Abstract
Background
Eluxadoline, a peripherally acting, mixed µ- and κ-opioid receptor (OR) agonist and δ-OR antagonist, is approved for treatment of adults with irritable bowel syndrome-diarrhea (IBS-D). About a third of IBS-D patients has bile acid diarrhea (BAD); opioids may stimulate TGR5 (bile acid) receptors.
Aim
To evaluate eluxadoline’s efficacy on altered bowel functions and safety in IBS-D patients with or without BAD.
Methods
In a single-center, phase 4, parallel-group, open-label study, patients with IBS-D (cohort 1) and patients with BAD were treated with eluxadoline, 100 mg tablets BID, with food for 4 weeks. Patients recorded bowel functions by electronic daily diary. BAD was based on fasting serum 7αC4 (> 52.5 ng/mL) or concurrent criteria of increased total or primary fecal BAs excreted in 48 h. We assessed efficacy on treatment compared to baseline in the two cohorts. Primary outcome measures were changes from baseline in average stool consistency Bristol Stool Form Scale (BSFS) score (range 1–7) and safety.
Results
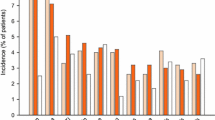
Mean changes from baseline in cohorts 1 and 2 (data presented in this order) were similar for: BSFS score averaged over 4 weeks’ treatment (− 1.25 and − 1.09); daily bowel movement frequency (− 1.48 and − 0.79); daily urgent bowel movements (− 0.52 and − 0.80); IBS-QoL (5.9 and 13.6); serum 7αC4 (− 5.59 and − 8.78 ng/mL). There were no deaths, serious treatment-emergent adverse events, or discontinuations due to adverse events during the study.
Conclusion
Eluxadoline is similarly efficacious in the treatment of IBS-D and BAD, and it appears to be safe and efficacious as documented in large clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pathophysiology of irritable bowel syndrome (IBS) is not completely understood, but it is thought to be multifactorial, with genetics, abnormal gut motility, visceral hypersensitivity, psychosocial distress, altered gut microbiome, and brain-gut interaction playing important roles in its development [1, 2]. Among patients with the diarrhea subtype of IBS (IBS-D), it is increasingly recognized that bile acid diarrhea (BAD) may be a potential cause of the chronic diarrhea [3,4,5,6]. In BAD, the failure to adequately reabsorb conjugated bile salts in the terminal ileum or increased hepatic synthesis of bile acids which has been associated with reduced ileal enterocyte production of the negative feedback control hormone, fibroblast growth factor-19 (FGF-19), [7,8,9,10] lead to excess bile acids entering the colon where they stimulate colonic secretion and motility [11]. Symptoms of BAD include watery diarrhea, bloating, fecal urgency, and fecal incontinence, which closely overlap the symptoms of functional diarrhea and IBS-D [12].
Recent evidence suggests that up to a third of patients who meet the Rome criteria for IBS-D has evidence of BAD, based primarily on 75selenium-homocholic acid taurine (SeHCAT) retention testing [3, 4, 6, 13]. Indeed, inclusion of a screen for BAD in patients with unexplained chronic diarrhea without rectal bleeding or symptoms suggestive of IBS-D could considerably reduce healthcare utilization as measured by the subsequent tests performed in the outpatient management of these patients. Thus, we have shown that, in a cohort of 936 out-patients, there was an average of more than 1 abdominal CT scan and 1 colonoscopy performed in the exclusion of organic disease prior to the correct diagnosis of BAD [14, 15].
Overall, IBS-D patients with and without evidence of BAD appear highly similar in demographic characteristics and assessments of well-being, except that patients with BAD tend to have a higher body mass index (BMI) [3, 16].
The potential overlap of IBS-D and BAD may have important implications for clinical practice, especially if an altered treatment course would be considered. Recognizing this, the most recent version of the Rome diagnostic criteria for IBS-D issued in 2016 (Rome IV) now recommends an assessment of bile acid status (e.g., excess synthesis, or reduced retention of bile acids) as a potential cause of chronic, watery diarrhea [17]. For patients with evidence of BAD, a bile acid sequestrant may be an appropriate treatment option, at least for the diarrheal symptoms, although poor tolerability and compliance can be problematic for the most economical bile acid binding agent, cholestyramine [11]. In addition, a recent study with colesevelam showed beneficial effects on biological markers of BAD, but the study was under-powered to detect improvements in patient response outcomes [18].
