Abstract
Purpose
To study how rod- and cone-driven responses depend on stimulus size in normal subjects and patients with retinitis pigmentosa (RP), and to show that comparisons between responses to full-field (FF) and smaller stimuli can be useful in diagnosing and monitoring disorders of the peripheral retina without the need for lengthy dark adaptation periods.
Method
The triple silent substitution technique was used to isolate L-cone-, M-cone- and rod-driven ERGs with 19, 18 and 33% photoreceptor contrasts, respectively, under identical mean luminance conditions. Experiments were conducted on five normal subjects and three RP patients. ERGs on control subjects were recorded at nine different temporal frequencies (between 2 and 60 Hz) for five different stimulus sizes: FF, 70°, 60°, 50° and 40° diameter circular stimuli. Experiments on RP patients involved rod- and L-cone-driven ERG measurements with FF and 40° stimuli at 8 and 48 Hz. Response amplitudes were defined as those of the first harmonic component after Fourier analysis.
Results
In normal subjects, rod-driven responses displayed a fundamentally different behavior than cone-driven responses, particularly at low temporal frequencies. At low and intermediate temporal frequencies (≤ 12 Hz), rod-driven signals increased by a factor of about four when measured with smaller stimuli. In contrast, L- and M-cone-driven responses in this frequency region did not change substantially with stimulus size. At high temporal frequencies (≥ 24 Hz), both rod- and cone-driven response amplitudes decreased with decreasing stimulus size. Signals obtained from rod-isolating stimuli under these conditions are likely artefactual. Interestingly, in RP patients, both rod-driven and L-cone-driven ERGs were similar using 40° and FF stimuli.
Conclusion
The increased responses with smaller stimuli in normal subjects to rod-isolating stimuli indicate that a fundamentally different mechanism drives the ERGs in comparison with the cone-driven responses. We propose that the increased responses are caused by stray light stimulating the peripheral retina, thereby allowing peripheral rod-driven function to be studied using the triple silent substitution technique at photopic luminances. The method is effective in studying impaired peripheral rod- and cone- function in RP patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that ERGs driven by different photoreceptor types exhibit substantially different luminance and chromatic sensitivities [1]. Hence, selective isolation of photoreceptor responses is vital for studying the functional characteristics of each photoreceptor type and the processing pathways to which they transmit their signals. The silent substitution technique [2,3,4], based on the principle of univariance, is a paradigm that enables the almost complete isolation of activity from specific photoreceptor subtypes, if the stimulator used is equipped with the same number of primaries as the number of different subtypes (i.e., four primaries are needed for the human retina) [5]. This technique not only allows each subtype to be stimulated with known strengths (i.e., amount of photoreceptor excitation expressed in terms of a cone or rod contrast) but also permits the comparison of different subtype-specific responses under identical states of adaptation [6]. The method has been used extensively in recent years as a means to analyze the physiological and psychophysical behaviors of each photoreceptor type, and the post-receptoral mechanisms to which they contribute [6,7,8].
Challa et al. [9] and Jacob et al. [10] revealed the potential spatial variation of the L- and M-cone-driven ERG profiles. ERGs recorded at temporal frequencies of 30 Hz and above, using full-field stimuli, yielded larger L/M ratios than those recorded with spatially restricted stimuli [9, 10], indicating that the peripheral retina is more strongly L-cone dominated than the central retina. This correlates with the change in the ratio of L- to M-opsin messenger RNA with retinal eccentricity [11]. Furthermore, response amplitudes monotonically decreased in proportion to stimulus size. In comparison, L/M ERG ratio was about unity at low temporal frequencies (12 Hz and below) [9, 10] and did not vary substantially with stimulus size. The response amplitudes were stable for circular stimuli down to 10° in diameter. Only for stimuli smaller than 10° in diameter, the response amplitude decreased and the phase changed with decreasing stimulus size [12].
To further these studies, it is also important to look at rod-driven ERGs, as they too play a vital role in basic and ophthalmological investigations. Low scotopic illumination under dark adaptation conditions allows the rod-driven function to be recorded. Recently, it was found that pure rod-only responses could be obtained up to mean retinal illuminances of around 800 Td using moderate temporal frequencies < 20 Hz and silent substitution stimuli [13, 14]. As seen in Maguire et al. [13] Fig. 5, beyond this luminance there was a qualitative change in the ERG signals, clearly indicating the intrusion of cone-based signals. Nevertheless, this opens up the possibility to study physiological mechanisms and pathophysiological changes in pathways that receive substantial rod inputs without the need for lengthy dark adaptation. Retinal disorders that may affect rod photoreceptor function, like retinitis pigmentosa (RP), congenital stationary night blindness (CSNB) and age-related macular degeneration (AMD), are monitored in clinics by assessing rod-mediated vision in patients with conventional dark-adapted ERGs [15,16,17]. The use of silent substitution stimuli may, therefore, be interesting for studying rod function in these diseases also at photopic conditions. Many diseases such as RP and AMD are thought to affect some parts of the retina more than others. It, therefore, will be interesting to use spatially restricted stimuli to study these diseases. Until now, rod ERGs has been measured with full-field stimuli. As rods are unevenly distributed over the retina and absent in the fovea, it would be interesting to explore how their ERG signals, measured over a broad range of temporal frequencies, change with different spatially restricted stimuli compared to ERGs mediated by L- and M-cones. In these retinopathies, there may be benefits from substantially increasing luminance, especially when fields are smaller, and the theme of this paper is to establish the feasibility of this approach.
