Abstract
We have used the post-bleach recovery of the ERG a-wave to estimate the time-course of regeneration of cone pigment, following bleaching exposures far stronger than in a previous study. We recorded the photopic electroretinogram a-wave from two subjects, in response to dim red flashes delivered following 1-min exposures to intensities ranging from 1.1 × 104 to 1.3 × 105 photopic cd m−2. The measured response amplitudes were “linearized” to derive estimates of pigment level. These estimated pigment levels were found to increase at an initially linear rate, consistent with a “rate-limited” model of photopigment regeneration. The extracted time-course was similar to that previously reported in densitometric studies of cone pigment regeneration after similarly intense exposures. On the other hand, the rate of regeneration was slower than measured in the same subjects following less intense bleaches. These results are consistent with the notion that cone pigment regeneration is slowed following very strong bleaching exposures, possibly as a result of depletion of a pool of retinoid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photopigment regeneration after bleaching depends on a healthy interaction between the photoreceptor outer segments and the retinal pigment epithelium (RPE) and, in the case of cones, the Müller cells and cone inner segments [1–5]; hence, the kinetics of pigment regeneration can provide valuable information on the function of the outer retina in health and disease. Traditionally, pigment recovery has been assumed to follow first-order kinetics, described by a single exponential component [6–8]. However, deviations from strictly first-order kinetics have been noted [9–11], whereby longer time constants are needed after larger bleaches.
In order to account for this kind of discrepancy in scotopic dark adaptation data, a “rate-limited” model of pigment regeneration was proposed [12], in which the initial slope of regeneration was limited to an approximately constant rate, independent of bleach level. If this is the case, and an exponential function is forced to fit the data, the required time constant will be found to increase with increasing bleach level.
The biochemical steps underlying pigment regeneration have been analysed by Lamb and Pugh [13], and a mathematical model for rate-limited behaviour has been described [13, 14]. One biophysical scenario that was shown to lead to rate-limited regeneration corresponded to the diffusion of 11-cis retinal from a “pool” of fixed concentration, via a resistive barrier, to the photoreceptor outer segments, where it combined with opsin. They showed that such a rate-limited model provided a good fit to rod and cone pigment regeneration data and also that it could explain the “S2” component of psychophysical rod dark adaptation, in healthy individuals and in a number of disease states [13].
Recovery of the electroretinogram (ERG) following bleaching exposures has been measured in a number of studies of human dark adaptation; these have included recovery of: the flicker ERG [15–17], the scotopic a-wave [18], the photopic a-wave [11, 14, 19] and the scotopic b-wave [20, 21]. Some of these studies have related ERG recovery to the kinetics of photopigment regeneration, but the interpretation is complicated by the fact that the ERG represents the summed electrical activity of the retina and is affected by factors other than simply the level of available photopigment.
In a previous study [14], we measured recovery of the amplitude (measured at a fixed time after flash presentation) of the photopic a-wave response to dim flashes delivered following a range of bleaching exposures. With certain assumptions (set out below, and evaluated in the Discussion), we used these amplitudes to estimate pigment levels. The deduced recovery of pigment level following a range of exposures could be fitted with the rate-limited model, with recovery proceeding initially at an approximately constant rate (i.e. linearly with time), irrespective of bleaching intensity or duration.
We found that data from previous studies of cone pigment regeneration using reflection densitometry were also better fit by the rate-limited model, rather than by a single exponential component. However, the initial rates in the densitometry studies were lower than those we found for the two subjects in our ERG study. Potential explanations include inter-subject variation as well as the possibility that the ERG method using photopic dim flashes may not be a reliable measure of cone pigment regeneration. Another possible factor, proposed in our previous study, is that regeneration rates may differ between the fovea (which was the focus of the densitometry studies), and when averaged across the whole retina (as evaluated in the ERG recoveries). An additional potential explanation stems from the fact that the bleaching intensities used in the densitometry studies (5.6–5.8 log photopic Td) were far higher than those used in our ERG study, and it is possible that the initial rate is slower following extremely intense bleaches.
