Abstract
The initial time course of the change in photoreceptor outer segment membrane conductance in response to light flashes has been modelled using biochemical analysis of phototransduction, and the model has been successfully applied to a range of in vitro recordings and has also been shown to provide a good fit to the leading edge of the electroretinogram a-wave recorded in vivo. We investigated whether a simple modification of the model’s equation would predict responses to the onset of steady illumination and tested this against electroretinogram recordings. Scotopic electroretinograms were recorded from three normal human subjects, using conductive fibre electrodes, in response to light flashes (0.30–740 scotopic cd m−2 s) and to the onset of steady light (11–1,900 scotopic cd m−2). Subjects’ pupils were dilated pharmacologically. The standard form of the model was applied to flash responses, as in previous studies, to obtain values for the three parameters: maximal response amplitude r max, sensitivity S and effective delay time t eff. A new “step response” equation was derived, and this equation provided a good fit to rod responses to steps of light using the same parameter values as for the flash responses. The results support the applicability of the model to the leading edge of electroretinogram responses: in each subject, the model could be used to fit responses both to flashes of light and to the onset of backgrounds with a single set of parameter values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Analysing the biochemical stages in the activation phase of the phototransduction cascade, Lamb and Pugh derived an equation (later termed the “LP model”) to describe the reduction in photoreceptor circulating current in response to a flash of light [1]. This expression has been shown to successfully fit responses obtained by suction pipette recording from both amphibian and mammalian photoreceptors (see for example [2] for fits to data obtained from previous studies on salamander and human rods).
The electroretinogram (ERG) represents electrical responses from the living eye, and the leading edge of the a-wave is regarded as being driven by the photoreceptor response. By assuming the a-wave to be proportional to the outer segment photocurrent, the parameters of phototransduction can be determined in vivo. The LP model has been shown to provide reasonable fits to both the scotopic and photopic a-waves [3–5]. However, several studies have shown that post-receptoral signals also make contributions to the a-wave [6–8]. Robson et al. [9] showed in 2003 that the macaque cone-driven a-wave contained post-receptoral contributions, even at relatively early times in the case of bright flashes. They showed that the rod-driven a-wave, however, appeared to represent the photoreceptor response up to near the peak. More recent evidence from that group suggests that the form of the initial peak and the subsequent sag appears to result from capacitive currents in the rod axons [10], but here we restrict consideration to the period prior to the peak.
In this study, we recorded electroretinogram responses to flashes of light and also to the onset of steady illumination (a “step” of light). The purpose was to explore whether the LP model could be extended to derive an expression describing photoreceptor responses to the onset of steady light. We concentrated on the leading edge of the rod-driven scotopic ERG, as this was more likely (than the cone-driven a-wave) to represent the photoreceptor current. Some of our findings have been published in preliminary form [11].
Methods
Stimuli and recording
The ERG was recorded by means of a conductive fibre electrode placed in the subject’s lower conjunctival fornix, beneath the lower eyelid. Stimuli were delivered via a white integrating sphere, viewed by the subject through a monocular viewing port, as described by Smith and Lamb [5]. Stimuli (flashes or steady illumination) were delivered from an array of up to 12 ultra-bright “white” LEDs (Luxeon™ LXHL-BW01, Lumileds, San Jose, CA, USA) that illuminated the sphere (providing a maximal steady intensity of 1,900 scotopic cd m−2). In addition, brighter flashes (up to 740 scotopic cd m−2 s) were delivered from a photographic flash gun (Mecablitz 60CT4, with N23 power pack; Metz), with a blue filter (450 nm peak, Lee 195), allowing maximal stimulation of the scotopic, but minimal stimulation of the photopic, system.
The duration of the flash stimuli ranged from 0.04 to 3 ms. Given that the ERG a-wave becomes obscured by other signals after a few tens of milliseconds, we were able to restrict the duration of the “step of light” to just 50 ms. Stimuli were repeated several times, so that responses could be averaged, and the inter-stimulus interval ranged from 5 to 60 s (for the brighter stimuli) to ensure recovery of the photoreceptors between stimuli.