Therefore, in the present study, we investigated the spatial properties of rod-driven ERGs generated by silent substitution stimuli of varying temporal frequencies and luminance and compared them with those driven by L- and M-cones in normal subjects and in RP patients. The spatial variation of L- and M-cone-driven ERGs was consistent with previous findings in normal subjects [10]. Data presented here showed that rod ERGs were recordable in normal subjects with spatially restricted stimuli for temporal frequencies below 20 Hz at a very high luminance (284 cd/m2) and with a high signal-to-noise ratio. In these conditions, the responses with spatially restricted stimuli were substantially larger than those to full-field stimuli. We propose that the responses are the result of indirect stimulation of the peripheral retina through stray light. At higher temporal frequencies, the putative rod responses are probably influenced by residual cone stimulation. In RP patients, we find indications that rods and cones are affected in the peripheral retina.
Methods
Subjects
Experiments were conducted on five normal subjects (age 29–57 years) and three RP patients (P1, P2 and P3; age 41–53 years). All normal subjects underwent a complete ophthalmological investigation and had normal color vision according to Farnsworth-Munsell D-15 color arrangement test and an Heidelberg Multi Color anomaloscope (Oculus Optikgeräte GmbH, Wetzlar, Germany). The RP patients were recruited from the retinal clinic of the University Hospital Erlangen. All patients underwent a complete clinical examination by an ophthalmologist, including best-corrected visual acuity, slit-lamp examination, electroretinography according to the standards of the International Society for Clinical Electrophysiology of Vision (ISCEV) [18], Goldman perimetry, optical coherence tomography (Spectralis OCT, Heidelberg Engineering, Heidelberg Germany) and fundus examination. All patients had a typical fundus appearance with bone spicules, characteristic visual field changes and highly reduced amplitudes in the ISCEV ERG allowing a clinical diagnosis of RP (see Table 1). Genetic tests were not performed. The disease was classified according to the pedigree as sporadic (simplex type) (P1 and P2) and autosomal recessive (multiplex type) (P3).
For the ERG recordings, the right eye of each subject was dilated with a drop of 0.5% tropicamide (Pharma Stulln). DTL fibers were used as active electrodes, placed on the lower conjunctiva and attached close to the inner and outer canthi. Ground and reference gold cup electrodes filled with electrode paste (DO Weaver & Company, USA) were placed on the forehead and ipsilateral temple, respectively, after the skin was scrubbed with Nuprep abrasive skin preparing gel (DO Weaver & Company).
All subjects were informed about the protocol and the purpose of experiments, and written consent was obtained. The experiments conducted in this study were in accordance with the tenets of the Declaration of Helsinki, and the protocol was approved by the local institutional ethics committee (medical faculty of the University Erlangen-Nürnberg).
Stimuli
The visual stimuli were generated with a Ganzfeld stimulator (Q450SC, Roland Consult, Brandenburg, Germany) that contained six differently colored light emitting diode (LED) arrays. The stimuli were controlled by a RETIport system (Roland Consult), which had the flexibility to independently control the mean luminance, contrast, phase and waveform of a light stimulus generated by each LED array. In the present study, the red, orange, green and blue LEDs were used to stimulate single photoreceptor types using the triple silent substitution paradigm [2, 3]. The emission spectra of the LEDs were measured using a CAS 140 spectroradiometer (Instrument Systems, Germany). The spectral outputs ± half bandwidth at half height were 638 ± 9, 594 ± 8, 523 ± 19 and 469 ± 11 nm for red, amber, green and blue LEDs, respectively. With the emission spectra and the photoreceptor fundamentals [1], mean photoreceptor excitations (expressed in cone or rod Td) and excitation modulation (expressed in cone or rod Michelson contrasts) were calculated via a linear transformation. The inverse transformation was used to determine the LED contrasts for a stimulus with desired cone or rod contrasts [3, 5, 6]. Three different stimulus combinations were generated to isolate L-cone-, M-cone-, and rod-driven responses at 19, 18 and 33% photoreceptor contrast, respectively. The luminance of the four primaries were: red (638 nm) 80 cd/m2, amber (594 nm) 160 cd/m2, green (523 nm) 40 cd/m2 and blue (469 nm) 4 cd/m2 giving a mean luminance of 284 cd/m2 for all three stimulus conditions. The mean chromaticity was also the same for all three stimulus conditions with 1931 CIE coordinates; x = 0.5951 and y = 0.3857. As a result, the state of adaptation was the same in all measurements. In a control experiment, additional mean luminances between 2.5 and 142 cd/m2 were used.