Eluxadoline is a novel, peripherally acting, mixed µ- and κ-opioid receptor (OR) agonist and δ-opioid receptor antagonist [19, 20]. By targeting multiple ORs, it induces visceral analgesia and decreases motility through actions on μ-OR, while adverse side effects attributed to excessive inhibition of gut motility related to the μ-OR agonist effects are mitigated by the actions on the κ- and δ-Ors [21, 22]. Eluxadoline was approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with IBS-D in February of 2015 [23]. In phase 3 trials, 2,427 patients with IBS-D were randomized to eluxadoline (75 mg or 100 mg) or placebo, twice daily, for 26 weeks or 52 weeks. There was significant improvement in the composite endpoint of abdominal pain and stool consistency with eluxadoline compared to placebo, but no improvement in abdominal pain alone [19]. Eluxadoline has demonstrated efficacy and safety in placebo-controlled phase 4 studies in patients with IBS-D and inadequate symptom relief with loperamide [24], and in the relief of worst abdominal pain in patients with IBS-D based on a post hoc analysis of 1,615 patients included in phase 3 trials [25].
Baseline characteristics of the phase 3 IBS-D patient population in which eluxadoline was tested for efficacy and safety [19] demonstrated high prevalence of both obesity and prior cholecystectomy. Given the known association of higher BMI with BAD [16] and given that cholecystectomy is a known risk factor for BAD, it is conceivable that some portion of patients enrolled in those studies may have had evidence of BAD. Since the eluxadoline phase 2B or phase 3 IBS-D studies did not evaluate patients’ bile acid absorption status [19, 20], it is unknown whether the effectiveness of eluxadoline may be different in patients with evidence of BAD. Indeed, it is possible that the overall effects of eluxadoline could have been diluted by a subset of nonresponsive patients suffering from BAD for whom a bile acid sequestrant might have been a more appropriate treatment option.
Given the established efficacy of eluxadoline compared to placebo in IBS-D based on prior trials (summarized above) and, conversely, the stimulation of TGR5 receptors by opioids that may theoretically diminish efficacy in patients with BAD, the overall hypothesis of this study was that the efficacy of eluxadoline is greater in patients without evidence of BAD compared to those with evidence of BAD.
The primary goal of the present study was to evaluate the efficacy on altered bowel function and safety of eluxadoline in IBS-D patients compared to those with evidence of BAD. A secondary goal was to evaluate the population pharmacokinetics (PK) of eluxadoline in IBS-D patients with and without evidence of BAD, determined from plasma concentration data extrapolated from dried blood sample analysis.
Methods
Study Design and Duration of Treatment and Participation in Study
This was a single-center, phase 4, parallel-group, cohort-controlled design, open-label study in patients with IBS-D fulfilling Rome IV criteria [17] with or without evidence of BAD. All patients were treated for 4 weeks with eluxadoline, 100 mg oral tablets, twice daily (BID), with food, in accordance with guidance from the Food and Drug Administration [23, 26]. The study was approved by the Mayo Clinic Institutional Review Board (IRB #17–009,507) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was registered in ClinicalTrials.gov #NCT03441581. The study was conducted exclusively at Mayo Clinic in Rochester, Minnesota, USA. The first patient was dosed on March 13, 2018, and the last patient completed study on April 28, 2020.
The 100 mg BID eluxadoline dose selected was consistent with USA and global labeling for the drug for IBS-D. The duration of treatment was chosen to be consistent with clinical practice in IBS, where a 1-month therapeutic study of a new agent is common, and was supported by results of the phase 3 studies which showed that a patient’s response to eluxadoline treatment over the first month was reasonably predictive of responsiveness over longer durations [19]. The total duration of the study was up to 11 weeks, which included a screening period (0–2 weeks), a pre-treatment period (2–3 weeks), a 4-week open-label treatment period, and a 2-week post-treatment safety follow-up period (Fig. 1).
Patient Eligibility Criteria
Inclusion Criteria
The main criteria were 24 adult men or women, aged 18–65 years inclusive, with diagnosis of IBS-D per Rome IV criteria [17], with > 25% of bowel movements with a Bristol Stool Form Scale (BSFS) score of 6 or 7 and < 25% of bowel movements with BSFS score of 1 or 2. Twelve patients had evidence of BAD, based on fasting serum 7a-hydroxy-4-cholesten-3-one (7αC4) level, or total fecal bile acids over 48 h at screening or within one calendar year prior to screening. The specific BAD criteria are detailed below. In addition, patients had an average daily BSFS score ≥ 5.0 or ≥ 25% of diary entry days with a BSFS score of 6 or 7 during the 14 days prior to day 1 of the study. Eligibility also required compliance with contraception in women of childbearing potential, completion of electronic diary on ≥ 10 of the 14 days prior to day 1, and lack of use of loperamide rescue medication on > 3 of the 14 days prior to day 1.