Isolation of rod-driven responses was achieved as follows: stimuli were first delivered in the dark (giving a response with both rod- and cone-driven contributions); identical stimuli were then delivered in the presence of a blue rod-saturating background (provided by a blue LED, giving a luminance of ~2.5 photopic and 35 scotopic cd m−2), to give the cone-driven response; the latter responses were subtracted from the former, to yield the isolated rod-driven response. Doubling the luminance of the rod-saturating background had little effect on the photopic responses, suggesting that the cones were minimally desensitised by such a background.
Subjects
Three normal subjects (authors of the present study, HCC, VSB and OM) participated (aged 20, 21 and 31), with no ocular morbidity other than refractive error (two subjects were myopes). The left eye was used, and the pupil was dilated pharmacologically with two drops of 1% tropicamide (as well as 2.5% phenylephrine in the case of one subject). The subject’s eye could be viewed via an infrared-sensitive video camera, allowing monitoring of eyelid position and measurement of pupil diameter.
The subjects gave written, informed consent. Ethical approval was obtained from the Cambridge Human Biology Ethics Committee, and the research adhered to the tenets of the Declaration of Helsinki (Code of Ethics of the World Medical Association).
Modelling the responses
Lamb and Pugh in 1992 [1] systematically modelled the kinetics of each activation stage in phototransduction following a light flash, at times sufficiently short that inactivation reactions could be ignored. At these early times, they predicted that in response to the brief flash: the number of activated rhodopsin molecules increases in a stepwise manner; the numbers of activated G proteins and phosphodiesterase (PDE) molecules increase linearly with time t; and the reductions in cyclic GMP and in circulating current then initially proceed as functions of t 2. The final equation describing the response r at time t after the flash is:
where r max is the maximal response amplitude (proportional to the summed circulating current of the rods in the dark), Φ is the number of photoisomerisations produced by the flash (proportional to the flash strength), A is an amplification constant, which combines the gains of the various steps in the phototransduction cascade, and t eff is an effective delay time, combining all the delays in the cascade, as well as any electrical filtering delay. It can be seen that Eq. 1 includes a static nonlinearity in the form of an exponential: the theoretical basis for expecting this form of nonlinearity is developed by Lamb and Pugh [1]. This form of model, in which an input proportional to the number of photoisomerisations passes through a linear filter whose output is subject to a static nonlinearity, is not unique, but Lamb and Pugh derived the above relation by considering the underlying molecular steps. Other, more complex, empirical models have been proposed that provide a good description of the response (e.g. Robson et al. [9]). The static nonlinearity in some previous models was hyperbolic [12] and not exponential.
In experiments on the intact eye, the number of photoisomerisations Φ can be calculated from the integrated flash strength Q at the retina (in units of Td s, i.e., troland s) according to Φ = Q K rod. However, the value of the conversion factor K rod is not known with certainty, and hence it is more straightforward to rewrite Eq. 1 in terms of flash strength as
where S is a sensitivity factor that is directly related to the conventional amplification constant A by S = A K rod.
One can follow the same analysis to predict the kinetics of each stage in response to onset of a step, rather than a flash, of light: here, the number of activated rhodopsin molecules will increase as a ramp, linearly with time; the number of activated G proteins and PDE molecules will increase parabolically as t 2; the reduction in cGMP and reduction in circulating current would then be expected to proceed initially as a function of t 3. The resultant expression, going through the same analysis, is as follows:
where I is the retinal intensity of the step (in Td, or trolands), and S is identically the same as in Eq. 2.
