Abstract
Background
Liver biopsy is the gold standard for staging liver fibrosis, but it has numerous drawbacks, mainly associated with bleeding and bile fistula risks. A number of non-invasive techniques have been investigated, but they all have their own disadvantages. To avoid the risks mentioned above and to improve the diagnostic value, we still need to search for a more accurate non-invasive method to evaluate the degree of liver fibrosis.
Aim
This study aimed to evaluate the diagnostic performance of FibroTouch versus other non-invasive fibrosis indexes in hepatic fibrosis of different aetiologies.
Methods
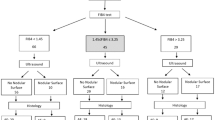
This study retrospectively enrolled 227 patients with chronic hepatic liver disease admitted to the first hospital of Lanzhou University from 2017 to 2020. Liver biopsy was performed in all of the patients, and their biochemical indicators were all tested. Non-invasive indexes including the fibrosis index based on four factors (FIB-4), the aminotransferase-to-platelet ratio index (APRI), and the gamma-glutamyl transpeptidase-to-platelet ratio index (GPRI) were all calculated. Transient elastography was performed using FibroTouch.
Results
The correlation between FibroTouch and the pathology of liver fibrosis was significantly higher than that between the non-invasive fibrosis indexes and the biopsy results (r = 0.771, p < 0.05). The area under the receiver operating curve (AUC) of FibroTouch was significantly higher than that of FIB-4, APRI, and GPRI for the diagnosis of significant fibrosis (≥ S2 fibrosis stage), advanced fibrosis (≥ S3 fibrosis stage), and cirrhosis (= S4 fibrosis stage) (p < 0.05). The patients were grouped according to different aetiologies. The diagnostic value of FibroTouch had much higher credibility in different fibrosis stages for different causes compared with other non-invasive indexes. The AUC of FibroTouch showed both higher specificity and higher sensitivity than FIB-4, APRI, and GPRI for different liver fibrosis stages with different aetiologies.
Conclusions
FibroTouch demonstrates the highest diagnostic value for liver fibrosis and cirrhosis among non-invasive methods, showing better results than FIB-4, APRI, and GPRI, and surpassed only by liver biopsy. FibroTouch is reliable in assessing liver fibrosis with different aetiologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The number of patients with cirrhosis is increasing each year, and the mortality rate for chronic liver diseases will continue to rise over the next few decades [1]. For the management of patients with chronic liver disease, the principle is to focus on delaying the occurrence of cirrhosis and associated complications, and to monitor for hepatocellular carcinoma as early as possible. Liver fibrosis is an essential stage of cirrhosis [2], and it is has various aetiologies, such as viral infection, alcohol, cholestasis, drugs, immune system disturbance, and iron deposition. Liver fibrosis can develop into cirrhosis and even hepatocarcinoma if it occurs in patients with chronic liver disease without appropriate intervention [3]. Timely diagnosis of liver fibrosis can also affect the options for optimizing treatment strategies. Estimating the degree of liver fibrosis in patients with chronic hepatitis B (CHB) enables the right timing of antiviral therapy, which will affect the long-term prognosis of patients. With the development of the current generation of direct-acting antiviral therapy for chronic hepatitis C (CHC), the fibrosis stage is no longer crucial for initiation of treatment for CHC patients. The European Association for the Study of the Liver (EASL) guidelines recommend that those patients who have been diagnosed with cirrhosis be screened for hepatocellular carcinoma (HCC). Therefore, all hepatitis C virus (HCV)-positive patients need to undergo evaluation of fibrosis stage as part of routine HCV care to exclude cirrhosis [4]. Additionally, the liver fibrosis stage of CHC patients before and after treatment with antiviral agents is still a meaningful status to monitor, not to mention that the more serious the liver fibrosis, the higher the possibility of progression to HCC. Confirmation of liver fibrosis changes related to chronic liver disease plays a decisive role in varicosity and liver cancer monitoring. A number of studies have demonstrated that liver fibrosis is a reversible process; with early discovery and treatment, standardized management can delay or even reverse disease progression [5].