Exclusion Criteria
Exclusion criteria included prior cholecystectomy, known or suspected biliary duct obstruction or sphincter of Oddi disease or dysfunction, history of alcoholism, alcohol abuse or alcohol addiction or drinking more than three alcoholic beverages per day, history of pancreatitis, structural diseases of the pancreas, mild, moderate, or severe hepatic impairment, inflammatory or immune-mediated gastrointestinal disorder, celiac disease or a positive serological test for celiac disease, known lactose or fructose intolerance associated with diarrhea, abdominal pain or discomfort that could confound assessments in the study, women who were currently pregnant or nursing or planned to become pregnant or nurse during the study, and known allergies or hypersensitivity to opioids.
Characterization of IBS-D Patients with Reference to Parameters of BAD
The criteria for BAD evolved with studies and related publications that were conducted during the course of this study; specifically, evidence that primary fecal bile acids [chenodeoxycholic acid (CDCA) and cholic acid (CA)] were significantly higher in patients with BAD and correlated with stool frequency and consistency [27, 28], and that primary bile acids > 10% identified patients with increased fecal weight (sensitivity 49% and specificity 91%) and rapid colonic transit (sensitivity 48% and specificity 87%) [29, 30].
Initial criteria for patients with evidence of BAD were: fasting serum 7αC4 level measured by HPLC–MS [31] ≥ 52.5 ng/mL or total fecal bile acids > 2337 micromoles/48 h (positive result) at screening or within one calendar year prior to screening. Elevated serum 7αC4 levels are significantly correlated with 75SeHCAT retention [32].
Patients without BAD were required to have a fasting serum 7αC4 level ≤ 47.1 ng/mL or total fecal bile acids < 2200 micromoles/48 h (negative result).
Final criteria for BAD were: fasting serum 7αC4 > 52.5 ng/mL or total fecal bile acids > 2337 micromol/48 h, or primary bile acids (fecal CDCA and CA) ≥ 10% in a 48-h fecal collection; or combination of primary bile acids (fecal CDCA and CA) ≥ 4% with total fecal bile acids ≥ 1,000 micromoles/48 h.
Patients without BAD were required to have at least one of the following at screening or within one calendar year prior to screening: fasting serum 7αC4 level ≤ 47.1 ng/mL; for those patients with fasting serum 7αC4 levels > 47.1 ng/mL but < 52.5 ng/mL, the fecal bile acid test had to be negative for BAD, that is, total fecal bile acids ≤ 2337 micromoles/48 h; primary bile acids (fecal CDCA and CA) < 10% in a 48-h fecal collection; and combination primary bile acids (fecal CDCA and CA) < 4% with total fecal bile acids < 1000 micromoles/48 h.
Prohibited Medications
Patients were required to avoid intake of bile acid sequestrants and 5-HT3 antagonists (e.g., alosetron or ondansetron) from 21 days prior to day 1 of study treatment, opioids or gastrointestinal preparations [including antacids containing aluminum or magnesium, docusate or antidiarrheal agents (except loperamide rescue as medication, see below)], anti-nausea agents, antispasmodic agents, bismuth, or prokinetic agents from 14 days, and rifaximin or other antibiotics (except for topical antibiotics) from 28 days prior to day 1 of study treatment.
Permitted and Rescue Medications
Antidepressants taken at stable doses for the 3 months prior to screening were permitted (at the same dose) during the study. Medications taken for the treatment of allergies, chronic medical conditions (e.g., hypothyroidism), and migraine headaches (excluding opioids) were permitted during this study. As-needed use of benzodiazepines for anxiety was permitted during the study.
Loperamide was permitted as rescue medication for acute treatment of diarrhea during the pre-treatment and open-label treatment periods and was recorded by patients in the eDiary. Loperamide, 2 mg, was permitted once, approximately every 6 h, with the following guidelines: total dose no more than 8 mg/day or 14 mg over 2 days, and no more than 11 unit doses (total 22 mg) over a continuous 7-day time period.
Study Visits
Table 1 provides a summary of the study visits. On day 1 (visit 3), eligible patients underwent baseline assessments, collection of fasting serum sample for serum 7αC4 determination, vital signs, weight, physical exam, urine pregnancy test for women of childbearing potential, completion of IBS-Quality of Life (QOL) Questionnaire, dried blood sample for pharmacokinetic (PK) analysis, and ingestion of eluxadoline, 100 mg BID (morning and evening), with food.
At week 2 (± 2 days, visit 4), patients returned to the study site for assessment of adverse events (AEs) and concomitant medications, and collection of dried blood samples for PK analysis pre-dose, and during the intervals 1 to 2 h, 3 to 4 h, and 5 to 8 h post-dose.
Patients returned to the study site at week 4 (± 2 days) after the completion of the.
treatment period and underwent the end-of-treatment assessments including vital signs, weight, physical examination, clinical laboratory tests (including chemistry, hematology), fasting serum 7αC4 level, review of AEs and concomitant medications, and urine pregnancy test for women of childbearing potential. Any unused study medication was counted and returned to the research pharmacy, and IBS-QOL questionnaire was completed. A mandatory post-treatment visit was performed 14 days after the last dose of study drug [week 6 (± 7 days)] for patients who completed the open-label treatment period.
