
We have previously shown that laparoscopic surgery can be safely performed in patients with colorectal cancer [11] and rectum, after conventional preoperative CRT with infusional FU [12]. Because the lack of data of surgical morbidity and oncologic results in rectal cancer patients, treated with capecitabine, irinotecan (CAPIRI) and concomitant RT followed by LTME, we designed a phase I study.
Methods and materials
Eligibility criteria and pre-treatment evaluation
Patients aged 18–80 years were enrolled in the study if they fulfilled the following inclusion criteria: histologically confirmed adenocarcinoma of the rectum located to less of 15 cm from the anal margin. Eastern Cooperative Oncology Group performance status ≤1, absence of previous oncologic treatments and adequate haematological, hepatic and renal function. Pregnant or lactating women, patients with synchronous colon cancer, those with a second neoplasia (except carcinoma in situ of cervix adequately treated or squamous or basocellular carcinoma of the skin), history of chronic diarrhoea, positive serology for HIV, ischemic heart disease in the six previous months, history of uncontrolled arterial hypertension and any contraindication for laparoscopic surgery were also excluded. The protocol was approved by the Hospital Clínic ethics committee. All patients provide written informed consent before entering the trial.
Pretreatment evaluation
All patients were evaluated prior to start the treatment, including history and physical examination, complete blood count, serum chemistry profile, chest X-ray, rectoscopy, endorectal ultrasonography and spiral abdominal and pelvic CT-scan. Weekly blood count and serum chemistry was repeated every week during CRT treatment.
Radiotherapy
Radiotherapy was delivered to the pelvis with a three-field technique (posterior and two lateral fields), using an energy of 6 or 18 MV from a linear accelerator while the patient was in the prone position, using an open tabletop (belly board) device for bowel exclusion. The total dose was 45 Gy, with a daily dose of 1.8 Gy administered 5 days each week, to the treatment isocenter (beam intersection point) according to the International Commission on Radiation Units and Measurements Report 50 guidelines. The target volume included the primary tumor, perirectal fat tissue, and the internal iliac and presacral lymph nodes. Customized blocks or multileaf collimators were used.
Chemotherapy
Patients received weekly irinotecan at a dose of 50 mg/m2 given over 30 min beginning on day 1 of RDT for five consecutive weeks. Patients received capecitabine orally bid within 30 min after a meal from Monday to Friday, which was planned to start at five different dose levels (DL): DL I (250 mg/m2/bid), DL II (375 mg/m2/bid), DL III (500 mg/m2/bid), DL IV (625 mg/m2/bid) and DL V (825 mg/m2/bid) during 5 weeks.
If a patient reported one of the following adverse events according to National Cancer Institute Common Toxicity Criteria (version 2.0), chemotherapy was to be interrupted if grade 4 haematological or grade ≥3 non-hematological toxicity occurs. Chemotherapy would be reassumed if adverse events had resolved to grade 0 or 1 with a dose-level reduction.
Surgery and pathologic examination
Surgical resection with selective or total mesorectal excision by laparoscopic approach was performed between 5 to 7 weeks after the completion of CAPIRI and radiotherapy. Pathologic dissection of each specimen was carried out as described by Quirke et al. [13]. This involved complete transverse slicing of the tumor and the segments above and below it at 3 to 5 mm intervals looking for continuous spread of tumor up to the CRM.
Study design
The primary objective of this phase I study was to determine the dose-limiting toxicities (DLTs) and the maximum-tolerated dose (MTD) of CAPIRI with RDT for rectal adenocarcinoma. Secondary end-point was to evaluate surgical complications and efficacy in terms of nodal retrieval and pathological complete response, with a mesorectal excision laparoscopic approach. At least three patients were enrolled per dose level, with this number increased to six if DLT occurred in one of three patients. Dose escalation was halted if DLT occurred in two of three patients at any dose level. DLT was defined as the presence of one or more of the following side effects: grade 4 neutropenia or thrombopenia; grade 3 or 4 febrile neutropenia; and any grade 3–4 non-haematological toxicity; during the weekly infusions or within the next 2 weeks after the last infusion. Maximum tolerated dose (MTD) was defined as the reached dose when two of each three or three of each six patients experience DLT, being evaluated after the five infusions and the next 2 weeks after the last infusion. The recommended dose is the dose level immediately lower to the MTD.
Because unacceptable toxicity was reported with CAPIRI and RT at doses over 500 mg/m2 bid of capecitabine [14], we made a protocol amendment on March 2005 stopping the trial at the dose level of 500 mg/m2 bid. The MTD was defined if DLTs occurred in three or more of nine patients entered on the III level.