ERG responses in normal observers were recorded for five different spatial stimulus configurations, viz., full-field (FF; no blockade of the light path except by the anatomical structures such as the nose or the eye brows) and 70°, 60°, 50° and 40° diameter circular stimuli. The spatial stimulus configurations were set up using black cardboard frames with various aperture sizes, placed at a 3 cm distance from the observer [10]. For each stimulus size, L-cone-, M-cone- and rod-driven ERG responses were recorded at nine different temporal frequencies: 2, 4, 8, 12, 16, 24, 36, 48 and 60 Hz. The recordings were performed over three sessions to minimize fatigue. In the RP patients, a subset of these stimuli was employed: rod- and L-cone-driven responses were measured with full-field and 40° stimuli. The temporal frequency was 8 and 48 Hz for the L-cone-isolating condition and 8 Hz for the rod-isolating condition.
ERG recordings
ERG signals recorded from subjects were amplified by a factor of 106, band-pass-filtered between 1 and 300 Hz cutoff frequencies, digitized with 2048 Hz sampling rate, averaged over 40–160 cycles (of 1 s each, excluding the first two cycles after stimulus onset to avoid onset artifacts) and stored by the RETIport system. The impedance of active and reference electrodes was maintained below 5 KΩ during measurements.
During offline analysis, the signals were Fourier-analyzed using a custom-written MATLAB script (R2011b, MathWorks, Massachusetts, USA), to extract the amplitude and phase of the fundamental frequency (f) component. Subsequent analyses were performed using Microsoft Excel 2010. The noise in the present experiment was defined as the average of amplitudes at the frequencies adjacent to the stimulus frequency (f + 1 and f − 1 Hz). The phases of ERG signals were discarded for further analysis when the signal-to-noise ratio (SNR) was smaller than two [19].
The fast Fourier transform (FFT) returns response phase in modulo of 360°. To estimate the absolute phase, it was assumed that for a particular temporal frequency, phases in all photoreceptor-driven responses were as close to each other as possible at a particular temporal frequency. Furthermore, the phase lag at a particular temporal frequency was larger than the phase lag in the adjacent lower frequency. Finally, the phases of the responses from all subjects to a particular stimulus were assumed to be less than 180° apart.
Results
Normal subjects
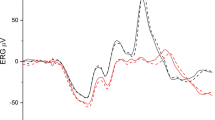
Figure 1 shows representative averaged responses to rod-isolating stimuli at three (of nine) temporal frequencies recorded from one subject for all the spatial stimulus configurations employed. At low temporal frequencies (2 and 8 Hz), rod responses to the spatially restricted circular stimuli were substantially larger than those to FF stimulation. Furthermore, the responses to the circular stimuli were of similar magnitude independent of aperture size. In contrast, the response to supposedly rod-isolating 48 Hz FF stimuli was larger than those to the circular stimuli, which were barely distinguishable from noise.
Figure 2 shows group-averaged amplitudes (average + SD; one sided because of the logarithmic scale of the amplitude axis) of the rod-, L- and M-cone-driven responses (defined as the fundamental component), elicited with the different stimulus sizes, plotted as a function of temporal frequency. In agreement with previously published results from our group [10], responses elicited by L- and M-cone-isolating stimuli at temporal frequencies up to about 16 Hz had similar amplitudes that, in addition, did not change much with stimulus size. At temporal frequencies above 16 Hz, L-cone-driven responses were larger than M-cone-driven ones, with the amplitudes of both showing a positive correlation with stimulus size. Differing ERG characteristics between low and high temporal frequency data have also been regularly reported in our previous findings [6, 10, 20, 21], which showed that two distinct mechanisms are involved in the generation of ERGs at these two frequency ranges.
The noise did not vary with stimulus size. Hence, noise data obtained from each subject at different stimulus sizes were averaged. It increased with decreasing temporal frequency indicating the presence of 1/f or ‘pink’ noise.
The rod-driven responses displayed a fundamentally different dependency on stimulus size than the cone-driven responses. As observed in the recordings shown in Fig. 1, responses at low temporal frequencies for FF stimuli were barely above the noise. At temporal frequencies below 16 Hz, the responses increased strongly when stimulus size was restricted to 70° rather than FF. A further decrease in stimulus size did not result in substantial changes in response amplitude. At low temporal frequencies, response amplitudes were about a factor of four larger when a smaller stimulus was used compared to FF stimulation. The data suggest that the responses mainly stem not from direct stimulation of the central retina but from stray light impinging on the peripheral retina, which is of course of considerably lower luminance. At temporal frequencies above 30 Hz, rod-driven responses decreased with decreasing stimulus size. A previous study suggested that stray light has a small influence on L- and M-cone-driven ERGs because the use of a white instead of a black surround resulted in a slight reduction in absolute response amplitudes of L- and M-cones [10]. In the present study, the experiments were conducted in a dark room and black cardboard was used for the field stop.