Efficacy Assessments
The efficacy of eluxadoline was based on eDiary measures related to improvements in IBS-D symptoms. Patients were required to access the eDiary each evening, preferably at the same time each day, to record daily IBS symptoms. At the start of the pre-treatment period, patients received full training on the use and completion of the daily eDiary.
Daily IBS Symptoms
Patients recorded the following symptoms in the daily eDiary during the pre-treatment period and throughout the 4 weeks of the open-label treatment period: stool consistency based on BSFS [33]; worst abdominal pain in the past 24 h on a 0 to 10 scale, where 0 corresponded to no pain and 10 corresponded to worst imaginable pain; and abdominal bloating in the past 24 h on a 0 to 10 scale, where 0 corresponded to no bloating and 10 corresponded to worst imaginable bloating. Patients were also asked to record the number of bowel movements, number of urgent bowel movements, and number of episodes of fecal incontinence over the past 24 h.
IBS-QOL Questionnaire
The impact of IBS on patients’ quality of life was assessed using the IBS-QOL Questionnaire [34, 35] which was completed at baseline (day 1) prior to administration of the study drug, and at week 4.
Serum 7αC4 Level
Fasting serum 7αC4 levels were measured (at least 8 h fasting) during screening, at baseline, and at week 4 (or end of treatment visit) to determine whether any changes occurred following treatment with eluxadoline. Fasting serum 7αC4 level obtained within one calendar year prior to screening could be used to determine BAD status.
Safety Assessments
Safety assessments were based on physical examination, vital signs, and clinical laboratory tests, specifically hematology group, clinical chemistry, and serum or urine pregnancy tests, as well as reported adverse effects. Particular attention was placed on evaluation of potential liver injury using Hy’s law [36].
Adverse Events
All AEs or serious AEs (SAEs) from the start of treatment until the follow-up visit were collected at the in-person visits to the study site, and as observed or reported spontaneously by study patients.
Pharmacokinetics
Dried blood samples for PK analysis were collected using a blood microsample collection device (Mitra™ cartridge in a mylar foil bag) at baseline (day 1, visit 3) prior to dispensing study drug, and during visit 4 at the following intervals post-dose: 1 to 2 h, 3 to 4 h, and 5 to 8 h. Samples were collected on the same day or on different days. For the post-dose samples, the date and time of study drug dose prior to each PK sample and the sampling times were recorded.
Data and Statistical Analysis
Primary outcome measures To evaluate the efficacy, safety, and tolerability over 4 weeks of treatment with eluxadoline, 100 mg BID, in IBS-D patients with evidence of BAD compared to IBS-D patients without evidence of BAD, the primary outcome measures were: change from baseline in average BSFS score on a range from 1 (separate hard lumps like nuts) to 7 (watery); and safety determined by adverse events, clinical laboratory tests, vital signs, and physical examinations.
Secondary outcome measures for the same group comparisons were changes from baseline over 4 weeks of treatment of the following: number of bowel movements, average daily worst abdominal pain score, abdominal bloating, number of daily urgent bowel movements, and episodes of fecal incontinence. In addition, changes from baseline in serum 7αC4 levels and IBS-QOL score at the end of the treatment period were compared in the two groups.
Pharmacokinetic (PK) outcomes: We evaluated the PK of eluxadoline as determined from plasma concentration data extrapolated from dried blood sample analysis. Plasma PK parameters in IBS-D patients with and without evidence of BAD were estimated using a population PK approach. An existing population PK model, previously constructed from 2,538 plasma PK observations obtained from 443 subjects in three clinical studies, was utilized to estimate individual post hoc structural model parameters (e.g., apparent clearance, apparent central volume of distribution, absorption rate, etc.) for the patients in the current study. Individual-level model parameters were then used to simulate the time-course of a 100 mg BID regimen at steady-state for each patient. Derived PK parameters [e.g., maximum plasma concentration (Cmax), minimum plasma concentration (Cmin), area under the plasma concentration–time curve during the dosing interval (AUCtau), and time to Cmax (tmax)] were then calculated from those individual-level simulations. Since all PK samples in the BAD study were obtained as dried blood samples and the PK sampling data utilized in the existing population PK model were from plasma samples, a conversion factor of 0.6753 was utilized, based on the correlation established in a prior phase 1 study.
Sample Size Considerations
While not powered for statistical significance, the study sample was considered sufficient to characterize the effects of eluxadoline on the altered bowel function of IBS-D patients with BAD and to qualitatively evaluate whether these patients responded differently to eluxadoline treatment. The sample size for this study was 24 patients (12 with BAD and 12 without BAD). No formal sample size estimation was calculated for this study, as it was not powered for inferential statistical analysis.