The purpose of the present study was to investigate recovery of the photopic dim-flash a-wave response in the same two subjects as in the previous study, following bleaching exposures up to 40 times stronger than used previously. The experiments were performed within 1–2 years after those of our previous study, so any effects of ageing on recovery are expected to be minimal. Our main findings are as follows: (1) recovery after these very strong bleaches still appears to follow an initially linear time-course, with the rate-limited model providing a better fit than a single exponential; (2) the deduced time-course of pigment regeneration is very similar to that seen in densitometry studies of cone pigment regeneration following bleaches of similar strength; and (3) recovery is slower than that recorded in the same subjects following less intense exposures. This last observation is not consistent with the biochemical model’s assumption of a constant pool of 11-cis retinal and suggests that very strong bleaches lead to slowed regeneration of pigment, either by reduction in the pool of 11-cis retinal or by some other mechanism. The possibility of depletion of pigment stores has been proposed in the past [6, 22].
Methods
Subjects
The subjects were the authors, aged 25 and 55 at the time of the experiments, with normal eyes apart from minor refractive errors. Ethical approval was obtained from the Cambridge Human Biology Research Ethics Committee, and the study conformed to the Declaration of Helsinki. The pupil of one eye was dilated with two drops of 1 % tropicamide (dilated pupil diameter around 7–8 mm).
Bleaching
Bleaching was delivered to the test eye using a “mini-ganzfeld” (as described by Thomas and Lamb [18]), a small cylinder, painted internally white, illuminated from an incandescent light source (with a yellow filter to cut out UV light) via fibre optic cables. In the present study, the luminance at the cornea (measured with an IL1700 photometer, International Light) ranged from 1.1 × 104 to 1.3 × 105 photopic cd m−2. For a pupil area of around 40 mm2, this corresponds to a retinal illuminance of 4.4 × 105 to 5.2 × 106 photopic Td. However, due to the Stiles-Crawford directional effect [23], the effective retinal intensity is likely to have been lower, and, taking an effective dilated pupil area of 20 mm2 (as used by Paupoo et al. [11]), we estimate that the effective retinal illuminance ranged from 2.2 × 105 to 2.6 × 106 Td.
The eye was exposed to the bleaching light for 1 min, and the subject attempted to keep his eyelids open throughout this time. The mini-ganzfeld (unlike the main ganzfeld) was not fitted with a video camera, so it was not possible to monitor the extent of eyelid closure or pupil constriction during the exposure. However, given the high intensity of illumination, we expect that the bleach would still have been near total, even with eyelid closure for part of the exposure (see next section).
Estimation of bleach levels achieved
To estimate the level of cone pigment bleaching achieved by the exposures, we can use inversion of Eqn (A12) derived in [14]. However, it is simpler to take advantage of the finding reported in Fig. 8 of that paper, that for intensities of 3,000 cd m−2 or more, the cone pigment level effectively reached steady-state well before the end of our 60 s exposure. In this case, the remaining fractional level of cone pigment, P 0, is given by Eqn (A16) of that paper, as
where v is the initial rate of regeneration following a total bleach, σ is the pigment photosensitivity, and I is the light intensity; the fractional bleach is B = 1 − P 0. Using the values of v ≈ 0.48 min−1 and σ−1 ≈ 700 cd m−2 min reported in [14], we calculate that our bleaching intensities of 1.1 × 104–1.3 × 105 cd m−2 should have left only 3–0.3 % of the cone pigment remaining, equating to bleaches of 97–99.7 %; hence, they can be regarded as “near-total” bleaches of cone pigment. For comparison, the cone bleaching levels in our previous study ranged from 27 to 96 %.
In the case of rod pigment, we need to invert Eqn (A12) of [14]. Substituting standard rod parameters of v = 0.085 min−1 and σ−1 = 106.8 Td s, as used by Cameron et al. [20], we calculate that our 1-min exposures would have bleached 98–99.6 % of rhodopsin. For comparison, the exposures used in our previous study would only have bleached 14–75 % of the rhodopsin.
Flash stimuli
Red flashes were delivered in the main ganzfeld (described by Smith and Lamb [24]), using a xenon flash gun fitted with a red filter (610 nm long pass). Dim test flashes (0.40 photopic cd m−2 s) were delivered before, and for several minutes after, each bleaching exposure; they were arranged in sets of 15 flashes every 10 s, at an inter-flash interval of 0.5 s, leaving a 3 s pause after the fifteenth flash to allow time for blinking.
A dim background (38 scotopic cd m−2, corresponding to approximately 1,600 scotopic Td), provided by a single blue LED (470 nm peak), was presented throughout to saturate the rod photoreceptors and hence isolate cone-system responses.
An infra-red-sensitive video camera at the rear of the main ganzfeld provided an image of the subject’s eye and allowed the pupil diameter and possible eyelid closure to be monitored [24].