Applicability of the model to ERG responses
Whilst the model describes the reduction in photoreceptor outer segment current, it has also been applied to the leading edge of the ERG a-wave. The rod-driven a-wave has been shown to arise largely from the photoreceptors, though the origin of the peak and subsequent sag are not yet clearly understood. Capacitance of the photoreceptor outer segment (and possibly the inner segment) is likely to have an effect, and a membrane capacitive time constant has been incorporated when modelling the a-wave response to bright flashes (see Results). Currents in the inner segment and axon may also contribute (investigated recently by Robson and Frishman [10], and it is likely that taking these into account will provide a more complete description of the a-wave, particularly at later times and when the contributions of post-receptoral cells have been removed (either pharmacologically in animal experiments or in human diseases affecting the inner retina). The assumption in this study is that the early part of the rod a-wave can be taken as proportional to the combined rod outer segment current up until almost the peak (prior to intrusion of post-receptoral signals or modification of the photoreceptor electrical response by changes in inner segment/axonal conductances), with the inclusion of a membrane capacitive time constant for bright stimuli. The close fit of the model to this early part of the a-wave suggests that this assumption is not unreasonable.
The fitting procedure was thus an ensemble fit to the “rising” phase of the scotopic-isolated responses (prior to the a-wave peak), using a single set of parameter values for a single subject. The fitting was done by eye and also using the nonlinear least squares fitting algorithm of Origin (OriginLab Corp).
Results
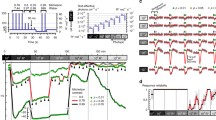
Figure 1 shows ERG responses (black traces) to brief white flashes (upper panel) and to the onset of steady illumination (lower panel) for one subject (HCC). The red traces show the theoretical predictions of Eq. 2 (upper panel) and Eq. 3 (lower panel), with the parameter values r max, S and t eff constrained to be the same in the two panels. (The parameter S is used, as in Eqs. 2 and 3, and in the Discussion, we convert this to the amplification parameter A, of Eq. 1). As the LED flashes lasted up to 3 ms, the responses have been shifted so that time zero corresponds to the midpoint of the flash. Modelling of flash responses was done assuming a flash of finite duration and also assuming an impulse flash (of very short duration), as assumed in Eq. 2. For the flash intensity and durations used, the predicted responses were very similar, so only those determined using Eq. 2 are shown. In both panels, the model curves are shown as solid over the onset phase of the response (which reflects the photoreceptor circulating current) and are continued as dashed thereafter. For both flashes and steps of light, the recorded ERG responses are accurately predicted over the onset phase by Eqs. 2 and 3, respectively, using a common set of parameter values (given in the legend). Thus, it appears that the new equation that we have derived for the step responses provided an adequate description of the experimental responses under the conditions of Fig. 1.
Upper panel shows scotopic-isolated ERG responses (black traces) to white flashes (11–251 scotopic Td s) from subject HCC (pupil area 34 mm2). The red curves show the fit of Eq. 2 to the responses (best-fitting parameters r max = −130 μV, S = 67 s−3 Td−1, t eff = 2.5 ms). The curves are solid over the time period where the fit is appropriate, during the initial down-going, “onset” phase of the response (corresponding to the initial photoreceptor response), and are continued as dashed curves thereafter. Lower panel shows responses (black traces) to steady illumination (427–72 400 scotopic Td) from the same subject. The red curves plot Eq. 3 using the same parameter values as in the upper panel
In Fig. 2, we extend the flash and step measurements to two further subjects (OM and VSB) and to higher flash strengths that were used in Fig. 1. Again, the ERG responses are plotted as the black traces, and the theoretical predictions are plotted as red traces, and for each subject, the three parameters r max, S and t eff have been constrained to the same values in the flash (upper) and step (lower) panels.