Statistical Analysis
All efficacy endpoints were summarized by the patient cohort [BAD (cohort 1) or non-BAD (cohort 2)] using descriptive statistics with standard deviation (SD), interquartile range or full range specified.
Changes in Conduct of the Study
The original protocol (dated October 19, 2017) was amended two times during the conduct of the study (Amendment 1, dated June 5, 2018, and Amendment 2, dated April 17, 2019). A summary of the major reasons for the protocol amendments and enrollment of patients by amendment is summarized in Supplemental Table A.
Results
Patient Demographics and Study Compliance
A total of 35 patients were screened; 24 patients with IBS-D were enrolled in the study and received investigational product [12 patients in cohort 1 (with evidence of BAD) and 12 in cohort 2 (without evidence of BAD)]. All 24 (100%) patients completed the study as planned, and no patient prematurely discontinued from the study. A high proportion of patients in both cohorts received investigational product per plan during the 4-week, open-label treatment period [> 90% compliance in both treatment cohorts, mean (SD) 95.86 (4.964) in cohort 1 and 92.07 (5.513) in cohort 2].
The mean age was 40.9 years (range 21–69 years) across both cohorts. The mean age by cohort was as follows: cohort 1 (n = 12), 41.6 years (range 32–50 years); and cohort 2 (n = 12), 40.2 years (range 21–69 years). Eight patients were female and 16 were male. Twenty-three patients were white, and 1 patient was black or African American.
Overall patients’ weight (SD) was 95.21 (21.830) kg (range 60.1–149.0 kg) and height (SD) was 171.20 (8.308) cm (range 158.5–185.7 cm), with an overall BMI (SD) of 32.54 (7.302) kg/m2 (range 20.8–47.4 kg/m2). There were no numerical differences in the demographic characteristics in the two cohorts, other than the numerically higher BMI in cohort 1 with BAD (Table 2).
Effects on Bowel Function
Numerical improvements in BSFS total score (primary efficacy parameter) averaged over 4 weeks of treatment with eluxadoline, 100 mg, were seen in both cohorts (patients with and without BAD) (Table 2).
The primary efficacy parameter (BSFS score) was the change from baseline in BSFS score averaged over 4 weeks of treatment and was similar in both cohorts: − 1.25 in cohort 1 (with evidence of BAD) and − 1.09 in cohort 2 (without evidence of BAD).
The secondary efficacy parameters were not numerically different at baseline or in response to treatment in the two cohorts (Table 2). Thus, change from baseline in daily bowel movement frequency averaged over 4 weeks of treatment was − 1.48 in cohort 1 (with evidence of BAD) and − 0.79 in cohort 2 (without evidence of BAD). Similarly, the change from baseline in daily urgent bowel movements averaged over 4 weeks of treatment was − 0.52 in cohort 1 (with evidence of BAD) and − 0.80 in cohort 2 (without evidence of BAD). Fecal incontinence during the treatment period was experienced by 4 (33.3%) patients in both cohorts (Table 2).
Effects on Abdominal Pain and Bloating
The change from baseline in daily worst abdominal pain score averaged over 4 weeks of treatment was − 0.12 in cohort 1 (with evidence of BAD) and − 1.28 in cohort 2 (without evidence of BAD).
The change from baseline in daily worst bloating averaged over 4 weeks of treatment was − 0.47 in cohort 1 (with evidence of BAD) and − 1.46 in cohort 2 (without evidence of BAD). For both daily worst abdominal pain and worst abdominal bloating, there appeared to be a numerically greater effect in patients without evidence of BAD compared to IBS-D with BAD.
Effect on Quality of Life (IBS-QOL Total Score)
IBS-QOL total scores at the end of the treatment period were 83.9 in patients with evidence of BAD and 84.7 in patients without evidence of BAD; the change from baseline was 8.8 and 13.2, respectively.
Use of Rescue Loperamide
Among the patients with IBS-D, 3 patients used 2 mg of loperamide for 1 or 2 days in the pre-treatment phase, and 2 of these 3 patients took 1 dose of loperamide 2 mg for 1 day in week 2 or 3 of eluxadoline treatment.
Among patients with BAD, 1 patient took 2 mg loperamide daily for 3 days in the pre-treatment phase, 1 patient took loperamide 2 mg for 1 day in week 1 of treatment, and 1 patient took 2 mg loperamide each day for 6 days in week one.
No patient exceeded the recommended limit of loperamide use.
Serum 7αC4 Levels
Mean serum 7αC4 levels at the end of the treatment period were 36.71 ng/mL in patients with evidence of BAD and 21.81 ng/mL in patients without evidence of BAD; the change from baseline was − 5.59 ng/mL and − 8.78 ng/mL, respectively (Table 3).
Safety: Adverse Events, Vital Signs, and Laboratory Tests
There were no deaths, serious treatment-emergent adverse events (serious TEAEs), or discontinuations due to AEs reported during the study (Table 4).