Results
Patient characteristics
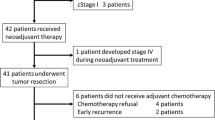
During the study period between February 2003 and February 2006, 172 patients with rectal cancer, underwent laparoscopic mesorectal excision in Hospital Clínic Barcelona. From these, 22 were included (see Table 1). Three patients with potentially resectable metastases in the lung, the right adrenal gland and a lymph node in the aortic bifurcation, were included in the trial. The median distance from the lower border of the tumour to the anal margin was 9 cm (4–15 cm).
Doses and safety
Seven patients received the dose level I (DL I) (250 mg/m2/bid), six the DL II (375 mg/m2/bid) and nine the DL III (500 mg/m2/bid). At DL I, one patient received only 1 week of chemotherapy treatment due to denied consent, but completed radiotherapy and was referred to other hospital for open surgical resection. This patient was excluded per protocol, for toxicity and laparoscopic-surgical complication analysis. At DL II, all patients received the 5 weeks of treatment. At the third level, one patient received only 1 week due to severe toxicity (grade 3 asthenia), and eight complete all treatment.
There were no deaths during treatment in any level. There were DLT in the three levels (see Table 2): one patient in DL I, two in DL II, and three in DL III. There were not grade 4 toxicities. No patients had to be hospitalised. The MTD was determined at DL II.
Surgery and pathologic assessment
Twenty-one patients underwent laparoscopic-assisted mesorectal excision (see Table 3). Conversion to open surgery was done in one patient (5%). Sphincter-preserving surgery was performed to 19 patients (90%). Postoperative complications developed in five patients. The rate of suture dehiscence was 9%. Other minor complications were a presacral abscess (1p), wound infection (1p) and acute urinary retention and a paralytic ileum in one patient. Patients with low-anterior resections undergo protective defunctioning stoma. There no were deaths due to surgical complications. Mean hospital staying was 6.6 days.
The mean number of lymph nodes retrieved were 11 (range, 4–21), and the mean distance to the distal resection margin was 2.3 cm (range, 0.2–6). Two patients had circumferential margin involvement (9%). Downstaging (defined as nodal downstaging (uN+ to pN0) or T stage downstaging (p.e. T3 to T2) was achieved in 57% of patients (see Table 4). Two patients in DL III achieved pathological complete remission (9%).
Survival
With a mean follow-up of 31 months (range 16–51), the 3-year disease specific survival rate was 91%. There have been three deaths, two due to metastasic progression, and one due to a vascular event, without evidence of disease relapse. One of the two patients with CMI involvement had a local relapse (5% of local recurrences). Among the three patients who showed initially metastatic involvement, one patient with a solitary lung metastases remain alive and without progressive disease after lung metastasectomy. Four of 19 patients with limited disease have progressed systemically (three with liver metastases and one with peritoneal spread).
Discussion
Few studies have investigated the use of concurrent irinotecan and capecitabine (CAPIRI) preoperatively in rectal cancer. Klautke et al. [15] defined the MTD with irinotecan 40 mg/m2 weekly and capecitabine at 750 mg/m2/bid continuous during 6 weeks, concurrently with radiotherapy (total dose 55.8 Gy). Hofheinz et al. [14] established the MTD of irinotecan 50 mg/m2 and capecitabine 500 mg/m2/bid including weekend, during 5 weeks, concurrently with radiotherapy (total dose 50.4 Gy). Three of seven patients included at 650 mg/m2/bid suffered grade 3 diarrhoea. In a recently published phase II trial for the same group, four patients had to be hospitalised due to grade 3 diarrhoea at 500 mg/m2/bid [16]. In our study with lower dose of capecitabine and radiotherapy, we have found grade 3 diarrhoea, in all the dose levels analysed and totally 28% of patients suffered grade 3 toxicities (14% diarrhoea). A similar range of toxicity has been reported also with oxaliplatin and capecitabine (CAPOX) doublet (grade III/IV diarrhoea 11–30%) [17–20]. Despite toxicity, 91% of patient complete treatment and no patients need to be hospitalised.
To our knowledge, this is the first report analysing surgical complications following laparoscopic approach with mesorectal excision, after doublet induction chemo-radiotherapy (CRT). Conversion rate to open surgery in this study (5%) compares favourably with our previously published experience with or without induction FU alone (20%) [12]. We could not rule out that the extensive experience gained for the surgical team between the two periods and differences in tumour characteristics, could influence favourably, current results. Additionally no major differences on surgical morbidity (26% vs. 23%) and hospital stance (6.8 ± 4.6 vs. 6.6 ± 2.1) were observed between our previous experience with conventional therapy and CAPIRI respectively. As it’s shown in Table 5, there is a significant difference favouring the laparoscopic approach compared with open procedures in terms of hospital stance. Anastomotic dehiscence is one of the major concerns in the two series with laparoscopic approach (5% with conventional therapy [12] and 9% with CAPIRI). Other authors have pointed out an increment of anastomosis leakage with LTME after induction CRT compared to LTME alone [21]. Anastomosis leakage is also one of the major problems (range between 3 to 12%) observed in some of the published series of patients treated with induction doublets [14, 16, 18, 19] and open-resection approach. Interestingly less than 3% anastomosis leakage has been published with capecitabine alone [22–24]. This suggests and increased risk of surgical morbidity with more complex combinations. Lymph node retrieval one of the major determinants of surgical quality is also comparable with other published data with open-surgery after doublets [16, 19].