Scotopic-isolated ERG responses (black traces) to blue flashes (upper panels, ranging in intensity from 60 to 36,000 scotopic Td s) and to onset of steady white illumination (lower panels, ranging in intensity from 1,300 to 95,000 scotopic Td) in two further subjects (OM and VSB, with pupil areas of 49 and 40 mm2, respectively). Red curves show the expected form of the response using Eq. 2 (upper panels) and using Eq. 3 (lower panels). A single set of parameter values was used for each subject (left-hand panels r max = −155 μV, S = 43 s−3 Td−1, t eff = 3.0 ms; right-hand panels r max = −147 μV, S = 45 s−3 Td−1, t eff = 3.0 ms). The solid red curves are adjusted to take into account a capacitive time constant (0.85 ms and 0.80 ms for left-hand and right-hand panels, respectively; see Text). The dashed red curves (indistinguishable from the solid curves for the majority of the responses) plot Eq. 2 without this adjustment
Using the LED stimulator, we were restricted to a maximum flash intensity of ~300 trolands (~6.6 scotopic cd m−2) before the flash duration became excessive, but in Fig. 2, we used the xenon flash gun to deliver flashes, allowing the flash strength to be up to 100 times greater than in Fig. 1. With the very rapid signal onset elicited by the brightest flashes, though, it is necessary to make explicit allowance for the capacitive time constant (see “Methods”) of the rod photoreceptors (rather than simply lump this in with other delays into t eff), and this may be done by numerical convolution [3, 4] or by analytical convolution [5]. We took both approaches and found them to be very similar. The solid red traces in the upper panels of Fig. 2 plot the resulting predictions (using numerical convolution) for the flash responses. Using this convolution approach, part of the effective delay time t-eff is replaced by an explicit capacitive time constant τ (of 0.80–0.85 ms in Fig. 2). The dashed red traces plot Eq. 2 without taking account of the capacitive time constant. The fit here is reasonable (it is difficult to distinguish them from the solid traces), except for the brightest flashes as expected. The solid red traces in the upper panels of Fig. 2 provide a good fit to the ERG a-wave measurements over a wide range of intensities and thereby provide a good test of the theory for flash responses in these two subjects.
The lower panels in Fig. 2 show responses (black traces) to the onset of steps of white light in the same subjects, and the red curves show the fit of Eq. 3, using the same parameter values as used for fitting the flash responses in the respective upper panels. Again, the model appears to closely predict the initial phase of the responses. The solid and dashed traces are indistinguishable, suggesting that the capacitive time constant did not need to be explicitly incorporated as the responses are not so rapidly rising. We would expect, however, that, had we been able to deliver a sufficiently bright light step, the fastest responses would require consideration of capacitance.
Discussion
We extended a model for the activation phase of photoreceptor responses to flashes of light to derive a new expression that would predict the response to the onset of a step of light. We tested this expression against human scotopic rod-driven ERG recordings to the onset of steps of light of different intensities and found that the responses did appear to proceed initially with the cube of time and could be described accurately by the new expression. Importantly, the flash responses and step responses from a given subject could be described using a common set of parameter values.
The parameter values derived in the present study are within the range of those reported in previous studies of the scotopic a-wave in human subjects where similar parameters have been described [3–5, 13]. The value for the amplification constant A can be derived from the sensitivity parameter S by dividing by the conversion factor, K rod, which was estimated as 8.6 photoisomerisations per rod per scotopic Td s by Breton et al. [13]. This would give values of A for our subjects ranging from 5.0 to 7.8 s−2, agreeing with previously reported values: Smith and Lamb reported values (mean ± standard deviation) ranging from 3.9 ± 1.6 to 6.9 ± 1.1 s−2 from their study [5] and previous studies [4, 13, 14]. Our values for the maximal response, r max, however, appear to fall below, or at the lower end of, the range (195 ± 39 to 463 ± 81 μV) reported in these previous studies. The conductive fibre electrode gives a lower amplitude response than other methods of ERG recording, and in our experiments, we chose to position the electrode deep in the lower conjunctival fornix. This gives a more stable recording, though one of the lower amplitude [15], and this may explain discrepancies in response magnitude between laboratories.