Figure 3 displays the stimulus size dependency of L-cone-, M-cone- and rod-driven ERG amplitude. FF responses were excluded because the size was difficult to estimate. The amplitude of each response type (excluding those to FF) is plotted as a function of circular stimulus diameter on a double logarithmic scale. At low temporal frequencies (8 and 12 Hz; Fig. 3; top panels), amplitude did not show a significant dependency on stimulus size. Please note that because FF data are not included, the smaller response amplitudes for FF rod-isolating stimuli cannot be observed in the plot. A positive correlation between the response amplitude and stimulus size was, however, apparent at 24 and 36 Hz. This is an additional indication that the responses are actually driven by residual cone activity. The positive correlation at 36 Hz of cone-driven response amplitudes with increasing stimulus size (i.e., L-cone slope = 1.71 and M-cone slope = 1.69 with R 2 = 0.78 and 0.82, respectively) was comparable to that described previously [10], whereas this is the first study to reveal the correlation between stimulus size and responses mediated by rod photoreceptors.
The logarithm of the rod-, L-cone- and M-cone-driven response amplitudes (blue triangle, red circle and green square symbols, respectively) plotted as a function of the logarithm of stimulus diameter for circular stimuli (i.e., excluding the FF condition) and for four representative frequencies viz. 8, 12, 24 and 36 Hz, respectively. Linear regressions and corresponding correlation coefficients (R 2) are shown color-coded to their respective data
Figure 4a (left column) displays the averaged ERG response phase (+ 1 SD) plotted as a function of temporal frequency for the five different spatial stimulus configurations. Figure 4b (right column) shows the same data, but here the response phase elicited by the different photoreceptor-isolating conditions is shown for FF and 70° diameter stimuli. Furthermore, a slope analysis was employed to describe the phase data in Fig. 4 and plotted in Fig. 5. Each data point in the plot represents the phase change divided by the frequency change at four adjacent temporal frequencies, calculated by performing regression fitting. These regressions, given as a function of the mean of the temporal frequencies, are given as an estimate of the local slopes at the temporal frequencies. In the low-frequency range (2–16 Hz), the rod response phase (bottom panel) decreased strongly with increasing temporal frequency, particularly for spatially restricted stimuli. The cone-driven response phases were similar to previous data [22]. The slopes at temporal frequencies above about 16 Hz were less steep for the cone- and rod-driven responses, indicative of a change in underlying physiological mechanisms. Furthermore, the FF rod-driven responses had a distinctly different phase relationship with higher frequencies compared to those measured with smaller stimuli.
a Averaged (+ 1 SD) phase of L-cone-, M-cone- and rod-driven responses (top, middle and bottom panels, respectively) recorded from each stimulus size plotted as a function of temporal frequency. Data for the different stimulus sizes are overlaid for each photoreceptor type and color-coded between panels. b Averaged phase of responses to FF and 70° stimuli. Here, the results with L-cone-, M-cone- and rod-isolating stimuli are plotted together for the two stimulus sizes
a Slope analysis of the phase plots displayed in Fig. 4. b Slope analysis of L-cone-, M-cone- and rod-driven responses phase plotted separately for FF and 70° stimulus size. Individual data points are a four-point linear regression as a function of the mean of four adjacent temporal frequencies
In agreement with previous data [22], M-cone-driven response phase was generally more advanced than that of L-cone-driven responses. The rod-driven responses had similar phase and slope variation to the L-cone-driven responses with FF stimuli, particularly at high temporal frequencies. This indicates that FF high-frequency rod-driven responses are probably residual cone-driven responses. With 70° stimuli, the three response types had distinct phase characteristics and the rod responses had phases that decreased more strongly with temporal frequency (especially from 2 to 24 Hz) than those driven by cones.
Control experiments
If we are correct in surmising that the increase in rod-driven responses with spatially restricted stimuli is caused by stray light, and not by interactions between central and peripheral responses, then the use of an illuminated non-modulating surround would be expected to have a large influence on the ERGs. To test this hypothesis, we performed a control experiment where the responses with a dark surround were compared with those in which the surround was created by illuminated white cardboard with a mean luminance of approximately 100 cd/m2. The rod-driven ERGs were recorded with two normal subjects (authors AA and JK) with black and illuminated white cardboard as a 40° field stop. In agreement with previous studies [13], we propose that the responses at frequencies above about 24 Hz are driven by cones through residual stimulation. Therefore, the control experiment was performed only for frequencies up to 24 Hz (i.e., 2, 4, 8, 12, 16 and 24 Hz). Figure 6 shows the rod-driven ERG response amplitude (left column) and phase (right column) recorded in the two subjects. The rod-driven ERG response amplitudes with white cardboard as field stop were substantially smaller than those recorded with black surround. The responses with white surround were barely above the noise in subject AA and about a factor of ten smaller in subject JK. These results strongly suggest that the increased rod-driven responses with spatially restricted stimulus sizes at low temporal frequencies and with dark surround are indeed caused by stray light stimulation of the peripheral retina.