Liver biopsy is the gold standard for the diagnosis of liver fibrosis and cirrhosis. However, it is an invasive examination that carries certain risks [6], such as haemorrhage and infection. In addition, the pathological results of liver biopsy need to be evaluated artificially, which is quite time-consuming and costly. Therefore, it is difficult to apply in clinical practice. There are several commonly used non-invasive approaches for evaluating liver fibrosis, including direct and indirect serum liver fibrosis marker tests, transient elastography (such as FibroScan), acoustic radiation force impulse (ARFI) imaging, and magnetic resonance imaging (MRI) [7]. However, they all have their own disadvantages. For example, FibroScan cannot avoid vessels, bile ducts, and calcification; thus, the test results do not match the reality in some cases. In addition, the probe must be replaced based on the weight of the patients, making the operation even more complicated [8]. ARFI is unreliable for obese patients and is less practical than transient elastography [9]. Diffusion-weighted imaging (DWI) is inherently a series of T2-weighted sequences that detect movement of protons in water molecules by applying opposite gradient pulses in each of three orthogonal directions, which is expensive, while the apparent diffusion coefficient (ADC) fails to discern the disease by stage [10]. Magnetic resonance elastography (MRE) is a promising tool for assessing liver fibrosis, and its diagnostic vale is much higher than that of DWI, but it still has some limitations. Firstly, the image post-processing is complex. Secondly, it is not suitable for patients with haemochromatosis, because iron causes signal loss and T2 shortening. Lastly, but no less important, it is also expensive [11]. Direct serum markers of liver fibrosis, such as hyaluronic acid and type III collagen, and non-invasive fibrosis indexes such as FIB-4 can be easily acquired at lower cost, but serological examination is susceptible to fluctuations with the circumstances of liver inflammation [12].
FibroTouch, a third-generation transient elastography technology, is the first device integrating 2D Doppler ultrasound imaging, transient elastography, and hepatic steatosis collection technology [13]. The innovative dynamic broadband scanning probe can be adjusted automatically according to the patient’s body type [14]. So, even though FibroTouch has only one probe, it performs well in obese patients. By influencing and guiding positioning, it can detect substantial stiffness of the liver more accurately and successfully than FibroScan. Moreover, FibroTouch has been reported to rival the collection success rate of FibroScan [15]. The device has now been used in many countries and regions. Its sensitivity and specificity for liver fibrosis and cirrhosis resulting from hepatitis B virus (HBV) have been verified in many reports [16, 17]. In contrast, few reports have focused on liver fibrosis with aetiologies other than CHB. In this study, the factors affecting the FibroTouch test results and its value in liver fibrosis evaluation were analysed by comparing FibroTouch with other non-invasive fibrosis indexes.
Patients and Methods
Patients
The study retrospectively included 227 patients with liver disease of diverse causes at the First Hospital of Lanzhou University during 2017–2020. Among those patients, there were 103 cases caused by chronic HBV infection, 52 cases caused by autoimmune liver diseases, and 72 cases of other liver diseases and unknown causes. There were 96 male and 131 female patients, and their average age was 40.94 ± 13.85 years. Patients who were pregnant or had liver cancer, disease complicated by ascites, or diseases related to other systems were all excluded from the study.
The study design was approved by the Ethics Committee of the First Hospital of Lanzhou University, and informed consent was obtained from all patients involved before the study was started.
Clinical Assessment
The age, sex, height, weight, and medical history of the patients were collected, and the body mass index (BMI) was calculated. The patients’ blood was tested with an Olympus AU640 automatic biochemical analyser (Olympus Diagnostic Systems, Tokyo, Japan), and aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), direct bilirubin (DBIL), serum albumin (Alb), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TCHO), triglyceride (TG), leukocyte (WBC), and platelets (PLT) were tested with a Mindray BC-3000Plus haematology analyser (Mindray, Shenzhen, China).