ERG recording
A conductive fibre electrode, placed in the lower conjunctival sac, was used to record ERG a-wave responses to the flashes, as described previously [24].
Estimation of post-bleach pigment levels from a-wave measurements
Our approach to estimating the pigment level assumes that the observed reduction in post-bleach response amplitude is due solely to the reduction in the effective intensity of the flashes resulting from pigment depletion. In the Discussion, we comment on the validity of this assumption for photopic dim-flash responses.
The compressive relation, rewritten from Eqn (7) of Mahroo and Lamb [14], is
where r is the response amplitude (measured at some fixed time after the flash), r max is the maximal value, Q is the flash quantity, Q 0 is a constant, and P is the fractional level of photopigment present. The values of r max and Q 0 were derived for each subject by recording responses to flashes of different intensity (both dimmer and brighter than the test-flash intensity) in dark-adapted conditions in an experiment of the kind illustrated in Fig. 1 of [14]. If we denote r DA as the dark-adapted test-flash response amplitude (i.e. when P = 1, either before, or upon complete recovery after, a bleach), we can rewrite Eq. (2) independent of Q/Q 0 as
Our ERG a-wave measurements can then be transformed into “linearized” estimates of cone pigment level, by inversion of Eq. (3), according to Eqn (10) of [14], rewritten here as
Kinetics of recovery
In fitting the post-bleach pigment regeneration curves, we chose to apply the fitting to the raw a-wave measurements, by compressing the predicted recoveries for P according to the compressive relationship measured prior to bleaching; that is, by substitution into Eq. (3). This enabled us to avoid having to transform the error bar estimates and thereby simplified our use of the least-squares fitting algorithm in Origin (OriginLab Corp.). We employed two alternative descriptions of pigment regeneration kinetics: either a single exponential, or the rate-limited formulation, described, respectively, by Eqns (11) and (A6) in [14]. Following the fitting procedure, we transformed the raw amplitude data into the scale of pigment level using Eq. (4) above.
In our previous fitting, the bleach level, B, was a free parameter, but in the present study, the exposures were so intense that it was appropriate to set B = 1. For the exponential description, the pigment kinetics are specified by a single free parameter: the time constant of recovery, τ. For the rate-limited description, there are potentially two free parameters specifying the pigment kinetics: v, the initial rate of regeneration following a total bleach, and K m, the fractional bleach at which regeneration proceeds at half the theoretical maximal rate. As we found previously [14], the best-fitting value of K m was around 0.2, but changing K m between 0.1 and 0.3 only weakly influenced the fits, so we chose to hold K m constant at 0.2 as in our earlier study, and accordingly, there was also only a single free parameter for the kinetics in the rate-limited case. Thus, when fitting either model, there were two free parameters: one for the kinetics (τ or v), and one for the final dark-adapted amplitude (r DA); in addition, a third parameter (r max) described the degree of response compression, but as its value had been determined in separate experiments, it was not a free parameter in the least-squares fitting.
Results
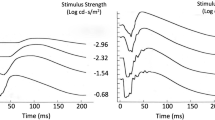
Figure 1a plots ERG a-wave responses from subject OARM to the dim red flashes after a 1-min exposure to a 4.0 × 104 photopic cd m−2 bleaching light. Responses are averaged from seven repetitions of the experiment using identical exposures. The smallest response was obtained immediately after extinction of the bleaching exposure, while the larger responses were obtained at progressively later times, up to 9 min post-bleach. The remaining panels plot the post-bleach time-course of recovery, either (in B–D) for response amplitude, or (in E–F) after conversion to our linearized estimate of pigment level. The lower two panels on the left present the raw amplitudes measured in each of the seven repetitions (B) and the average amplitudes for the first two and the last two repetitions (C); these controls will be considered subsequently.
