Abstract
Background
Active Crohn’s disease increases the risk of strictures, fistulas, and abscesses. Less than 30% of patients with Crohn’s disease achieve endoscopic remission on any therapy. Tofacitinib may be a therapeutic option for patients with refractory Crohn’s disease.
Aims
We aimed to evaluate the safety and effectiveness of off-label tofacitinib for refractory Crohn’s disease.
Methods
We retrospectively assessed adverse events and clinical/endoscopic response after therapy.
Results
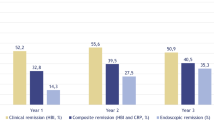
Forty-four patients were included in the safety analysis and 35 were included in the clinical and/or endoscopic assessments. The mean age was 41.8 years and the mean disease duration was 17.4 years. All patients had prior biologic exposure. Adverse events were reported in 52.3% of patients; 13.6% had ≥ 1 serious adverse event after a median 54.6 weeks of treatment. Seventy percent achieved clinical response after a mean 29.4 (SD 15.1) weeks, and 33.3% achieved clinical remission after a mean 33.4 (SD 17.6) weeks of therapy. Endoscopic improvement occurred in 25.0%, endoscopic remission in 12.5%, and endoscopic healing in 4.2% of patients after a mean 52.0 (SD 15.0) weeks of therapy. The mean Simple Endoscopic Score in Crohn’s disease significantly improved from 23.1 ± 3.7 to 18.0 ± 13.7 after treatment (P = .02).
Conclusions
In the short term, tofacitinib appears well tolerated. The most common adverse event was minor infection. One serious infection and one colorectal cancer occurred. While half of patients reported adverse events, this likely reflects the severe refractory disease in this population and no new safety events were observed. Tofacitinib achieved clinical and endoscopic improvement in some patients with refractory Crohn’s disease. Further research is needed to understand the long-term safety and efficacy of tofacitinib in Crohn’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) activity increases the risk of strictures, abscesses, and fistulas which require surgical intervention, resulting in risk of short gut syndrome, malnutrition, and chronic symptoms [1]. Less than 30% of patients with CD achieve endoscopic remission on any currently available therapy [2, 3]. Biologic efficacy may be lower in patients with hypoalbuminemia or high inflammatory burden as these are associated with inadequate therapeutic drug levels. Small molecules such as tofacitinib have good oral bioavailability and age, gender, body weight, or disease severity at baseline do not appear to affect the clearance or plasma levels of tofacitinib [4], which may offer some advantage in patients with prior failure of biologic therapy. Therefore, it is critical to understand how we can utilize novel agents to achieve higher rates of endoscopic remission.
Tofacitinib (Xeljanz, Pfizer Inc.) is a pan Janus kinase (JAK) inhibitor FDA-approved for ulcerative colitis (UC) [5]. Two randomized trials evaluating tofacitinib in CD did not show clinical response [6]; however, lack of endoscopic assessment and high placebo response rate limited trial interpretation. The tofacitinib CD trials did report a statistically significant improvement in C-reactive protein (CRP) in patients treated with tofacitinib 10 mg PO BID as compared to placebo (P < 0.0001), suggesting that tofacitinib may improve objective disease activity measures. This warrants further evaluation to understand the clinical and endoscopic response to tofacitinib in patients with CD. Additionally, other JAKs are in clinical trials for CD [7] and retrospective studies suggest tofacitinib may be effective in CD, though data are limited to case series and case reports [8, 9]. With regard to safety, prior randomized clinical trials in UC and CD describe the safety profile of tofacitinib. Risks of tofacitinib include cardiovascular events including venous thromboembolic events (VTE), shingles, and malignancy [10].
Given the objective improvement reported in the tofacitinib CD trials, we utilized off-label tofacitinib in patients with CD that was refractory to other FDA-approved therapies who were at high risk of disease complications. We report our data on the safety and effectiveness of tofacitinib for in a cohort of patients with severe, refractory CD.
Methods
Patient Population and Ethics
We received institutional review board approval for this retrospective cohort study. Eligible patients received care between 2017–2019 at an academic center or county hospital in Washington State, were ≥ 18 years old, were prescribed tofacitinib for CD, and had at least one outpatient encounter after starting tofacitinib.
Patient sex, age, disease duration, location, and behavior, age at diagnosis, concomitant medications, prior CD therapy, tobacco use, and medical/surgical history were collected from medical records. Harvey Bradshaw Index (HBI) [11], Short Inflammatory Bowel Disease Questionnaire (SIBDQ), C-reactive protein (CRP), hematocrit, and albumin were collected prior to and after therapy. Patients in our practice complete HBI and SIBDQ questionnaires at each clinic encounter per standard practice. Simple Endoscopic Activity Score in CD (SESCD) [12] was collected prior to and after therapy from colonoscopy reports. SESCD is scored at the time of procedure per standard practice.