Non-serious TEAEs were reported by 11 (91.7%) patients with evidence of BAD (cohort 1) and by 7 (58.3%) patients without evidence of BAD (cohort 2). The higher incidence of non-serious TEAEs that was reported by patients in cohort 1 was largely driven by an increase in the incidence of TEAEs in the gastrointestinal System and Organization Controls (SOC) in cohort 1, compared to cohort 2. The higher incidence of TEAEs in cohort 1 was contributed to by TEAEs that were reported in other SOCs at low rates (≤ 2 patients) and which were absent in cohort 2, making interpretation difficult.
Overall, shifts from a normal laboratory value at baseline to an abnormal value at the end of the treatment period were infrequent and occurred at a similar rate in both cohorts. A post-baseline procedure coding system of abnormal laboratory values was reported for 1 patient [in cohort 1; glucose percent (%) > 1.4 × ULN]. No patients met potential Hy’s law criteria for significant drug-induced liver injury.
Mean changes from baseline to the end of the treatment period in vital sign parameters were small and similar in both cohorts. A potentially clinically significant vital sign value was reported for 1 patient (in cohort 2; diastolic blood pressure ≤ 50 and decrease of ≥ 15 mmHg).
The safety findings were consistent with the known safety profile of eluxadoline and support the conclusion that eluxadoline is safe and well tolerated in patients with a diagnosis of IBS-D who have evidence of BAD.
Pharmacokinetics
Overall, the derived PK parameters (Cmax, Cmin, AUCtau, and Tmax) showed relatively high inter-individual variation (% CV > 80%), but they were comparable between patients with and without evidence of BAD (Table 5). Variations in weight, sex, and gender among the 24 patients were investigated for potential relationships with the individual estimates of structural PK model parameters; like the prior population PK analysis, no statistically significant relationships were found.
Estimated Sample Required to Document Difference in Eluxadoline Efficacy in the Two Cohorts
Based on the observed data in the current study, in order to demonstrate statistical significance in the two cohorts for on-treatment difference in the (pre-specified primary endpoint) average stool consistency (BSFS) of 0.14, the sample size required would be 290 per group.
Discussion
In this open-label study of eluxadoline in patients with IBS-D, 12 patients in cohort 1 (with evidence of BAD) and 12 patients in cohort 2 (without evidence of BAD) completed the study as planned, and no patients prematurely discontinued the study. There were no numerical differences in the demographic characteristics in the two cohorts, other than the numerically higher BMI in cohort 1 with evidence of BAD; this is consistent with prior reports in patients with IBS-D with BAD at our medical center [16]. After 4 weeks of treatment with eluxadoline, 100 mg BID, numerical improvements were seen for both the primary (BSFS total score) and secondary efficacy endpoints in IBS-D patients with and without evidence of BAD. The safety findings were consistent with the known safety profile of eluxadoline and support the conclusion that eluxadoline is safe and well tolerated in patients with a diagnosis of IBS-D who have evidence of BAD. In addition, the pharmacokinetic profiles were not significantly different in the two subgroups.
Although there is no evidence that opioid mechanisms modify the synthesis, secretion or absorption of bile acids, opioids do bind to the TGR5 receptor (bile acid receptor) to reduce pruritus [37], and bile acids activate TGR5 on sensory nerves, stimulating the release of neuropeptides in the spinal cord that transmits itch and analgesia. It is conceivable that opioid-activation of the TGR5 receptors that modify colonic motility and stool water [38] could increase colonic motility or secretion, and opioid agents might conceivably aggravate diarrhea associated with a separate mechanism, that is, BAD. In fact, prior studies documented the association of genetic variation in the TGR5 gene and faster colonic transit [39].
Nevertheless, our study suggests that the antidiarrheal effects of eluxadoline are documented relative to baseline in patients with IBS-D who have evidence of BAD. This information is relevant, as it suggests that screening for BAD would be unnecessary prior to considering treatment with eluxadoline in patients with IBS-D. This consideration is relevant since it has been estimated that 25–33% of patients with IBS-D have evidence of BAD.
The conduct of the study was somewhat complicated by the prospective validation of eligibility criteria for the diagnosis of BAD. Thus, two patients who were included in the non-BAD cohort actually had some evidence of BAD: one was based on total fecal bile acids (1022 µmol/48 h, with 15.7% primary bile acids), and a second patient was based on increased proportion of primary bile acids (54.7%, with normal total bile acids of 511 µmol/48 h) in 48-h stool collections. However, given that they did not fulfill the criteria at the time of recruitment, we have retained them in the non-BAD cohort, consistent with an intention-to-treat analysis.