Liver Biopsy and Histological Assessment
Liver biopsy was performed by qualified doctors as guided by colour ultrasound with 5 ml of 2% lidocaine for local anaesthesia. The patients laid on their backs and underwent liver biopsy with a 16G biopsy needle (TSK, Japan) following ultrasound guidance. Approximately 1.5–3 cm liver specimens were collected and then fixed with 10% formalin, embedded in paraffin, and stained with haematoxylin and eosin (HE). Then, histopathological analysis was performed independently by two experienced pathologists; if any results were disputed, a third experienced specialist was necessarily involved to reach agreement. The liver inflammation and fibrosis staging complied with the METAVIR scoring system: S0, no fibrosis; S1, portal fibrosis without septa; S2, portal fibrosis with rare septa; S3, many septa including central vein-portal bridge fibrosis and portal-portal bridge fibrosis without cirrhosis; and S4, cirrhosis [18].
Patients with S2 or higher were considered to have 'significant fibrosis', patients with S3 or higher were considered to have ‘advanced fibrosis’, and patients with S4 were considered to have cirrhosis [19].
FibroTouch (Wuxi Hisky Medical Technologies Co., Ltd., Wuxi, China) was operated by an experienced nurse and applied to detect the liver stiffness of all patients within 1 week after liver biopsy. An ultrasonic probe was placed in the area covering the seventh to the ninth intercostal spaces from the right anterior axillary line to the midaxillary line of the patients who needed ultrasonic examination. Marks were left on uniform hepatic tissues of proper thickness that were free from either artery, bile ducts, or cysts. The device was then switched to elastography mode, and patients were asked to hold their breath for 3 s. When the image became stabilized, liver stiffness measurement (LSM) was conducted. The measurement was carried out 10 times, and the median was considered the final value and was expressed as the value of elasticity (kPa). A reliable LSM was defined as more than 10 valid shots, a success rate of at least 60%, and an IQR < 30% [20]. The fat attenuation parameter (FAP) value was also acquired.
Calculation
The fibrosis index based on four factors (FIB-4), aminotransferase-to-platelet ratio index (APRI), and gamma-glutamyl transpeptidase-to-platelet ratio index (GPRI) values were determined.
FIB4 = [age (yrs) × AST (U/L)]/[PLT (× 109/L) × ALT (U/L)1/2].
APRI = ([AST/ULN*]/platelet count (109/L)) × 100
GPRI = [GGT/ULN*]/platelet count (109/L) × 100
In our laboratory, the upper limit of normal (ULN) of AST and ALT was 49 IU/L, and the ULN of GGT was 69 U/L.
Statistics
The data are expressed as the average ± standard deviation (mean ± SD). The relevance between the two test values was tested with Spearman’s or Pearson’s rank correlation. With pathological grading of liver fibrosis as the gold standard, the receiver operating characteristic (ROC) curve was applied to determine the area under the curve (AUC), cut-off value, sensitivity, and specificity. Differences were considered significant when p < 0.05.
Results
Patient Characteristics
In total, 227 patients with liver disease of different aetiologies were included in the study, including 103 cases of chronic hepatitis B, 52 cases of autoimmune liver diseases, and 72 cases of unknown cause and other liver diseases such as hepatitis C virus (HCV) infection, drug-induced liver injury, non-alcoholic fatty liver disease (NAFLD), progressive familial intrahepatic cholestasis (PFIC), Wilson’s disease, alcoholic liver disease, and Budd-Chiari syndrome. Their clinical characteristics are summarized in Table 1.
Diagnostic Value of LSM, FIB-4, APRI, and GPRI in Liver Fibrosis
Based on liver pathological results, LSM, FIB-4, APRI, and GPRI were all positively correlated with the fibrosis stage (p < 0.01). The correlation coefficient between LSM and liver pathology grade was the highest (r = 0.771, p < 0.01), followed by FIB-4 (r = 0.493, p < 0.01), GPRI (r = 0.438, p < 0.01), and APRI (r = 0.415, p < 0.01), as shown in Fig. 1.