Rod-driven ERG response amplitude (left column) and phase (right column) recorded from two normal subjects for 40° stimulus size with black (black circles) and white (gray circles) cardboard as a field stop. The dashed black and gray lines are the noise data acquired with black and white cardboard, respectively
The data displayed in Fig. 2 seemingly contradict the observation of Maguire et al. [13] who showed that the responses above about 800 photopic Td (corresponding to about 16 cd/m2 luminance assuming an 8-mm-diameter pupil) are not rod driven. The data in this report suggest that a 284 cd/m2 stimulus may elicit strong responses from rods. The explanation for this discrepancy is almost certainly due to the stray light effect described above. In agreement with the Maguire et al. data, we found very small rod-driven responses, close to noise, with FF stimuli. To further link our data with those of Maguire et al., we performed a second control experiment in which the effect of varying mean luminance on the responses to FF and restricted stimuli was studied. The experiment was conducted again with two subjects AA and JK. Rod-isolating ERGs were measured for four different mean luminances (2.5, 10, 35.5, 142 and 284 cd/m2) at nine different temporal frequencies (same frequency range which was used in the main experiment) for FF and 40° stimulus size. The low mean luminances were achieved by introducing appropriate neutral density filters. Figure 7 shows the rod-driven ERGs obtained from the second control experiment. In agreement with the data of Maguire et al., the FF rod-driven ERGs in sub 1 and sub 2 (left panels) showed approximately a four- to fivefold increase in rod-driven ERG amplitudes between 2 to 8 Hz when measured with 2.5 and 10 cd/m2 (corresponding to about 125 and 500 Td) as compared to 284 cd/m2 mean luminance. In contrast, the rod-driven ERGs recorded with 40° stimulus size (right panels) showed maximal responses at 284 cd/m2 mean luminance that systematically decreased with a decreasing mean luminance. Above 16 Hz, rod-mediated ERGs at all mean luminances to FF and 40° stimulation were very small. To see the effect of mean luminance more clearly, we replotted the 8 Hz rod-isolating ERG amplitudes for FF and 40° stimulus size as a function of mean luminance in Fig. 8. Clearly, luminance had opposite effects on the responses to FF and 40° diameter stimuli. The FF rod-driven response amplitudes increased with decreasing luminance, which is in close agreement with Maguire et al. In contrast, there was a positive correlation between mean luminance and response amplitude for the smaller stimuli. This can be explained by an increase in stray light with increased luminance.
RP patients
Table 1 provides a clinical characterization of the three RP patients who participated in this study. Pedigree analysis showed sporadic (simplex type) RP in subjects P1 and P2. Subject P3 has a brother who is also affected, suggesting an autosomal recessive or X-linked type of RP (multiplex type). The standard ISCEV dark-adapted 3.0 cd s/m2 flash ERG was extinguished in all patients. The light-adapted flicker ERG responses were extinguished in P1 and P2 and strongly reduced in amplitude in P3. As the signals were so small, implicit times were non-informative. As mentioned above, the measurements with L-cone- and rod-isolating conditions were performed only with two stimulus configurations (FF and 40°) and two temporal frequencies (8 and 48 Hz). Figure 9 shows the amplitudes of the ERG responses elicited by a 8 Hz rod (upper panel)- and L-cone (8 and 48 Hz; lower panel)-isolating stimuli recorded from normal (average + 1 SD) and the three individual RP patients (P1, P2 and P3) with FF and 40° stimulus size. 48 Hz responses to rod-isolating conditions were not included because these responses were probably contaminated by residual cone-driven responses. Overall, in contrast to the absence of measurable responses using the ISCEV standards, the rod- and L-cone-isolating conditions elicited robust responses in the RP patients in several conditions. The L-cone-driven responses to the 40° stimuli in the patients were of similar amplitude as in normal subjects, indicating that the central retina was functional. In contrast with responses measured in normal subjects, where FF and 40° stimuli strongly differed for 8 Hz rod-isolating and for 48 Hz L-cone-isolating stimuli, the responses with FF and 40° stimuli were similar amplitudes in the RP patients in all conditions. This indicates that the additional stimulation of the peripheral retina did not lead to response increases and that both rod- and cone-driven ERG pathways were non-functional in the retinal periphery.
Averaged response amplitudes to rod- and L-cone-isolating stimuli recorded from normals (average ± SD) and three RP patients (P1, P2 and P3) at 8 and 48 Hz (L-cone-driven ERGs only) with FF and 40° stimulus size. An asterisk indicates a significant difference; ns indicates a nonsignificant difference
In Fig. 10, the corresponding ERG response phases for rod- and L-cone-isolating stimuli in Fig. 9 are shown. Phases were averaged for normal subjects and plotted separately for each RP patient (P1, P2 and P3) for each particular frequency (8 and 48 Hz). The rod-driven response at 8 Hz in normal subjects displayed a substantial phase difference between responses to FF and 40° stimuli, in that the FF responses were considerably more phase-advanced relative to the 40° stimuli. In the RP group on the other hand, the reduction in stimulus size did not result in a significant phase change. The L-cone-driven response phases were similar for the two stimulus sizes in both normal subjects and RP patients. This finding is in favor of the notion that the responses obtained from the patients originate from the central retina.