One of the objectives of more intensive induction regimens lies to reduce the incidence of positive circumferential resection margins (CRMs) in patients with high-risk of fascia involvement [25]. This issue has never been tested face to face, over standard treatment with 5-fluouracil and radiotherapy. Our results are also in the range of published literature with open [17, 18, 20] or laparoscopic procedures [26], but EUS staging performed in our study unfortunately does not allowed to establish the pre-treatment risk of CRM involvement.
Three strategies have been used, trying to improve pCR. The first one is to add oxaliplatin or irinotecan to fluoropyrimidines. pCR with CAPIRI or CAPOX combinations range between 5–19% [14, 16, 17–20]. Our results, 9% pCR, are therefore in the range of published data after CAPOX or CAPIRI therapies. Indeed the RTOG randomized trial, suggested a benefit of CAPOX vs CAPIRI in terms of pCR (21% vs 10%) with a similar pattern of toxicity [27]. The second approach is to apply neoadjuvant chemotherapy before CRT doublets. Only one trial has being published with a slightly better pCR (24%; 95%CI, 14%–36%) [28]. The third approach add to CRT new agents like cetuximab or bevacizumab, but the percentage of pCR are still disappointing (5–25% pCR) [29–32]. Globally these results, seems quite similar than those obtained with capecitabine alone, but the toxicity profile and surgical complications, favour capecitabine monotherapy.
In conclusion, treatment with CAPIRI and radiotherapy has proved activity in the neoadjuvant setting of rectal carcinoma, but it’s hampered for its toxicity, and therefore further development in phase II or III trials should be probably considered with caution, specially in the view of the activity and toxicities observed with irinotecan/capecitabine combinations compared with full dose capecitabine monotherapy. Laparoscopic surgery seems a safe procedure after doublet induction therapy with complications and oncologic results similar than those obtain with open surgery-procedures.
References
Kapiteijn E, Marijnen CAM, Nagtegaal I et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646. doi:10.1056/NEJMoa010580
Sauer R, Becker H, Hohenberger W et al (2004) German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740. doi:10.1056/NEJMoa040694
Bosset J-F, Collette L, Calais G et al (2006) Chemotherapy with radiotherapy in rectal cancer. N Engl J Med 355:1114–1123. doi:10.1056/NEJMoa060829
Leroy J, Jamali F, Forbes L et al (2004) Laparoscopic total mesorectal excision (TME) for rectal cancer. Surg Endosc 18:281–289. doi:10.1007/s00464-002-8877-8
Wu X-W, Sun Y-M, Hua Y-B, Shen L-Z (2004) Laparoscopic versus conventional open resection of rectal carcinoma: a clinical comparative study. World J Gastroenterol 10:1167–1170
Rich TA, Skibber JM, Ajani JA et al (1995) Preoperative infusional chemoradiation therapy for stage T3 rectal cancer. Int J Radiat Oncol Biol Phys 32:1025–1029. doi:10.1016/0360-3016(95)00020-Y
Dunst J, Reese T, Sutter T et al (2002) Phase I trial evaluating the concurrent combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol 20:3983–3991. doi:10.1200/JCO.2002.02.049
Chen AY, Choy H, Rothenberg ML (1999) DNA topoisomerase I-targeting drugs as radiation sensitizers. Oncology 10:39–46
Klautke G, Feyerherd P, Ludwig K et al (2005) Intensified concurrent chemoradiotherapy with 5-fluorouracil and irinotecan as neoadjuvant treatment in patients with locally advanced rectal cancer. Br J Cancer 92:1215–1220. doi:10.1038/sj.bjc.6602492
Navarro M, Dotor E, Rivera F et al (2006) A phase II study of irinotecan (CPT-11) and 5-fluorouracil (5-FU) concomitantly with preoperative radiotherapy (RT) in patients (pts) with locally advanced resectable rectal cancer. Int J Radiat Oncol Biol Phys 66:201–205. doi:10.1016/j.ijrobp.2006.04.007
Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J (2002) Laparoscopic-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer. Lancet 359:2224–2229. doi:10.1016/S0140-6736(02)09290-5
Delgado S, Momblan D, Salvador L et al (2004) Laparoscopic-assisted approach in rectal cancer. Lessons learned from >200 patients. Surg Endosc 18:1457–1462. doi:10.1007/s00464-003-8831-4
Quirke P, Durdey P, Dixon MF et al (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumor spread and surgical excision. Lancet 2:996–999. doi:10.1016/S0140-6736(86)92612-7