Averaged response amplitudes and estimated pigment levels, following repeated bleaching exposures at a single strength, for subject OARM. a averaged responses to dim red flashes (0.4 photopic cd m−2 s) following a 1-min bleaching exposure to yellow light of 4.0 × 104 photopic cd m−2. Pupil diameter ~8 mm; estimated cone pigment bleach >99 %. Traces are averaged over successive time windows of 20 s (i.e. they include two groups of 15 responses to flashes at 0.5 s intervals), and over 7 repetitions of the exposure; hence, each trace is the average of up to 210 responses. All measurements of response amplitude in subsequent panels were made over the indicated window, 14–15 ms after flash delivery. b raw response amplitudes for the seven separate repetitions used for a. c Response amplitudes averaged from the first two (filled squares) and the last two (open squares) of the seven repetitions used for a. Error bars plot S.E.M. d points plot the amplitudes (mean ± S.E.M.) of the traces in a, as a function of post-bleach time. The S.E.M. here is calculated for up to 210 responses, that is, taking into account all 7 exposures. The curves plot the least-squares best fits obtained, assuming a full bleach (B = 1) and with response compression according to Eq. (3) using the measured r max = −17 μV (see “Methods” section), when pigment regeneration followed either of two kinetic forms, as follows. Red curve single exponential (r DA = −11.0 μV and τ = 2.30 min or τ−1 = 0.43 min−1). Solid black curve rate-limited recovery (r DA = −10.6 μV, K m = 0.2, v = 0.32 min−1). Dashed curve rate-limited recovery with v constrained to 0.5 min−1. e,f points plot estimates of pigment level transformed from d using Eq. (4), and curves plot the kinetics of pigment regeneration for the parameters fitted in that panel. e Exponential pigment regeneration kinetics. f Rate-limited pigment regeneration kinetics. The points differ slightly between the panels because fitting with the two models gave slightly different estimates of r DA
Figure 1d plots the amplitudes of the averaged responses in panel A, measured over the indicated window of 14–15 ms post-flash delivery, against post-bleach time. On the assumption that the observed reduction in response amplitude is due to pigment depletion, we fitted the recovery predicted by the two kinetic models for pigment regeneration, with compression according to the response intensity relation that had been measured for dark-adapted flashes (see “Methods” section). The red curve plots the best fit to the measured recovery on the assumption that pigment regenerates as a first-order process (single exponential)—the legend gives the value of the time constant, τ, and also its reciprocal, the rate constant, τ−1, to facilitate comparison with the rate parameter of the rate-limited model, v, which also has units of min−1. The solid black curve is the best fit on the assumption of rate-limited regeneration. The rate-limited model appears to provide a better fit visually, and this is confirmed by the chi-square goodness of fit parameter (0.87 for the rate-limited and 1.90 for the exponential, with 22 degrees of freedom in both cases).
It is interesting that the magnitude of the initial rate v, determined as 0.32 min−1, is considerably (36 %) lower than the value of 0.5 min−1 that we found previously [14] for this subject (OARM), across a range of lower bleach strengths (exposure durations ranging from 5 s to several minutes, and intensities ranging from 700 to 3,000 cd m−2). For comparison, the dashed black curve plots the best-fitting rate-limited recovery when the initial rate v was constrained to be 0.5 min−1; this curve clearly provides a poor fit.
The raw measurements of response amplitude in Fig. 1d may be converted to predictions of pigment level, by transforming according to Eq. (4) as shown in the lower panels on the right. Note, though, that our curve-fitting was always done in terms of the raw amplitudes, to avoid the need to transform the error bar estimates. Figure 1e plots the fit of pigment recovery obtained using a single exponential, while Fig. 1f plots the fit for the rate-limited model. Again, the superiority of the fit obtained using the rate-limited model is apparent by visual inspection.
Our finding that the initial rate of recovery for very strong bleaches is lower than we previously found using a range of weaker bleaches suggests that very strong bleaches lead to a slowing of pigment regeneration, possibly as a result of depletion of some pool of retinoid (see “Discussion” section). In this case, the question arises as to whether the seven bleaches that we delivered in the experiment of Fig. 1 had cumulative effects; for example, through successive reductions in the pool. This possibility is explored in the lower panels on the left of Fig. 1. Figure 1b superimposes the recovery of response amplitude from each of the seven individual exposures. The later recoveries are not obviously different from the earlier ones. Figure 1c attempts to improve the signal-to-noise ratio by averaging separately the first two and the last two exposures. The comparison here is a little clearer, and one can more confidently assert that the last two recoveries are not appreciably slower than the first two—if anything they may be a little faster, although within the bounds of error. We conclude that the effect of a very strong bleach, in eliciting slower regeneration than seen after smaller bleaches, is not augmented by further repetitions of that bleach at intervals of 10–15 min.