Discussion
In the present study, we recorded ERGs from five healthy trichromats and three RP patients under conditions of cone and rod isolation using the triple silent substitution technique. The aim of this study was fourfold. Firstly, we wanted to provide further evidence that the triple silent substitution can be used to achieve isolation of rod responses and to explore what the possible limitations of this isolation might be. Secondly, we sought to study how rod-driven responses are related to responses obtained from L- and M-cone-isolating stimuli when using spatially restricted stimuli. Thirdly, we wanted to investigate how rod-driven responses depended on stimulus size and temporal frequency and lastly, we wanted to study if these stimuli could be used to identify compromised function in retinitis pigmentosa.
Effect of stimulus size on rod- and cone-driven responses
We previously have shown that triple silent substitution can be useful to isolate photoreceptor-driven responses [22]. The cone-driven responses described here and their dependency on stimulus size and temporal frequency are in agreement with previous data [10, 23]. In contrast, the responses to putatively rod-isolating stimuli displayed a different dependency on stimulus size and temporal frequency compared to the cone-driven responses. In the low-frequency region and for FF stimuli, rod signals were barely above the noise. At high temporal frequencies, responses were measurable. The phases of the responses were close to those of cone-driven responses. Furthermore, similar to cone-mediated responses, the rod-driven response amplitudes at high temporal frequencies decreased with decreasing stimulus size. From these observations, we conclude that in FF conditions the responses are probably mainly driven by residual L-cone signals caused by small errors and variabilities in cone fundamentals [24]. This conclusion is in agreement with those of Maguire et al. [13, 14], who found that rod-driven responses cannot be reliably measured at these high luminances. Also, the responses to smaller stimuli with frequencies above about 20 Hz can probably be attributed to residual cone-driven responses.
Responses to rod-isolating stimuli were increased by a factor of about four when recorded with restricted stimulus sizes in normals. The response phase also changed strongly when the stimulus was decreased from FF to 70°. Neither response amplitude nor response phase changed dramatically when stimulus diameter was further decreased from 70° to 40°. These response characteristics were also substantially different from cone-driven response properties. From this, we conclude that the responses under these conditions are indeed driven by the rods. With spatially restricted stimuli, the rod response amplitudes vs. temporal frequency characteristics were similar to those found by others [13]: The response amplitudes were relatively constant up to about 10 Hz and monotonically decreasing amplitudes with increasing temporal frequency for the region beyond 10 Hz. We propose that the larger rod-driven responses with restricted stimuli are caused by stray light stimulating the retinal areas around the stimulus. The mean retinal illuminance in these areas is substantially smaller compared to that within the stimulus. With full-field stimuli, stray light plays no role. Because of the high retinal illuminances, the rod-driven responses are too small to be measured explaining why in FF condition the responses are mainly determined by residual cone responses. Conversely, it can be concluded that the different dependency of low temporal frequency responses to rod-isolating high luminance stimuli on stimulus size compared to those to cone-isolating conditions indicates that the responses are truly rod driven. The data also indicate that rod-driven responses may contaminate cone-driven ERG signals to spatially restricted stimuli when they do not silence the rods. We have indeed found indications of this intrusion in unrelated experiments with small stimuli [12].
We would like to give a model that quantitatively describes the stray light hypothesis. For simplicity, we assume that the retina is spatially homogeneous. Further, we assumed that the local retinal illuminance (I L) at distance x from the edge of the stimulus can be described as follows:
where a and b are free parameters.
In which I S is the retinal illuminance of the stimulus. The dependency of rod-driven ERG responses to full-field stimuli (R FF) as a function of retinal illuminance (I FF) can be described by the data given by Maguire et al. (their Fig. 8). These data are replotted in Fig. 11 and are fitted with the following function:
The local response (R L) at distance x from the edge of the stimulus is assumed to be proportional to the full-field response at the local illuminance:
Finally, we assume that the total response to the stray light (R SL) is the sum of the local responses:
This model was fitted to the responses as a function of stimulus luminance. The fits are shown in Fig. 8. There were three free parameters in the fits: a, b and c. The number of free parameters is large when considering the limited number of data points (five) for each subject. Furthermore, there are a large number of assumptions. Therefore, the model should be considered with some caution. However, we found that the fitting parameters were strongly constrained by the data, so that a unique set was obtained for each subject (JK a: 111.2 Td, b: 0.96, c: 0.33; AA a: 116.5 Td, b: 0.95, c: 0.23). In any case, the model shows that the measured responses with restricted stimuli can be quantitatively explained by a stray light effect. However, more data are necessary for a more concrete description and to decrease the number of assumptions.