Adverse events (AE), defined as any untoward medical occurrence during the study period, were collected during therapy until 8 weeks after discontinuation or date of last data capture. Serious AEs (SAE) were AEs that resulted in death, were life threatening, required inpatient hospitalization, prolonged hospitalization, or resulted in significant disability. AE causality was assessed by investigators as not related, unlikely related, possibly related, probably related, and highly probably related to tofacitinib.
Patients were considered to have nonresponse to therapy if they had no meaningful improvement in clinical or endoscopic disease activity. Patients were considered to have inadequate response if they had partial response, but had ongoing disease activity resulting in a change in therapy.
Endpoints
Endpoint definitions were predefined: active clinical disease (baseline HBI ≥ 5); clinical response (≥ 3 point decrease in HBI); clinical remission (HBI ≤ 4); active endoscopic disease (baseline SESCD ≥ 6, or ≥ 4 for isolated ileal disease); endoscopic improvement (≥ 50% decrease in SESCD); endoscopic remission (SESCD ≤ 3); and endoscopic healing (SESCD = 0).
Statistical Analysis
Statistical analysis (Stata Statistical Software: Release 16, StataCorp LLC, College Station, TX) included descriptive statistics (numbers, frequencies, and means with standard deviations) for demographics/characteristics, paired t-test for changes in means, proportions for outcomes rates and adverse events, and chi-square test to assess differences between proportions. When multiple values were available, the lowest score was utilized. Surgically absent bowel was scored as zero. Scores were excluded from analysis if missing, if surgical anatomy invalidated the score (HBI), or if the patient did not meet the pre-defined active clinical or endoscopic disease score at baseline. All patients who met eligibility criteria were included in the safety analysis. For the clinical/endoscopic response analysis, patients were required to have disease activity prior to treatment, which was pre-defined for HBI and SESCD. Patients were also required to have available scores (HBI or SESCD) both prior to and after treatment with tofacitinib so that response could be calculated.
Results
Baseline Characteristics and Treatment Duration
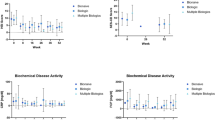
Administrative databases identified 11,047 patients; 44 patients met inclusion criteria. The mean disease duration was 17.4 years (range 0–59), 34.1% of patients had penetrating or stricturing disease, 27.3% had history of perianal fistula, 43.2% had history of gastrointestinal surgery, and 93.2% had prior failure of ≥ 2 biologics (Table 1). Concomitant medications were methotrexate (n = 3) and corticosteroids (n = 15). The indication to start tofacitinib was luminal disease (n = 30), symptoms (n = 7), cross-sectional imaging (n = 5), pyoderma gangrenosum (n = 1), and CD related arthropathy (n = 1). The median treatment duration estimated by Kaplan–Meier drug survival was 382 days (Fig. 1). At the end of data collection, 47.7% (n = 21) discontinued tofacitinib due to inadequate response (n = 6), nonresponse (n = 5), intolerance (n = 3), infection (n = 3), patient choice (n = 2), malignancy (n = 1), and pregnancy (n = 1) (Table 2).
Approach to Treatment
Tofacitinib was utilized in the context of off-label, compassionate care in patients with refractory CD with prior failure of FDA-approved therapies. Over 90% of patients had failed ≥ 2 biologic therapies. About 93.2% had prior exposure to tumor necrosis factor antagonist therapy, 70.5% had prior exposure to anti-integrin therapy, and 68.2% had prior exposure to anti-IL 12/23 therapy. Three patients initiated tofacitinib after failure of one biologic agent: 1 male with severe CD and comorbid multiple sclerosis contraindicating tumor necrosis factor therapy with nonresponse to vedolizumab and cyclosporine, 1 male with severe Crohn’s colitis with primary nonresponse to infliximab, and 1 male with severe small bowel CD requiring hospitalization and total parenteral nutrition with primary nonresponse to intravenous steroids, cyclosporine, and vedolizumab. Patients initiated tofacitinib 10 mg PO BID for at least 8 weeks, then reduced to 5 mg PO BID if clinical remission was achieved, per prescribing provider.
Adverse Events
Of patients treated with tofacitinib, 52.3% had ≥ 1 AE after a median 54.6 weeks of treatment, most commonly urinary tract infection (n = 5) and upper respiratory tract infection (n = 4). One patient had uncomplicated shingles and two had cytomegalovirus (CMV) on colonoscopic biopsies without symptoms of systemic illness. Thirteen percent (n = 6) had ≥ 1 serious AE (SAE). One patient had stage IV cecal carcinoma and one patient developed colonic dysplasia. No patients experienced VTE (Table 3).