Diagnostic Value Between FibroTouch and the Non-invasive Fibrosis Indexes in Liver Fibrosis
The patients were divided into three groups by pathological grading of liver fibrosis: significant fibrosis (≥ S2), advanced fibrosis (≥ S3), and cirrhosis (S4). The diagnostic accuracy of LSM, FIB-4, APRI, and GPRI is shown in Table 2 and Fig. 2. LSM differed with stages (p < 0.05). In the three groups, compared with FIB-4, APRI, and GPRI, the AUC of LSM was significantly higher than that of other non-invasive fibrosis indexes of liver fibrosis (p < 0.05).
Diagnostic Value of FibroTouch in Patients with Chronic Liver Disease of Different Aetiologies
The patients were divided into three groups by aetiology: CHB group, autoimmune liver diseases group, and other/unknown liver disease group. The diagnostic accuracy of LSM, FIB-4, APRI, and GPRI in patients of the three groups is shown in Table 3 and Fig. 3. Among the three groups, the AUC of LSM was significantly high, especially in S3 and S4. The cut-off value for LSM varies according to different causes of liver diseases, as shown in Table 3 and Fig. 3.
a1 FibroTouch LSM ROC curve analysis for significant fibrosis in CHB; a2 FibroTouch LSM ROC curve analysis for advanced fibrosis in CHB; a3 FibroTouch LSM ROC curve analysis for cirrhosis in CHB. b1 FibroTouch LSM ROC curve analysis for significant fibrosis in autoimmune liver diseases; b2 FibroTouch LSM ROC curve analysis for advanced fibrosis in autoimmune liver diseases; b3 FibroTouch LSM ROC curve analysis for cirrhosis in autoimmune liver diseases. c1 FibroTouch LSM ROC curve analysis for significant fibrosis in unknown and other liver diseases; c2 FibroTouch LSM ROC curve analysis for advanced fibrosis in unknown and other liver diseases; c3 FibroTouch LSM ROC curve analysis for cirrhosis in unknown and other liver diseases
Factors Affecting LSM
Factors that affect the expression of LSM were also assessed. The LSM was significantly correlated with age, ALT, AST, and TBIL levels (r = 0.365, 0.267, 0.286, and 0.285, respectively; all p < 0.05). Sex, BMI, and FAP exhibited no impact on LSM (p > 0.05).
Discussion
Chronic liver diseases will inevitably proceed to cirrhosis, which is characterized by high morbidity and mortality in countries around the world [21, 22]. As mentioned above, early diagnosis of liver fibrosis is critical to improving the patient’s prognosis [23]. The methods used to measure liver fibrosis remain limited; they include invasive liver biopsy, non-invasive liver elastography, direct fibrosis serum markers, liver fibrosis models, and the internationally well-recognized FIB-4, APRI, and GPRI [9, 24]. FibroTouch is the latest elastography technology for assessing liver fibrosis [8, 19, 25, 26], however, most of the reports have concentrated on CHB patients, while they have rarely mentioned its application in evaluating liver fibrosis of other causes. In view of this limitation, this study aimed to determine the diagnostic value of FibroTouch for liver fibrosis among patients with chronic liver diseases of different aetiologies.