Maguire et al. [13] characterized the retinal illuminances and temporal frequencies at which the responses can be considered to be purely rod driven without intrusion from cone-driven responses and without the need for extensive dark adaptation for FF conditions. We here extend these conditions beyond those recommended by Maguire et al. to fully photopic stimuli with spatially restricted stimuli. The responses obtained, although due to a stray light effect, are highly reliable with large signal-to-noise ratio and with relatively little inter-individual variability. This offers the possibility to use them to study the functional integrity of the peripheral retina. This stimulus, therefore, may be interesting for studying diseases that affect the peripheral retina such as retinitis pigmentosa. The comparison of the responses measured in normal subjects and RP patients is discussed in the next section.
Effect of RP on Rod- and L-cone-driven ERGs at different stimulus size
It has been known that early RP mainly affects the scotopic vision in the peripheral retina [25]. Our measurements using rod-isolating stimuli at 8 Hz support this notion. The stimulation of peripheral rods by stray light in RP patients did not result in a substantial response amplitude increase as was found with normal subjects. In addition, the 48 Hz L-cone-driven responses in RP patients were not different for full-field or 40° stimuli. Such a difference was clearly present in the normal subjects. The two effects indicate that the responses originating in the peripheral retina are affected in the RP patients. The data also indicate that both rods and cones are involved in RP.
Interestingly, L-cone-driven responses at 8 Hz with FF and 40° stimuli were significantly above the noise level in RP patients. Moreover, the responses were not reduced when compared with responses measured in the normal subjects. This suggests that functionality was preserved in the central retina of the RP patients. As we have shown previously [12], the ERGs recorded from the central retina strongly reflect red–green chromatic processing. We have previously found that the ERG responses to 30 Hz stimuli presented on a CRT screen reflect chromatic mechanisms in RP patients and mainly luminance mechanisms in normal subjects [26]. The preservation of central retinal mechanisms in RP patients, displaying relatively strong opponent processing, as is shown in the present paper, may explain this result. The standard ERG techniques recommended by ISCEV [27] do not contain any chromatic information and therefore may be too insensitive to detect these substantial responses from the central retina.
The finding of preserved ERG responses from the central retina is slightly unexpected because, apart from the (nearly) extinguished standard ISCEV ERG, visual acuity was reduced in all patients. This suggests a certain amount of central retinal involvement in RP. However, Jacobson and coworkers have found that almost 25% of patients with simplex, multiplex or autosomal recessive RP had relatively normal central retinal function according to static chromatic perimetry and ocular coherence tomography [28] even though some of these patients also had severely reduced visual acuity. In RP, peripheral loci of reduced cone and normal rod function have been demonstrated using rod- and cone-mediated multifocal ERGs [29]. The central 40° of the retina seems to have preserved relatively normal function.
Conclusion
In conclusion, it is possible to isolate and study rod-driven ERGs using triple silent substitution technique at photopic (284 cd/m2) luminances using spatially restricted stimuli. We propose that the responses are not elicited by direct stimulation of photoreceptors but through the stray light that reaches the peripheral retina. The increased responses with smaller stimuli in normal subjects with the rod-isolating conditions validate that a fundamentally different mechanism drives the ERGs in comparison with the cone-driven responses where such an increase is not observed. This paradigm may be useful in a clinical setting when peripheral signals are of interest (e.g., with retinitis pigmentosa). Our data suggest that in RP, not only the peripheral rods but also the peripheral cones are affected. Assuming that RP affects the central retina to a lesser extent may explain why ERG signals reflecting chromatic mechanisms may still be preserved in RP patients.