There are limitations to our study including the open-label nature of the study and the absence of a control treatment group in estimating the efficacy of eluxadoline. Even though the overall efficacy of eluxadoline in IBS-D had been previously documented in large phase 2B and phase 3 studies, the objective of this study was to obtain an assessment of the changes from baseline in the two cohorts of IBS-D (with or without BAD). However, it is worth noting that, for the primary bowel function endpoint of stool consistency, as well as for the secondary endpoints, the numerical values of the changes from baseline clearly overlap for both cohorts, as shown in the detailed data provided in Table 2. The inability to identify differences may be due to the relatively small number of patients within each cohort. The mechanism for improved benefit in bowel symptoms does not appear to be related to bile acid synthesis, since serum 7αC4 levels at the end of the treatment period were, on average, 36.71 ng/mL in patients with evidence of BAD and 21.81 ng/mL in patients without evidence of BAD. Similarly, the median changes from baseline were − 5.59 ng/mL and − 8.78 ng/mL, respectively, and were no different in the two cohorts. The only possible numerical difference in the two cohorts is the apparently greater change from baseline in worst abdominal pain and worst bloating in the cohort without BAD. The post hoc power calculation based on the observed effects in the two cohorts suggests that a sample size of 290 patients per group would be required to establish a significant difference in stool consistency, the primary study endpoint. Alternative hypotheses to explain the lack of difference in the effects of eluxadoline between those patients with IBS and BAD and those without BAD include either a limited role of BAD in the pathophysiology of IBS, or the possibility that diagnostic testing for BAD available in the United States is suboptimal, as the “gold standard” 75SeHCAT retention is not available. However, we believe that the latter consideration is unlikely since the methods used for inclusion in the study, fasting serum 7αC4 level or total fecal bile acids over 48 h, have been thoroughly examined in prior studies [15, 16, 28,29,30] and are associated with important clinical indicators such as increased stool weight and fat, faster colonic transit, and impact on health care utilization.
In conclusion, eluxadoline is efficacious in the treatment of IBS-D with or without BAD, and it appears to be safe and efficacious as documented in large clinical trials. While opioids may stimulate TGR5 (bile acid) receptors, the current data document the similarity in clinical efficacy in two cohorts of patients with symptoms consistent with IBS-D with or without evidence of BAD. The information from this study suggests that testing for BAD is not indicated prior to considering eluxadoline for treatment of IBS-D, since the outcome of treatment is similar in IBS-D patients with or without BAD, though studies in larger numbers of patients would be required to confirm the current findings.
References
Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635.
Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958.
Aziz I, Mumtaz S, Bholah H, Chowdhury FU, Sanders DS, Ford AC. High prevalence of idiopathic bile acid diarrhea among patients with diarrhea-predominant irritable bowel syndrome based on Rome III criteria. Clin Gastroenterol Hepatol. 2015;13:1650–1655.
Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2015;64:84–92.
Camilleri M. Bile acid diarrhea: prevalence, pathogenesis, and therapy. Gut and Liver 2015;9:332–339.
Valentin N, Camilleri M, Altayar O et al. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut 2016;65:1951–1959.
Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, Le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194.
Hofmann AF, Mangelsdorf DJ, Kliewer SA. Chronic diarrhea due to excessive bile acid synthesis and not defective ileal transport: a new syndrome of defective fibroblast growth factor 19 release. Clin Gastroenterol Hepatol. 2009;7:1151–1154.
Pattni SS, Brydon WG, Dew T et al. Fibroblast growth factor 19 in patients with bile acid diarrhea:a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38:967–976.
Chang C, Jiang J, Sun R, Wang S, Chen H. Downregulation of serum and distal ileum fibroblast growth factor19 in bile acid diarrhoea patients. Dig Dis Sci. 2021. https://doi.org/10.1007/s10620-021-07042-x.
Camilleri M, Vijayvargiya P. The role of bile acids in chronic diarrhea. Am J Gastroenterol. 2020;115:1596–1603.
Islam RS, DiBaise JK. Bile acids: an underrecognized and underappreciated cause of chronic diarrhea. Practical Gastroenterol. 2012;36:32–44.
Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol. 2009;30:707–717.
Turner JM, Pattni SS, Appleby RN, Walters JR. A positive SeHCAT test results in fewer subsequent investigations in patients with chronic diarrhoea. Frontline Gastroenterol. 2017;8:279–283.
Vijayvargiya P, Gonzalez Izundegui D, Calderon G, Tawfic S, Batbold S, Camilleri M. Fecal bile acid testing in assessing patients with chronic unexplained diarrhea: implications for healthcare utilization. Am J Gastroenterol. 2020;115:1094–1102.
Camilleri M, Busciglio I, Acosta A et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol. 2014;109:1621–1630.
Lacy BE, Mearin F, Chang L et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407.
Vijayvargiya P, Camilleri M, Carlson P et al. Effects of colesevelam on bowel symptoms, biomarkers, and colonic mucosal gene expression in patients with bile acid diarrhea in a randomized trial. Clin Gastroenterol Hepatol. 2020;18:2962-2970.e6.