Comparison with previous densitometry data
The bleaching intensity in the present study is similar to that used in some previous studies of cone pigment regeneration using retinal densitometry. The open squares in Fig. 2 re-plot the densitometry measurements from Fig. 2 of van Norren and van der Kraats [25] following a 2-min exposure to 5.6 log photopic Trolands. In the present study, the exposure for subject OARM was for 1 min at 5.9 log photopic Td (assuming an effective pupil area of 20 mm2), which yields almost exactly the same integrated exposure, of 7.7 log photopic Td s, and an estimated bleach level of >98 %. The filled squares in Fig. 2 re-plot our estimates from Fig. 1f of the pigment level following this exposure, and it can be seen that the recoveries obtained using densitometry and the ERG are very similar. The densitometric readings are fairly direct measurements of pigment level, whereas our approach involves certain assumptions in estimating the pigment level from ERG responses (see “Discussion” section), including the assumption that post-receptoral signals are similar before and after the bleach. The similarity between the two measures of recovery supports the notion that our method indeed yields an estimate of the post-bleach recovery of pigment levels.
Comparison of recoveries measured using the ERG and using retinal densitometry. Filled symbols re-plot the estimates of cone pigment recovery from Fig. 1f, using the right-hand ordinate. The 1-min exposure to 4.0 × 104 cd m−2 corresponds to ~5.9 log photopic trolands (assuming an effective dilated pupil area of ~20 mm2 after adjustment for the Stiles-Crawford effect). Open symbols re-plot the densitometry measurements from Fig. 2 of van Norren and van de Kraats [25], using the left-hand ordinate, and were obtained after a 2-min exposure to 5.6 log photopic trolands. The ordinates have been scaled such that a density of 0.42 corresponds to a full complement of pigment
Exposures of different intensity
Figure 3 shows recoveries for subject TDL following 1-min exposures to three different levels of intense bleaching illumination (1.1 × 104, 4.4 × 104 and 1.3 × 105 cd m−2). The symbols plot response amplitude, measured at a fixed time after flash delivery, as a function of post-bleach time. The left-hand panels show the fit to recovery assuming that pigment regenerates according to the rate-limited model—with v constrained to be the same for all three bleach strengths, and with K m held at 0.2 (see “Methods” section); the right-hand panels show the fit according to a single exponential, with the time constant constrained to be the same for all three bleach strengths. Once again, the rate-limited model provides a closer fit visually, and the chi-square goodness of fit parameter confirms this (1.25 for the rate-limited, and 2.52 for the exponential, with 66 degrees of freedom in both cases). The rate v that fitted these recoveries best was 0.39 min−1, again considerably (17 %) slower than the rate of 0.47 min−1 found for the same subject across a range of lower intensity bleaching exposures (ranging from 5 s to several minutes in duration, and between 500 and 3,000 cd m−2 in intensity) [14].
Recoveries following bleaching exposures of different intensities for subject TDL. Points plot amplitudes (mean ± S.E.M.) of responses to the dim red flashes (delivered according to the protocol in Fig. 1) following 1-min exposures to three intensities (1.1 × 104, 4.4 × 104 and 1.3 × 105 photopic cd m−2); pupil diameter ~7 mm. Points are averaged over successive time windows 20 s wide, and over three repetitions of each intensity, so each point is averaged from up to 90 responses. In the left-hand panels pigment regeneration was assumed to follow rate-limited kinetics (K m = 0.2, v = 0.39 min−1, B = 1 for all recoveries). In the right-hand panels, a single exponential process was assumed (τ = 1.85 min, corresponding to τ−1 = 0.54 min−1, and B = 1 for all recoveries). In determining the best fits, response compression according to Eq. (3) was applied, using the measured value of r max = −26 μV
If the rate v varies with bleaching level, then it may not be valid to constrain the value of v to be the same for the three different exposure intensities in Fig. 3. By visual inspection, it does appear that recovery from the least intense of these backgrounds (top row) is marginally faster than that from the higher intensity backgrounds. However, the scatter in the data precludes a firm conclusion in this regard.
Discussion
The results of this study support the notion that cone pigment regeneration, as estimated using the ERG photopic dim-flash response, follows rate-limited rather than exponential kinetics, proceeding at an initial rate that is approximately constant. However, the limiting rate appears to be markedly lower (by 17–36 %) for very strong exposures, of the order of 104 to 105 cd m−2 viewed through a dilated pupil for 1 min, than for smaller bleaches. The estimated kinetics are very similar to those from previous studies of cone pigment recovery using reflection densitometry.
In our earlier study [14], we commented on the finding that a lower rate was needed to fit pigment regeneration data from densitometry studies compared to recovery measured using our ERG approach and speculated that regeneration might be slower in the foveal or parafoveal region (the focus of the densitometry studies) than when averaged across the whole retina (as assessed by the ERG study). However, in light of our new results, we feel it more likely that the discrepancy resulted from differences in the strength of the bleaches between the two studies.
