Abstract
Background
Dieulafoy’s lesion (DL) is a rare but increasingly recognized cause of severe upper GI hemorrhage (SUGIH). There is little consensus regarding the endoscopic approach to management of bleeding from DL.
Aims
Our purposes were to compare 30-day outcomes of patients with SUGIH from DL with Doppler endoscopic probe (DEP) monitoring of blood flow and guided treatment versus standard visually guided hemostasis (VG).
Methods
Eighty-two consecutive DL patients with SUGIH were identified in a large CURE Hemostasis database from previous prospective cohort studies and two recent RCTs at two university-based medical centers. 30-day outcomes including rebleeding, surgery, angiography, death, and severe medical complications were compared between the two treatment groups.
Results
40.2% of DL bleeds occurred in inpatients. 43.9% of patients had cardiovascular disease, and 48.7% were taking medications associated with bleeding. For the entire cohort, 41.3% (26/63) of patients treated with VG had a composite 30-day outcome as compared to 10.5% (2/19) of patients treated with DEP (p = 0.017). Rebleeding occurred within 30 days in 33.3% and 10.5% of those treated with VG and DEP, respectively (p = 0.051). After propensity score matching, the adjusted 30-day composite outcome occurred in 39.0% in the VG group compared to 2.6% in the DEP group (p < 0.001). Adjusted 30-day rebleeding occurred in 25.3% in the VG group versus 2.6% in the DEP group (p < 0.001).
Discussion
DL patients with SUGIH were frequently inpatients and had severe cardiovascular comorbidities and recurrent bleeding. Lesion arterial blood flow monitoring and obliteration are an effective way to treat bleeding from DL which reduces negative 30-day clinical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dieulafoy’s lesion (DL) is a large-caliber, submucosal artery that fails to undergo the progressive narrowing that is characteristic of most arteries in the GI tract. Endoscopically, it is difficult to diagnose unless there is active bleeding because there is no ulceration and lesions are small and often obscured by pooled blood or clots. DL is an important and often unrecognized cause of severe upper gastrointestinal hemorrhage (SUGIH). While claims data attribute 1–2% of upper GI bleeding diagnoses to DLs, recent studies report them as a more common cause of SUGIH, accounting for more than 10% [1, 2].
First-line therapy for DL is endoscopic hemostasis with angiographic embolization and surgery as salvage modalities for failed endoscopic therapy or recurrent bleeding [3]. There is no consensus about the best endoscopic treatment for bleeding from DLs. Options include injection with epinephrine or sclerosing agents; thermal treatment with heater probe (HP), multipolar electrocautery (MPEC) or argon plasma coagulation (APC); and mechanical therapy with endoscopic band ligation (EBL) and through-the-endoscope hemoclips (HC) [4,5,6,7,8,9]. Over-the-scope clip (OTSC) is an emerging endoscopic therapy which is promising for treatment of ulcer bleeding and potentially for DL [10,11,12]. Previous reports of different treatments for DL in randomized controlled trials (RCT) are small in size [7,8,9, 13]. This is probably because DLs are relatively rare compared to peptic ulcers.
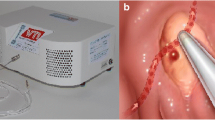
Arterial blood flow monitoring with the Doppler endoscopic probe (DEP) is reported to be an effective guide for definitive endoscopic hemostasis of severe GI bleeding for non-variceal upper GI lesions including ulcers and DL [2, 14]. Because DLs are large, submucosal arteries that bleed through a small mucosal defect without an ulceration, the use of DEP is well suited for mapping the arterial location and as a guide for documenting obliteration of arterial flow by therapeutic techniques (Fig. 1) [2].
a Gastric body DL in a patient with liver cirrhosis, b pre-treatment use of the Doppler probe adjacent to the DL, c DL after treatment with four standard hemoclips, d fundal DL identified in retroflexion e in an inpatient bleeder. Use of DEP to monitor arterial blood flow f. DL after treatment with OTSC
Our aims in this study were to compare 30-day clinical outcomes of patients with SUGIH from DL treated with standard visually guided endoscopic treatment (VG) to those patients with DEP-guided treatment (DEP) in a large cohort of DL patients and also a subgroup from recent RCTs.
Methods
All consecutive patients hospitalized at UCLA-Ronald Reagan Medical Center and the VA-West Los Angeles Medical Hospital for the management of SUGIH between December 1987 and July 2020 who had urgent upper endoscopy were assessed by the CURE Hemostasis Research Group (e.g., a bleed team consulted upon for patients with acute GI hemorrhage). Patients were consented for either cohort studies or recent RCTs of severe non-variceal upper GI hemorrhage (NCT00732212 and NCT03065465), and data were prospectively collected [2, 12]. All studies were approved by the Institutional Review Boards at both institutions.