Lembo AJ, Lacy BE, Zuckerman MJ et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med. 2016;374:242–253.
Dove LS, Lembo A, Randall CW et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145:329-338.e1.
Sobolewska-Włodarczyk A, Włodarczyk M, Storr M, Fichna J. Clinical potential of eluxadoline in the treatment of diarrhea-predominant irritable bowel syndrome. Ther Clin Risk Manage. 2016;12:771–775.
Varga EV, Navratilova E, Stropova D, Jambrosic J, Roeske WR, Yamamura HI. Agonist-specific regulation of the delta-opioid receptor. Life Sci. 2004;76:599–612.
FDA Approved Drug Products. 2015 [Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=206940.
Brenner DM, Sayuk GS, Gutman CR et al. Efficacy and safety of eluxadoline in patients with irritable bowel syndrome with diarrhea who report inadequate symptom control with loperamide: RELIEF Phase 4 Study. Am J Gastroenterol. 2019;114:1502–1511.
Lembo AJ, Covington PS, Dove LS, Andrae DA. Effects of treatment with eluxadoline on abdominal pain in patients with IBS-D: Additional post hoc analyses of Phase 3 trials. Neurogastroenterol Motil. 2020;32:e13774.
VIBERZI™ Prescribing information Allergan USA, Inc. Irvine, CA 926122015; https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7821bd40-4c84-4984-951b-6436ae20421a
Wong BS, Camilleri M, Carlson P et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–1015.
Shin A, Camilleri M, Vijayvargiya P et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1270-1275.e1.
Vijayvargiya P, Camilleri M, Carlson P et al. Performance characteristics of serum C4 and FGF19 measurements to exclude the diagnosis of bile acid diarrhoea in IBS-diarrhoea and functional diarrhoea. Aliment Pharmacol Ther. 2017;46:581–588.
Vijayvargiya P, Camilleri M, Chedid V et al. Analysis of fecal primary bile acids detects increased stool weight and colonic transit in patients with chronic functional diarrhea. Clin Gastroenterol Hepatol. 2019;17:922–929.
Camilleri M, Nadeau A, Tremaine WJ et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734-e43.
Eusufzai S, Axelson M, Angelin B, Einarsson K. Serum 7 alpha-hydroxy-4-cholesten-3-one concentrations in the evaluation of bile acid malabsorption in patients with diarrhoea: correlation to SeHCAT test. Gut 1993;34:698–701.
O’Donnell LJD, Heaton KW. Pseudo-diarrhea in the irritable bowel syndrome: patients’ records of stool form reflect transit time while stool frequency does not. Gut 1988;29:A1455.
Drossman DA, Patrick DL, Whitehead WE et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007.
Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. https://doi.org/10.1023/a:1018831127942.
FDA Guidance for Industry, Drug-Induced Liver Injury: Premarketing Clinical Evaluation, July2009https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm 174090.pdf
Alemi F, Kwon E, Poole DP et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513–1530.
Alemi F, Poole DP, Chiu J et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 2013;144:145–154.
Camilleri M, Shin A, Busciglio I et al. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am J Physiol Gastrointest Liver Physiol. 2014;307:G508–G516.
Acknowledgments
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding
This study was supported by Allergan. Michael Camilleri is supported by Grant R01-DK115950 from National Institutes of Health for studies on bile acid diarrhea. The study was facilitated by the nursing core of the Clinical Research and Trials Unit at Mayo Clinic, supported by CCaTS Grant UL1-TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author information
Authors and Affiliations
Contributions
PV: recruitment, care of patients, authorship. MB-L: recruitment and retention of patients, records management. SLN: recruitment and retention of patients, records management. IB: recruitment, regulatory affairs (IRB). RB: clinical pharmacology. AM: clinical development. TJ: pharmacokinetics. MC: principal investigator; regulatory affairs (IRB); senior author.
Corresponding author
Ethics declarations
Conflict of interest
Michael Camilleri receives research support unrelated to this manuscript from Takeda, Vanda, Arena, and Novartis. Priya Vijayvargiya, Margaret Breen-Lyles, Sara Linker Nord, Daniel Maselli, and Irene Busciglio have no conflicts of interest. Ramesh Boinpally is an employee of AbbVie Inc.; Anna Muslin and Timothy J. Carrothers were employees of AbbVie Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Mayo Clinic Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual patients included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-022-07386-y.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vijayvargiya, P., Breen-Lyles, M., Nord, S.L. et al. Safety and Efficacy of Eluxadoline in Patients with Irritable Bowel Syndrome-Diarrhea With or Without Bile Acid Diarrhea: Open-Label Study. Dig Dis Sci 67, 3911–3921 (2022). https://doi.org/10.1007/s10620-022-07379-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07379-x