The research results indicate that FibroTouch, FIB-4, APRI, and GPRI are all positively correlated with the hepatic pathology MATEVIA scoring system. The correlation of FibroTouch was significantly higher than that of other non-invasive fibrosis indexes, which is consistent with previous research results [19]. In addition, compared with FIB-4, APRI, and GPRI, FibroTouch has higher accuracy, sensitivity, and specificity in diagnosing fibrosis in patients with chronic liver disease [27]. In previous studies, the non-invasive fibrosis indexes of liver fibrosis were highly recognized [28, 29]. According to studies, the AUC of FBI-4, APRI, and GPRI was 0.865, 0.91, and 0.93 in the diagnosis of significant fibrosis; 0.87, 0.91, and 0.93 in the diagnosis of advanced fibrosis; and 0.728, 0.836, and 0.842 in the diagnosis of early cirrhosis, respectively [28, 30]. However, we obtained different results in our ROC curve analysis: 0.610, 0.624, and 0.540 in the diagnosis of significant fibrosis; 0.718, 0.694, and 0.768 in the diagnosis of advanced fibrosis; and 0.684, 0.660, 0.615 in the diagnosis of early cirrhosis, respectively; our data showed diagnostic values much lower than those reported previously. Yang et al. [8] also reported the limitations of digital fibrosis models in predicting liver fibrosis and cirrhosis. These limitations may be attributed to the inclusion of only two or three fibrosis-related indicators. Furthermore, AST is also related to liver inflammation and may not accurately reflect liver fibrosis, and PLT counting suffers from a large overlap between mild and severe liver fibrosis. In addition, crucial factors such as age are not included in the formula. The results may also be related to the research objectives in previous studies that had focused more on the CHB-infected group and also excluded patients with extremely low platelet counts. In our study, in order to reflect real situations, patients in all states of illness were included.
Regarding the ROC values, FibroTouch showed moderate performance in diagnosing significant fibrosis (AUC 0.732) in the CHB group. However, the diagnosis of advanced fibrosis and cirrhosis with the FibroTouch was excellent (AUC 0.943, 0.922, respectively). As PPVs were in the range of 67.5–88.9% and NPVs were in the range of 73.7–100% for LSM, these are accurate for predicting liver fibrosis stages. The cut-off value was 6.3 kPa in significant fibrosis, 8.4 kPa in advanced fibrosis, and 12.55 kPa in cirrhosis in the CHB group. These results are similar to those of related reports [8, 13, 15]; the cut-off values for CHB in those studies were 5.8–9.1 kPa for significant fibrosis, 7.6–10.7 kPa for advanced fibrosis, and 12.5–14 kPa for cirrhosis. However, the results were lower than those shown in the guidelines for the prevention and treatment of chronic hepatitis B (2019) [31] and consensus on clinical application of transient elastography for the detection of liver fibrosis (2018) [32]. The guidelines recommended an optimal cut-off value in CHB of 9.4 kPa in significant fibrosis, 12.4 kPa in advanced fibrosis, and 17 kPa in cirrhosis when ALT was abnormal but lower than 5*ULN. The optimal cut-off value is 6.0 kPa in significant fibrosis, 9.0 kPa in advanced fibrosis, and 12 kPa in cirrhosis when ALT is completely normal. Our study also included some cases with abnormal ALT but < 5*ULN; thus, the cut-off value in this study is included in the two situations mentioned above. FibroTouch also showed outstanding performance in diagnosing fibrosis stages in autoimmune liver diseases. The AUC of LSM performed by FibroTouch was 0.899, 0.909, and 0.920, respectively, which was similar to the result for FibroScan shown in a systematic review (AUC 0.9, 0.91, 0.89 for significant liver fibrosis, advanced liver fibrosis, and cirrhosis, respectively) [33]. However, the ROC value in the autoimmune liver diseases group in the study was lower than that shown in a previous report [8]. In that report, the LSM was performed by FibroTouch but the number of cases was 16, which probably led to higher AUC. The optimal cut-off value was 6.3 kPa in significant fibrosis, 8.4 kPa in advanced fibrosis, and 12.55 kPa in cirrhosis in the autoimmune liver diseases group, which is similar to the result shown in the systematic review mentioned above [33]. The optimal cut-off value was different in different aetiologies; thus, it is necessary to choose the optimal cut-off value according to the corresponding aetiology before using FibroTouch for fibrosis evaluation, which can improve the probability of reliable test results. Studies with larger sample size are still needed for further confirmation.
Further analysis of the factors that affect the LSM expression levels tested by FibroTouch showed that age and ALT, AST, and TBIL levels were all correlated with LSM performance, similar to the factors influencing FibroScan [34, 35]; however, the effect of BMI on the diagnostic value of FibroTouch was less than that for FibroScan. These results indicate that the expression of LSM tested by FibroTouch may be affected by liver inflammation rather than by obesity.