Use of the photopic a-wave to estimate pigment levels
The photopic dim-flash a-wave response, measured at 14–15 ms post-flash, is generated in part by the cone photoreceptors, together with a substantial, possibly greater, contribution from OFF-bipolar cells [26]. The assumption in this study has been that the reduction in post-bleach amplitude is due entirely to a reduction in the effective intensity of the flashes, as a result of photopigment depletion. Other factors that could also account for the reduction in amplitude and thus affect the validity of our assumption are as follows: changes within the photoreceptors themselves (outer or inner segment), or within the OFF-bipolar cells, or at the synapse linking them.
Changes within the photoreceptor outer segments that might influence the interpretation would include reduction in photoreceptor circulating current, reduction in the amplification of the activation phase of phototransduction, or a speeding up of recovery of the photoresponse. In rods, light-generated products (metarhodopsins and opsin) activate the phototransduction cascade in darkness, eliciting an “equivalent background” that can desensitize the rods. As there is emerging evidence that similar activation and desensitization may occur in amphibian cones [28, 29], we now consider the situation in human cones. Cone photoreceptor circulating current, as assessed with bright-flash ERG a-wave responses, is reduced during a bleaching exposure, but (in contrast to rod current) has been shown to recover extremely rapidly following such exposures [11], so would be expected to be back to the dark-adapted level in the post-bleach period. Thus, in contrast to rods, the equivalent photoproducts in cones either do not cause a reduction in circulating current or are removed so rapidly that the current is able to recover immediately. Regarding the amplification of the activation phase of the photoresponse, we have no reason to expect any reduction in this in cones following such bleaching exposures, although this has not been explicitly tested; human rod responses do not appear to show a reduction in amplification during or after bleaching exposures [18]. Thirdly, considering the recovery phase of the photoresponse, although this does accelerate with bright light and may remain accelerated following extinction of the bleaching light, our amplitude measurements were made at an early time (14–15 ms) after flash delivery, where any acceleration of the recovery phase should have little influence.
It has been shown that the peak/sag nature of the ERG a-wave is determined in part by capacitive currents [27], in addition to conductance changes in the photoreceptor inner segment and axon, and so any post-bleach modification of these contributions might have some effect. However, at least in the case of rods, these changes only cause significant effects at times after the peak of the a-wave, so that the scotopic a-wave faithfully reflects outer segment current almost until the peak. We are therefore assuming it is unlikely that post-bleach changes in such contributions would significantly affect the cone a-wave at our early measurement times. The similarity in shape of post-bleach responses, compared with pre-bleach responses to dimmer flashes [14], lends support to this assumption.
Another possible confounding factor is the OFF-bipolar cell contribution. Should the gain of cone to bipolar cell transmission change after a bleach, this would affect our interpretation of the a-wave responses. We addressed this previously [14], by estimating recovery using the bright-flash a-wave, measured at extremely early times following the flash, when the post-receptoral contribution is expected to be minimal. We found that the recovery was very similar to that obtained using dim flashes. Our assumption is that this finding also holds for the very intense exposures used in the present study, although this has not been explicitly tested.
Finally, the close correspondence between the recovery that we derived from the ERG and that previously derived using reflection densitometry (Fig. 2) would also suggest that our measurements do indeed estimate pigment recovery.
Slowed rate of regeneration
The finding that cone pigment appears to regenerate more rapidly following smaller or shorter bleaches is not new. Rushton and Henry [6] proposed a readily accessible store of 11-cis retinal as one possible explanation of rapid regeneration following a short bleaching exposure. Smith et al. [9] presented data consistent with the rate of regeneration increasing as the proportion of bleached photopigment decreased, and the Rushton-Henry proposition of depletion of a store 11-cis retinal was invoked later to explain such observations [22].
Although a rate-limited model of pigment regeneration satisfactorily explains the form of the recovery kinetics observed previously for bleaches of cone pigment from less than 30 % to more than 90 % [14], the present study shows that it does not provide a complete description that extends to even higher levels. After very strong bleaches, the initial rate of recovery appears to be lower, though the data are still better fitted by the rate-limited expression than by an exponential recovery.