Effectiveness
HBI decreased significantly from 11.5 ± 6.5 to 7.5 ± 5.9 (P < 0.01) after a mean 33.3 (SD 17.0) weeks of tofacitinib treatment, 70.8% of patients had clinical response after a mean 29.4 (SD 15.1) weeks, and 33.3% achieved clinical remission after a mean 33.4 (SD 17.6) weeks of treatment. Mean SESCD significantly decreased from 23.1 ± 13.7 to 18.0 ± 13.7 (P = 0.02), 25.0% had endoscopic improvement, 12.5% achieved endoscopic remission, and 4.2% endoscopic healing after a mean 52.0 (SD 15.0) weeks of treatment (Fig. 2). Mean CRP decreased from 40.1 ± 21.0 to 32.2 ± 38.5 (RR: 0–10 mg/L; P = 0.36) and 30.8% normalized CRP; mean albumin significantly increased from 3.0 ± 0.3 to 3.5 ± 0.4 (RR: 3.5–5.2; P < 0.01) and 55.6% normalized albumin; and 45.5% normalized hematocrit [Supplemental information, Table 4]. One patient who initiated tofacitinib for pyoderma gangrenosum had visual improvement, but not resolution of lesions after therapy.
Clinical and endoscopic response after tofacitinib therapy among patients with refractory Crohn’s disease. Endpoint definitions: active clinical disease (baseline HBI ≥ 5); clinical response (≥ 3-point decrease in HBI); clinical remission (HBI ≤ 4); active endoscopic disease (baseline SESCD ≥ 6, or ≥ 4 for isolated ileal disease); endoscopic improvement (≥ 50% decrease in SESCD); endoscopic remission (SESCD ≤ 3); and endoscopic healing (SESCD = 0). Clinical response was assessed after a mean 29.4 (SD 15.1) weeks and clinical remission after a mean 33.4 (SD 17.6) weeks of tofacitinib treatment. Endoscopic response was assessed after a mean 52.0 (SD 15.0) weeks of tofacitinib treatment
Discussion
Refractory CD increases the risk of strictures, fistulas, and abscesses that require surgery and lead to chronic symptoms and disability. Currently only three medication classes are FDA-approved for CD. As the majority of patients do not achieve remission on any one therapy, it is important to understand if other clinically available medications may improve outcomes in patients with refractory CD. Our study reports that tofacitinib was well tolerated in the short-term with no new safety signals, and that treatment resulted in improvement in clinical and endoscopic disease activity in some patients with refractory CD. These results add to current data reporting the potential role of tofacitinib in CD [8, 9].
We report that the short-term safety of tofacitinib in CD is not different from the risk profile previously reported in pivotal trials [6, 13]. Safety concerns for tofacitinib include increased risk of VTE, shingles, and malignancy [5, 6, 10, 14, 15]. We observed no VTE during our study. We identified one case of shingles and no systemic varicella-zoster, although 43.2% of patients received shingles vaccination. We identified two cases of CMV, neither with systemic symptoms of CMV-related disease. Patients were treated with valganciclovir and tofacitinib was discontinued; however, it was not clear that CMV was pathogenic or significantly contributed to the patients’ symptoms. However, this raises concern about risk of viral infection associated with tofacitinib and warrants further study. The colorectal malignancy and colonic dysplasia reported in our study occurred in patients with longstanding severe colitis. While causation was felt to be possibly related given the short duration of tofacitinib therapy prior to cancer diagnosis, causation cannot be ruled-out and warrants further study. While our study reported that > 50% of patients experienced an AE, this reflects the severe and refractory nature of the population under study, as well as a thorough review of the medical record for all untoward events during tofacitinib therapy. Many AEs were assessed as not related to tofacitinib therapy. No unexpected or new AE’s were identified during our study period.
Our study population had refractory, complicated CD with a long duration of disease (mean 17 years), > 90% having failed ≥ 2 biologics, and > 40% with history of gastrointestinal surgery. The refractory disease and failure of multiple prior therapies were primary influencing factors in recommending off-label tofacitinib; we do not advocate this regimen as first-line treatment among patients naïve to advanced therapy or with additional available options. Yet, even in the setting of longstanding, severely medically refractory disease, 70.8% of patients in our study achieved clinical response, which is similar to tofacitinib induction trials in CD (69.8%) [6] and higher than another retrospective study of tofacitinib in refractory CD (46.6%) [9]. In our study, 25.0% of patients achieved endoscopic improvement on tofacitinib. These preliminary results suggest that tofacitinib may be effective for patients with refractory CD.