Although the diagnostic value of FibroTouch is significantly higher than that of the other non-invasive fibrosis indexes, its use in liver fibrosis evaluation and diagnosis should be combined with multiple indicators, especially in patients with ascites or narrow intercostal space [36] given the inherent limitations, and it is helpful for improving its diagnostic accuracy and stability [37].
The study still has many limitations. For example, it is a single-centre study with a small sample size that may have resulted in certain inaccuracy. However, the gold standard, liver biopsy, can contain certain errors due to limited sampling and operator differences. In addition, patients with chronic liver diseases other than CHB and autoimmune liver disease may not be classified and evaluated for the accuracy of FibroTouch due to the limited samples. Some clinical studies have demonstrated that BMI (≥ 28 kg/m2) has an effect on the diagnostic value of FibroTouch [14], but it was less still than that on FibroScan. However, this study showed that BMI had no effect on LSM. This result may be caused by the small sample size, especially the shortage of patients with NAFLD. We will involve more patients to clarify the diagnostic value in the above conditions in the future.
In summary, FibroTouch is a novel transient elastography technology. FibroTouch and FibroScan have good consistency in the evaluation of the degree of liver fibrosis, but the success ratio was significantly higher and the measurement duration was significantly shorter with FibroTouch than with FibroScan [14, 15]. FibroTouch was also significantly better than other non-invasive fibrosis indexes of liver fibrosis, namely FIB-4, APRI, and GPRI, based on our data. FibroTouch should thus be highly recommended for use.
References
Sharma S, Khalili K, Nguyen GC. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J Gastroenterol 2014;20:16820–16830.
Pencheva B, Mihajlov R, Ruseva A et al. Reference values of certain serum indicators of liver fibrosis. Clin Lab 2017;63:1793–1800.
Fricker J. European association for the study of the liver: international liver congress 2017 meeting. Lancet Oncol 2017;18:713.
European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392–420.
Shiha G, Sarin SK, Ibrahim AE et al. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 2009;3:323–333.
Gwon D 2nd, Ko GY, Yoon HK et al. Hepatocellular carcinoma associated with membranous obstruction of the inferior vena cava: incidence, characteristics, and risk factors and clinical efficacy of TACE. Radiology. 2010;254:617–626.
Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142:1293-1302.e4.
Yang XZ, Gen AW, Xian JC, Xiao L. Diagnostic value of various noninvasive indexes in the diagnosis of chronic hepatic fibrosis. Eur Rev Med Pharmacol Sci 2018;22:479–485.
Motola DL, Caravan P, Chung RT, Fuchs BC. Noninvasive biomarkers of liver fibrosis: clinical applications and future directions. Curr Pathobiol Rep 2014;2:245–256.
Onur MR, Poyraz AK, Bozdag PG, Onder S, Aygun C. Diffusion weighted MRI in chronic viral hepatitis: correlation between ADC values and histopathological scores. Insights Imaging 2013;4:339–345.
Li J, Venkatesh SK, Yin M. Advances in Magnetic Resonance Elastography of Liver. Magn Reson Imaging Clin N Am 2020;28:331–340.
Chon YE, Choi EH, Song KJ et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS ONE 2012;7:e44930.
Wong GL. Prediction of fibrosis progression in chronic viral hepatitis. Clin Mol Hepatol. 2014;20:228–236.
Zeng J, Sun WL, Chen GY et al. Efficiency of FibroScan and FibroTouch in liver stiffness measurement and fat quantification: a comparative analysis]. Zhonghua Gan Zang Bing Za Zhi. 2016;24:652–658.
Xu Y, Liu Y, Cao Z et al. Comparison of FibroTouch and FibroScan for staging fibrosis in chronic liver disease: Single-center prospective study. Dig Liver Dis. 2019;51:1323–1329.
Ou X, Wang X, Wu X et al. Comparison of FibroTouch and FibroScan for the assessment of fibrosis in chronic hepatitis B patients]. Zhonghua Gan Zang Bing Za Zhi. 2015;23:103–106.