Lamb and Pugh [13] proposed a biophysical basis for rate-limited regeneration, in which the delivery of 11-cis retinal to the outer segments is limited by diffusion from a pool of constant concentration in the retinal pigment epithelium (RPE). In this model, the initial rate v of recovery following a total bleach is proportional to C/R where C is the concentration of 11-cis retinal in the RPE pool, and R is the resistance to its movement between the pool and the photoreceptor outer segments. Thus, a reduction in rate, seen after very strong bleaches, might be explained by a reduction in C and/or by a rise in R. We feel that depletion of C is more likely, as there is no particularly reason to suspect any change in resistance to retinoid movement. In future, it might be possible to test the potential role of pool depletion versus resistance by examining the slope of the “S2” component of cone psychophysical dark adaptation, because this slope is predicted to be affected only by C and not by R [13].
It is important to bear in mind that the specific biophysical model mentioned in the previous paragraph is only one of several potential mechanisms that could underlie the observed limitation to the rate of pigment regeneration. Other likely explanations would include possible rate limitations in the chain of enzymatic reactions producing 11-cis retinal. Hence, it is entirely possible that the reduced rate of regeneration observed after very strong bleaches might be caused by a reduction in the concentration of a retinoid species other than 11-cis retinal (for example, 11-cis retinol or retinyl ester).
The near-total bleaches (97–99.7 % of the pigment) in our present study elicited a regeneration rate v that was around 17–36 % lower than we found in our previous study [14] where we estimated the strongest exposures to have bleached 89 and 96 % for the two subjects. It may at first seem surprising that a relatively modest increase in fractional bleach level could elicit such a substantial change in regeneration rate, but we think that factors other than just the bleach level may be involved. For example, the concentration of 11-cis retinal in the rod outer segments is expected to have been around tenfold lower initially, and it is conceivable that this could have led to some transient alteration of the outer segment properties. Alternatively, the intense bleach may have had some other effect, perhaps through a direct action on any of the proteins of the retinoid cycle, or possibly by affecting another protein in the RPE such as RGR (see [30]).
Location of the presumed pool of retinoid
It is now known that, in contrast to rods that have access only to 11-cis retinal from the RPE, cones have access to an additional source of 11-cis retinol from Müller cells [1] and further that cones, but not rods, can convert 11-cis retinol to retinal. A recent study in salamander retina has suggested that this pathway appears to have comparable capacity to the more conventional RPE pathway [3]. Might the existence of this second pathway help explain our finding of a lower rate of cone pigment regeneration after extremely strong bleaches?
One possibility we suggest is that the strong bleaches used here lead to a reduction in the extent to which the cones can access retinoid from the RPE pool. This might result from depletion of the RPE store of retinoid, due to the much higher level of rod bleach (see “Methods” section for estimated levels). Alternatively, it might result from greater competition for the available RPE pool from the heavily retinoid-depleted rods, which are far more numerous than the cones. On this basis, the regeneration of cone pigment after very strong bleaching of the rods may depend primarily on the Müller cell and cone inner segment pathway, with a reduced contribution from the RPE pathway. The finding that regeneration rates are similar after seven repetitive intense bleaches (Fig. 1b, c) suggests that the retinoid pool available for cone pigment regeneration in such conditions is completely restored within 10–15 min of dark adaptation (the repetition interval between the bleaching exposures). If this restoration time was much more rapid than the regeneration time for rhodopsin, then it might indicate that the cones are only weakly reliant on the RPE pathway. However, we are unable to make such an assertion, because the rhodopsin regeneration time is in fact comparable, with more than 95 % regenerating within 15 min of a total bleach (see Fig. 9 of [13]).