This study was limited by retrospective study design with variable time to assessment, lack of comparator, small sample size, inherent bias in clinical decision-making, and difficulty in blinding data interpretation. A weakness of our data is that some patients did not have a complete set of assessments available based on pre-defined endpoints. This includes missing clinical data (HBI), biochemical data (CRP) or endoscopic data (SESCD). Additionally, we excluded patients with data that did not qualify per our inclusion criteria (e.g., patients with low symptom index but active ulceration on endoscopy would be included in the endoscopic assessment but not the clinical assessment). Thus, not all patients were included in the clinical and endoscopic analysis and some patients without adequate clinical data were only included in the safety analysis.
Strengths of our data include assessment of patient-reported symptoms, and objective evaluation of CRP and endoscopic response to therapy. Another strength compared to available literature, includes the utilization of a validated clinical endpoint (HBI) rather than a subjective physician assessment as reported in other publications. Further randomized controlled trials are indicated to support the efficacy and safety of tofacitinib and other JAK inhibitors in CD.
In conclusion, our study reports that tofacitinib was well tolerated. The most common AE was minor infection. One serious infection, one case of uncomplicated shingles, and one malignancy were reported; however, no patients reported cardiovascular events in our study. Overall, the safety profile in our study is similar to that previously reported in tofacitinib trials. The long-term safety of tofacitinib in CD is unknown and warrants further research. We report that tofacitinib is effective in achieving clinical and endoscopic improvement in some patients with refractory CD. Our data suggest that tofacitinib may be a viable option to improve outcomes in patients who are refractory to standard therapy, in whom ongoing disease places them at risk of disease complications and further surgery. Larger studies of longer duration are necessary to understand the true benefits and risks of tofacitinib in CD.
References
Thia KT, Sandborn WJ, Harmsen WS et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–1155.
Sandborn WJ, Feagan BG, Rutgeerts P et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–721.
Feagan BG, Sandborn WJ, Gasink C et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med 2016;375:1946–1960.
Pérez-Jeldres T, Tyler CJ, Boyer JD et al. Targeting Cytokine Signaling and Lymphocyte Traffic via Small Molecules in Inflammatory Bowel Disease: JAK Inhibitors and S1PR Agonists. Front Pharmacol 2019;10:212.
Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:496–497.
Panes J, Sandborn WJ, Schreiber S et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–1059.
Rogler G. Efficacy of JAK inhibitors in Crohn’s disease. J Crohns Colitis 2020;14:S746-s754.
Bauer CM, Barnes EL, Herfarth HH. Tofacitinib in the treatment of Crohn’s-like disease of the pouch. Am J Gastroenterol 2020;115:2116–2117.
Fenster M, Alayo QA, Khatiwada A et al. Real-world effectiveness and safety of tofacitinib in Crohn's disease and IBD-U: a multicenter study from the TROPIC consortium. Clin Gastroenterol Hepatol 2020
Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine Xeljanz, Xeljanz XR (tofacitinib), in Drug Safety Communication. 2021. FDA: https://www.fda.gov/drugs/drug-safety-and-availability/initial-safety-trial-results-find-increased-risk-serious-heart-related-problems-and-cancer-arthritis
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1:514.
Daperno M, D’Haens G, Van Assche G et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004;60:505–512.
Sandborn WJ, Su C, Sands BE et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–1736.
Sandborn WJ, Panes J, D'Haens GR et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2018
Sandborn WJ, Panés J, Sands BE et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50:1068–1076.
Funding
This study did not receive specific funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SL, KC-S, AS, and KK. The first draft of the manuscript was written by SL and KC-S and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Scott Lee receives grant and research support from the following: AbbVie Pharmaceuticals, Janssen Pharmaceuticals, Inc., Takeda Pharmaceuticals, Inc., Bristol Myers Squibb Pharmaceuticals, Inc., Pfizer Pharmaceuticals, Inc., Atlantic Pharmaceuticals, Ltd., Gilead Sciences, Inc., Tetherex Pharmaceuticals, Arena Pharmaceuticals, Shield Therapeutics PLC and is a consultant for UCB Pharma, Cornerstones, Janssen Pharmaceuticals, Inc., Takeda Pharmaceuticals, Inc., and Eli Lilly Company. Kindra Clark-Snustad has been a consultant for Pfizer Pharmaceuticals, Inc., Takeda Pharmaceuticals, Inc., AbbVie Pharmaceuticals, and Bristol Myers Squibb Pharmaceuticals, Inc. Kendra J. Kamp is supported, in part, by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Program, at the University of Washington (Grant Nr. T32DK007742). For the remaining authors no conflicts of interest were declared.
Ethical approval
Declarations: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the University of Washington Human Subjects Division.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-022-07448-1.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S.D., Singla, A., Harper, J. et al. Tofacitinib Appears Well Tolerated and Effective for the Treatment of Patients with Refractory Crohn’s Disease. Dig Dis Sci 67, 4043–4048 (2022). https://doi.org/10.1007/s10620-022-07444-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07444-5