References
Stockman A, MacLeod DIA, Johnson NE (1993) Spectral sensitivities of the human cones. J Opt Soc Am A 10:2491–2521
Estevez O, Spekreijse H (1982) The “silent substitution” method in visual research. Vis Res 22(6):681–691
Estevez O, Spekreijse H (1974) A spectral compensation method for determining the flicker characteristics of the human colour mechanisms. Vis Res 14(9):823–830
Donner KO, Rushton WAH (1959) Retinal stimulation by light substitution. J Physiol 149:288–302
Shapiro AG, Pokorny J, Smith VC (1996) Cone-rod receptor spaces with illustrations that use CRT phosphor and light-emitting-diode spectra. J Opt Soc Am A 13(12):2319–2328
Kremers J (2003) The assessment of L- and M-cone specific electroretinographical signals in the normal and abnormal human retina. Prog Retin Eye Res 22(5):579–605
Kremers J, Scholl HPN (2001) Rod-/L-cone and rod-/M-cone interactions in electroretinograms at different temporal frequencies. Vis Neurosci 18:339–351
Ripamonti C, Woo WL, Crowther E, Stockman A (2009) The S-cone contribution to luminance depends on the M- and L-cone adaptation levels: silent surrounds? J Vis 9(3):1–16
Challa NK, McKeefry D, Parry NR, Kremers J, Murray IJ, Panorgias A (2010) L- and M-cone input to 12 and 30 Hz flicker ERGs across the human retina. Ophthalmic Physiol Opt 30(5):503–510
Jacob MM, Pangeni G, Gomes BD, Souza GS, Filho MDS, Silveira LC, Maguire J, Parry NR, McKeefry DJ, Kremers J (2015) The spatial properties of L- and m-cone inputs to electroretinograms that reflect different types of post-receptoral processing. PLoS ONE 10(3):e0121218
Hagstrom SA, Neitz J, Meitz M (1997) Ratio of M/L pigment gene expression decreases with retinal eccentricity. In: Cavonius CR (ed) Colour vision deficiencies XIII. Documenta Ophthalmologica Proceedings Series vol 59. Springer, Netherlands, pp 59–65
Martins CM, Tsai T, Barboni MT, da Costa MF, Nagy B, Ventura DF, Kremers J (2016) The influence of stimulus size on heterochromatic modulation electroretinograms. J Vis 16(8):1–11
Maguire J, Parry NRA, Kremers J, Kommanapalli D, Murray IJ, Mckeefry D (2016) Rod electroretinograms elicited by silent substitution stimuli for the light-adapted human eye. Trans Vis Sci Tech 5(4):13. https://doi.org/10.1167/tvst.5.4.13
Maguire J, Parry NR, Kremers J, Murray IJ, McKeefry D (2017) The morphology of human rod ERGs obtained by silent substitution stimulation. Doc Ophthalmol 134:11–24
Berson EL, Gouras P, Gunkel RD (1968) Rod responses in retinitis pigmentosa, dominantly inherited. Arch Ophthalmol 80(1):58–67
Berson EL, Gouras P, Gunkel RD, Marianthopoulos NC (1969) Rod and cone responses in sex-linked retinitis pigmentosa. Arch Ophthalmol 81(2):215–225
Scholl HPN, Langrová H, Weber BH, Zrenner E, Apfelstedt-Sylla E (2001) Clinical electrophysiology of two rod pathways: normative values and clinical application. Graefes Arch Clin Exp Ophthalmol 239(2):71–80
Marmor MF, Zrenner E (1995) Standard for clinical electrophysiology (1994 update). Doc Ophthalmol 89:199–210
Meigen T, Bach M (1999) On the statistical significance of electrophysiological steady-state responses. Doc Ophthalmol 98(3):207–232
Kremers J, Link B (2008) Electroretinographic responses that may reflect activity of parvo- and magnocellular post-receptoral visual pathways. J Vis 8(15):11–14
Kommanapalli D, Murray IJ, Kremers J, Parry NR, McKeefry DJ (2014) Temporal characteristics of L- and M-cone isolating steady-state electroretinograms. J Opt Soc Am A 31(4):A113–A120
Kremers J, Pangeni G (2012) Electroretinographic responses to photoreceptor specific sin wave modulation. J Opt Soc Am A 29:306–313
Tsai TI, Jacob MM, McKeefry D, Murray IJ, Parry NR, Kremers J (2016) Spatial properties of L- and M-cone driven incremental (On-) and decremental (Off-) electroretinograms: evidence for the involvement of multiple post-receptoral mechanisms. J Opt Soc Am A 33(3):A1–11
Huchzermeyer C, Kremers J (2017) Perifoveal S-cone and rod-driven temporal contrast sensitivities at different retinal illuminances. J Opt Soc Am A 34(2):171–183
Gouras P, Carr RE (1964) Electrophysiological studies in early retinitis pigmentosa. Arch Ophthalmol 72(1):104–110
Scholl HPN, Kremers J (2000) Large phase differences between L-cone and M-cone driven electroretinograms in retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:3225–3233
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Jacobson SG, Roman AJ, Aleman TS, Sumaroka A, Herrera W, Windsor EA, Atkinson LA, Schwartz SB, Steinberg JD, Cideciyan AV (2010) Normal central retinal function and structure preserved in retinitis pigmentosa. Invest Ophthalmol Vis Sci 51(2):1079–1085
Holopigian K, Seiple W, Greenstein VC, Hood DC, Carr RE (2001) Local cone and rod system function in patients with retinitis pigmentosa. Inves Ophthalmol Vis Sci 42(3):779–788
Acknowledgements
The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. rer. biol. hum” for AJA. NRAP’s participation was facilitated by the Manchester Biomedical Research Center and the Greater Manchester Comprehensive Local Research Network.
Funding
Bundesministerium fur Bildung und Forschung (BMBF) provided financial support in the form of project funding to JK (Grant#: 01DN14009). Deutsche Forschungsgemeinschaft (DFG) provided financial support in the form of project funding to JK (Grant#: KR1317/13-1). The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Aher, A.J., McKeefry, D.J., Parry, N.R.A. et al. Rod- versus cone-driven ERGs at different stimulus sizes in normal subjects and retinitis pigmentosa patients. Doc Ophthalmol 136, 27–43 (2018). https://doi.org/10.1007/s10633-017-9619-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-017-9619-5