References
Mata NL, Radu RA, Clemmons RC, Travis GH (2002) Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron 36:69–80
Miyazono S, Shimauchi-Matsukawa Y, Tachibanaki S, Kawamura S (2008) Highly efficient retinal metabolism in cones. Proc Nat Acad Sci 105:16051–16056
Wang JS, Estevez ME, Cornwall MC, Kefalov VJ (2009) Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci 12:295–302
Kolesnikov AV, Tang PH, Parker RO, Crouch RK, Kefalov VJ (2011) The mammalian cone visual cycle promotes rapid M/L-cone pigment regeneration independently of the interphotoreceptor retinoid-binding protein. J Neurosci 31:7900–7909
Takahashi Y, Moiseyev G, Chen Y, Nikolaeva O, Ma J-X (2011) An alternative isomerohydrolase in the retinal Müller cells of a cone-dominant species. FEBS J 278:2913–2926
Rushton WA, Henry GH (1968) Bleaching and regeneration of cone pigments in man. Vision Res 8:617–631
Hollins M, Alpern M (1973) Dark adaptation and visual pigment regeneration in human cones. J Gen Physiol 62:430–447
Coile DC, Baker HD (1992) Foveal dark adaptation, photopigment regeneration, and aging. Vis Neurosci 8:27–39
Smith VC, Pokorny J, van Norren D (1983) Densitometric measurement of human cone photopigment kinetics. Vision Res 23:517–524
Burns SA, Elsner AE (1985) Color matching at high illuminances: the color-match-area effect and photopigment bleaching. J Opt Soc Am A: 2:698–704
Paupoo AA, Mahroo OA, Friedburg C, Lamb TD (2000) Human cone photoreceptor responses measured by the electroretinogram a-wave during and after exposure to intense illumination. J Physiol 529:469–482
Lamb TD (1981) The involvement of rod photoreceptors in dark adaptation. Vision Res 21:1773–1782
Lamb TD, Pugh EN Jr (2004) Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 23:307–380
Mahroo OAR, Lamb TD (2004) Recovery of the human photopic electroretinogram after bleaching exposures: estimation of pigment regeneration kinetics. J Physiol 554:417–437
Padmos P, Norren DV (1972) The vector voltmeter as a tool to measure electroretinogram spectral sensitivity and dark adaptation. Invest Ophthalmol 11:783–788
Binns A, Margrain TH (2005) Evaluation of retinal function using the Dynamic Focal Cone ERG. Ophthalmic Physiol Opt 25:492–500
Wood A, Margrain T, Binns A (2011) The effect of bleach duration and age on the ERG photostress test. Graefes Arch Clin Exp Ophthalmol 249:1359–1365
Thomas MM, Lamb TD (1999) Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram. J Physiol 518:479–496
Kenkre JS, Moran NA, Lamb TD, Mahroo OA (2005) Extremely rapid recovery of human cone circulating current at the extinction of bleaching exposures. J Physiol 567:95–112
Cameron AM, Mahroo OA, Lamb TD (2006) Dark adaptation of human rod bipolar cells measured from the b-wave of the scotopic electroretinogram. J Physiol 575:507–526
Ruseckaite R, Lamb TD, Pianta MJ, Cameron AM (2011). Human scotopic dark adaptation: Comparison of recoveries of psychophysical threshold and ERG b-wave sensitivity. J Vis. 11(8)
Coolen AC, van Norren D (1988) Kinetics of human cone photopigments explained with a Rushton-Henry model. Biol Cybern 58:123–128
Stiles WS, Crawford BH (1932) Equivalent adaptational levels in localized retinal areas. In: Report of a joint discussion on vision, physical society of London. Cambridge University Press, Cambridge, pp 194–211 (reprinted in Stiles WS (1978) Mechanisms of colour vision. Academic Press, London)
Smith NP, Lamb TD (1997) The a-wave of the human electroretinogram recorded with a minimally invasive technique. Vision Res 37:2943–2952
van Norren D, van de Kraats J (1989) Retinal densitometer with the size of a fundus camera. Vision Res 29:369–374
Robson JG, Saszik SM, Ahmed J, Frishman LJ (2003) Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 547:509–530
Robson JG, Frishman LJ (2011) The a-wave of the electroretinogram: importance of axonal currents. Invest Ophthalmol Vis Sci 52:E-Abstract 692
Cornwall MC, Matthews HR, Crouch RK, Fain GL (1995) Bleached pigment activates transduction in salamander cones. J Gen Physiol 106:543–557
Estevez ME, Kolesnikov AV, Ala-Laurila P, Crouch RK, Govardovskii VI, Cornwall MC (2009) The 9-methyl group of retinal is essential for rapid Meta II decay and phototransduction quenching in red cones. J Gen Physiol 134:137–150
Wenzel A, Oberhauser V, Pugh EN Jr, Lamb TD, Grimm C, Samardzija M, Seeliger MW, Remé CE, von Lintig J (2005) The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem 280:29874–29884
Acknowledgments
We thank Julal Syed for help with experiments. Supported by grant 034792 from the Wellcome Trust, by a Merck, Sharpe and Dohme studentship to OARM, and by ARC Centres of Excellence grant CE561903.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahroo, O.A.R., Lamb, T.D. Slowed recovery of human photopic ERG a-wave amplitude following intense bleaches: a slowing of cone pigment regeneration?. Doc Ophthalmol 125, 137–147 (2012). https://doi.org/10.1007/s10633-012-9344-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-012-9344-z