Yuan L, Shao J, Hao M et al. Correlation between liver hardness testing results obtained by FibroTouch and FibroScan and liver pathological stage]. Zhonghua Gan Zang Bing Za Zhi 2014;22:425–429.
Tian A, Pu K, Li B et al. Weighted gene coexpression network analysis reveals hub genes involved in cholangiocarcinoma progression and prognosis. Hepatol Res 2019;49:1195–1206.
Nan Y, Niu X, Wang R et al. microRNA-1273g-3p is a useful non-invasive test for the prediction of liver fibrosis in patients with chronic hepatitis C. Exp Ther Med 2019;17:1817–1824.
Duan WJ, Wang XZ, Ma AL et al. Multicenter prospective study to validate a new transient elastography device for staging liver fibrosis in patients with chronic hepatitis B. J Dig Dis 2020;21:519–525.
EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-460.
Zalesak M, Francis K, Gedeon A et al. Current and future disease progression of the chronic HCV population in the United States. PLoS ONE 2013;8:e63959.
Tapper EB, Challies T, Nasser I, Afdhal NH, Lai M. The performance of vibration controlled transient elastography in a US cohort of patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2016;111:677–684.
Karlas T, Dietrich A, Peter V et al. Evaluation of Transient Elastography, Acoustic Radiation Force Impulse Imaging (ARFI), and Enhanced Liver Function (ELF) Score for Detection of Fibrosis in Morbidly Obese Patients. PLoS ONE 2015;10:e0141649.
Wang YQ, Cao WJ, Gao YF, Ye J, Zou GZ. Serum interleukin-34 level can be an indicator of liver fibrosis in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2018;24:1312–1320.
Deng H, Wang CL, Lai J, Yu SL, Xie DY, Gao ZL. Noninvasive diagnosis of hepatic steatosis using fat attenuation parameter measured by FibroTouch and a new algorithm in CHB Patients. Hepat Mon 2016;16:e40263.
Wang R, Ren W, Zhao S et al. Clinical study on FibroTouch and multi-parameter model for diagnosis of hepatic fibrosis in patients with chronic liver disease]. Zhonghua Gan Zang Bing Za Zhi 2015;23:265–269.
Zeremski M, Talal AH. Noninvasive markers of hepatic fibrosis: are they ready for prime time in the management of HIV/HCV co-infected patients. J Hepatol 2005;43:2–5.
Weng H, Mertens PR, Gressner AM, Dooley S. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol 2007;46:295–303.
Wang RQ, Zhang QS, Zhao SX et al. Gamma-glutamyl transpeptidase to platelet ratio index is a good noninvasive biomarker for predicting liver fibrosis in Chinese chronic hepatitis B patients. J Int Med Res 2016;44:1302–1313.
[The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi 2019;27:938–961.
[Consensus on clinical application of transient elastography detecting liver fibrosis: a 2018 update]. Zhonghua Gan Zang Bing Za Zhi 2019;27:182–191.
Wu S, Yang Z, Zhou J et al. Systematic review: diagnostic accuracy of non-invasive tests for staging liver fibrosis in autoimmune hepatitis. Hepatol Int 2019;13:91–101.
Das K, Sarkar R, Ahmed SM et al. “Normal” liver stiffness measure (LSM) values are higher in both lean and obese individuals: a population-based study from a developing country. Hepatology 2012;55:584–593.
Coco B, Oliveri F, Maina AM et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat 2007;14:360–369.
Degos F, Perez P, Roche B et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:1013–1021.
Shiha G, Ibrahim A, Helmy A et al. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int 2017;11:1–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, X., Tian, A., Li, J. et al. Diagnostic Value of FibroTouch and Non-invasive Fibrosis Indexes in Hepatic Fibrosis with Different Aetiologies. Dig Dis Sci 67, 2627–2636 (2022). https://doi.org/10.1007/s10620-021-07049-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07049-4