Hofheinz RD, Gerstenberg B, Wenz F et al (2005) Phase I trial of capecitabine and weekly irinotecan in combination with radiotherapy for neoadjuvant therapy of rectal cancer. J Clin Oncol 23:1350–1357. doi:10.1200/JCO.2005.04.171
Klautke G, Küchenmeister U, Foitzik T et al (2006) Concurrent chemoradiation with capecitabine and weekly irinotecan as preoperative treatment for rectal cancer: results from a phase I/II study. Br J Cancer 94:976–981. doi:10.1038/sj.bjc.6603053
Willeke F, Horisberger K, Kraus-Tiefenbacher U et al (2007) A phase II trial of capecitabine and irinotecan in combination with concurrent pelvic radiotherapy (Caplri) as neoadjuvant treatment of locally advanced rectal cancer. Br J Cancer 96:912–917. doi:10.1038/sj.bjc.6603645
Glynes-Jones R, Sebag-Montefiore D, Maughan TS et al (2006) A phase I dose escalation study of continuous oral capecitabine in combination with oxaliplatin and pelvic irradiation (XELOX-RT) in patients with locally advanced rectal cancer. Ann Oncol 17:50–56. doi:10.1093/annonc/mdj031
Machiels JP, Duck L, Honhon B et al (2005) Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadioOxCape study. Ann Oncol 16:1898–1905. doi:10.1093/annonc/mdi406
Rodel C, Liersch T, Hermann EM et al (2007) Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 25:110–117. doi:10.1200/JCO.2006.08.3675
Rutten H, Sebag-Montefiore D, Glyne-Jones R et al. Capecitabine, oxaliplatin, radiotherapy, and excision (CORE) in patients with MRI-defined locally advanced rectal adenocarcinoma: results of an international multicenter phase II study. J Clin Oncol 24:153s, 2006 (suppl; abstr 3528)
Morino M, Parini U, Giraudo G et al (2003) Laparoscopic total mesorectal excision in a consecutive series of 100 patients. Ann Surg 237:335–342. doi:10.1097/00000658-200303000-00006
Kim JC, Kim TW, Kim JH et al (2005) Preoperative concurrent radiotherapy with capecitabine before total mesorectal excision in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 63:346–353. doi:10.1016/j.ijrobp.2005.02.046
Krishnan S, Janjan N, Skibber J et al (2006) Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 66:762–771. doi:10.1016/j.ijrobp.2006.05.063
De Paoli A, Chiara S, Luppi G et al (2006) Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: a multicentric phase II study. Ann Oncol 17:246–251 . doi:10.1093/annonc/mdj041
Salerno G, Daniels IR, Moran BJ et al (2006) Clarifying margins in the multidisciplinary management of rectal cancer: the MERCURY experience. Clin Radiol 61:916–923 . doi:10.1016/j.crad.2006.06.005
Pachlivanides G, Gouvas N, Tsiaoussis J et al (2007) Lymph node clearance after total mesorectal excision for rectal cancer: laparoscopic versus open approach. Dig Dis 25:94–99. doi:10.1159/000099176
Wong SJ, Winter K, Meropol NJ et al RTOG 0247: A randomized phase II study of neoadjuvant capecitabine and irinotecan versus capecitabine and oxaliplatin with concurrent radiation therapy for locally advanced rectal cancer. Proc ASCO 2008; 4021
Chau I, Brown G, Cunningham D et al (2006) Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 24:668–674. doi:10.1200/JCO.2005.04.4875
Machiels JP, Sempoux C, Scalliet P et al (2007) Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Ann Oncol 18:738–744. doi:10.1093/annonc/mdl460
Rodel C, Arnold D, Hipp M et al (2008) Phase I–II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys 70:1081–1086. doi:10.1016/j.ijrobp.2007.07.2356
Czito BG, Bendell JC, Willett CG et al (2007) Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: phase I trial results. Int J Radiat Oncol Biol Phys 68:472–478. doi:10.1016/j.ijrobp.2007.02.001
Hofheinz RD, Horisberger K, Woernle C et al (2006) Phase I trial of cetuximab in combination with capecitabine, weekly irinotecan, and radiotherapy as neoadjuvant therapy for rectal cancer. Int J Radiat Oncol Biol Phys 66:1384–1390. doi:10.1016/j.ijrobp.2006.07.005
Authors’ Disclosures of potential conflicts of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ugidos, L., Delgado, S., Conill, C. et al. Phase I trial of neoadjuvant chemoradiotherapy (CRT) with capecitabine and weekly irinotecan followed by laparoscopic total mesorectal excision (LTME) in rectal cancer patients. Invest New Drugs 27, 262–268 (2009). https://doi.org/10.1007/s10637-008-9192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9192-6