In this study, we used the initial phase of the ERG as an approximate measure of the massed responses of human rod photoreceptors in vivo. However, the ERG contains responses from other retinal cells, and so it is not certain that the recorded responses are free from contributions from other cells even at very early times. Previous studies in macaques do suggest that the scotopic a-wave (in contrast to the photopic a-wave) response to flashes does appear to represent the photoreceptor response up to the peak: although post-receptoral signals contribute—a negative inner retinal signal and the early positive-going rod bipolar cell signal—they appeared to cancel one another out initially, hence not affecting the magnitude of the response [9]. It was in view of these previous findings—that the scotopic ERG provides a more faithful representation of the rod response than the photopic ERG does of the cone response—that we concentrated on scotopic responses at early times. Also, as discussed in the Methods, the ERG a-wave is likely to be modified by factors in addition to the photoreceptor outer segment current (including inner segment and axonal currents [10]); however, this study has taken the good fit of the model to the initial part of the a-wave response to flashes as a basis for assuming that this part of the a-wave dose reflects outer segment current, modified by the incorporation of a single, “capacitive” time constant in the case of bright stimuli. Although there is no direct evidence in the literature to show that the scotopic ERG responses to steps of illumination do indeed accurately reflect signals from the rod photoreceptors, our results certainly support that view. Thus, the fact that these responses could be fit with the same parameters as the flash responses supports the notion that the scotopic ERG response to onset of steps of light reflects the photoreceptor response up to, or close to, the initial a-wave peak.
The present study also brings out the importance of flash duration in affecting the form of the response. ERG studies often use long flash durations, sometimes deliberately to separate on and off components of a response [16]. However, a long flash duration of several tens of milliseconds will not only alter the timing of on and off components, but will also alter the initial form of the photoreceptor response itself (a long flash effectively becomes a step of light), and so direct comparisons of response-amplitudes may need to take this into account. The expressions derived in this study demonstrate explicitly how the forms differ and they allow equivalent phototransduction parameters to be extracted from both forms of response, facilitating quantitative comparison.
References
Lamb TD, Pugh EN Jr (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol 449:719–758
Lamb TD, Pugh EN Jr (2006) Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci 47:5137–5152
Hood DC, Birch DG (1995) Phototransduction in human cones measured using the alpha-wave of the ERG. Vis Res 35:2801–2810
Cideciyan AV, Jacobson SG (1996) An alternative phototransduction model for human rod and cone ERG a-waves: normal parameters and variation with age. Vis Res 36:2609–2621
Smith NP, Lamb TD (1997) The a-wave of the human electroretinogram recorded with a minimally invasive technique. Vis Res 37:2943–2952
Bush RA, Sieving PA (1994) A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 35:635–645
Jamison JA, Bush RA, Lei B, Sieving PA (2001) Characterization of the rod photoresponse isolated from the dark-adapted primate ERG. Vis Neurosci 18:445–455
Robson JG, Frishman LJ (1996) Photoreceptor and bipolar cell contributions to the cat electroretinogram: a kinetic model for the early part of the flash response. J Opt Soc Am A Opt Image Sci Vis 13:613–622
Robson JG, Saszik SM, Ahmed J, Frishman LJ (2003) Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 547:509–530
Robson JG, Frishman LJ (2011) The a-wave of the electroretinogram: importance of axonal currents. Invest Ophthalmol Vis Sci 52: E-Abstract 692
Mahroo OAR, Ban VS, Bussmann BM, Copley HC, Lamb TD (2011) A model for the electrical responses of human rod photoreceptors to the onset of steady backgrounds. Proc Physiol Soc 22:C08
Penn RD, Hagins WA (1972) Kinetics of the photocurrent of retinal rods. Biophys J 12(8):1073–1094
Breton ME, Schueller AW, Lamb TD, Pugh EN Jr (1994) Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci 35:295–309
Hood DC, Birch DG (1994) Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci 35:2948–2961
Hébert M, Vaegan LachapelleP (1999) Reproducibility of ERG responses obtained with the DTL electrode. Vis Res 39:1069–1070
Naarendorp F, Williams GE (1999) The d-wave of the rod electroretinogram of rat originates in the cone pathway. Vis Neurosci 16:91–105
Acknowledgments
We thank David Cunningham, Sara Haenzi, Rebecca Rewbury, Oshini Shivakumar and Mathew Vithayathil for assistance with experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahroo, O.A.R., Ban, V.S., Bussmann, B.M. et al. Modelling the initial phase of the human rod photoreceptor response to the onset of steady illumination. Doc Ophthalmol 124, 125–131 (2012). https://doi.org/10.1007/s10633-012-9316-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-012-9316-3