SUGIH was defined as clinical evidence of upper GI bleeding (melena, hematemesis, and/or hematochezia with or without NG tube aspiration of blood) along with syncope, hypotension, shock, and tachycardia not explained by another cause; a decrease in hemoglobin from baseline of ≥ 2 g/dL; and/or transfusions of ≥ 2 units of packed red blood cells (RBC).
In cases where mucosal visualization with the therapeutic upper endoscope was limited, a larger channel upper endoscope or a BioVac Direct Suction device (US Endoscopy, OH) was utilized to remove blood and clots from the stomach. Other maneuvers including gastric lavage and/or repositioning to the right side down were performed when necessary in intubated patients to improve mucosal visualization of the gastric fundus. After it became available in January 2008, the Doppler endoscopic probe was used for arterial blood flow detection, mapping the underlying artery, and documenting post-treatment obliteration of arterial blood flow.
We retrospectively screened the CURE database for DL as the final diagnosis of SUGIH. Lesions were classified as a DL if an artery protruded through a mucosal defect without surrounding ulceration or mass. We did not include arteries with surrounding mucosal ulceration (classified as peptic ulcers) or UGI tumors with protruding arteries. Every report and all endoscopic photographs and/or videos of DL cases were reviewed by the principal investigator (D.J.) and the co-investigators of the CURE Hemostasis Research Group to confirm the endoscopic diagnosis of DL.
CURE Hemostasis endoscopy attendings utilized dilute epinephrine pre-injection (1:20,000 in saline) in patients with either spurting bleeding (Forrest 1A) or oozing bleeding (Forrest 1B) to control or slow bleeding and provide better visualization prior to other more effective treatments (MPEC, HP, HC, or OTSC) as previously described [2]. Also, DLs with adherent clots had pre-injection with epinephrine prior to cold guillotining off the clots and more effective endoscopic treatments, as previously described [2, 15].
Patients were classified into two groups according to whether arterial blood flow was monitored with DEP and used as a guide to definitive hemostasis or visually guided treatment alone was utilized [2, 14]. The VG group included patients that underwent treatment with thermal coagulation (MPEC or HP) or HC, with or without epinephrine pre-injection. In the DEP group, patients were evaluated pre- and post-treatment with Doppler and were found to have a negative signal post-treatment. The treatments utilized included MPEC, HP, HC, and/or OTSC with or without epinephrine pre-injection.
Medical records of DL patients were prospectively followed-up from the date of diagnosis and treatment. For DL patients included in two recent RCTs, the patient, managing physicians, and healthcare staff were blinded as to the type of endoscopic treatment (VG vs. DEP-guided). These physicians made all subsequent decisions about rebleeding, use of surgery or angiography, level of care, and whether to recommend repeating the endoscopy. Data from the medical record were entered onto standard CURE research forms, and all data were de-identified and entered onto computer data files by experienced data managers in a large CURE prospective database of SUGIH.
The primary outcomes of this study were 30-day DL-related rebleeding and a composite 30 day outcome of rebleeding, surgery, angiography, severe complications of bleeding, or all-cause mortality within 30 days. The components of the composite outcomes have been used as primary and secondary outcomes in recent CURE RCTs and have been previously described as clinically important [2, 12, 16]. Secondary outcomes included hospital and ICU days, transfusions of RBCs, and mortality caused by GI hemorrhage. These outcomes were evaluated in both the entire patient cohort and separately in RCT patients.
Statistical Methods
SAS 9.4 (SAS, Inc, Cary, NC) and R 3.5.2 (R project for statistical computing; https://www.r-project.org/) were used for data management and statistical analyses. The p-values for comparing proportions in VG versus DEP groups were computed using Fisher’s exact test. The p-values for comparing continuous variables such as age between the two groups were computed using either t-tests if the data had a normal distribution or the corresponding non-parametric Wilcoxon rank sum test. Rebleed-free and composite event-free curves were computed using the Kaplan–Meier method for time to event data, and the p-values for their comparison were computed using the log rank test.
Propensity score weights were used in order to control for possible confounding by race, early/late time period, fundal location of DL, use of any of six antithrombotic or anticoagulant drugs, age and lowest hemoglobin. The propensity score was computed via logistic regression on visual versus DEP treatment [17]. The p-values for comparing propensity weight adjusted 30 day rebleeding or composite outcome between the two groups were computed using the Rao–Scott method, analogous to adjusting for survey weights [18].
Results
Patient Characteristics
The baseline clinical characteristics of 82 DL patients are presented in Table 1. The mean age was 61.7 years. DEP patients were older than the VG group (70.6 vs. 58.9; p = 0.003). Patients had a mean American Society of Anesthesiology (ASA) score of 3.3. 43.9% had cardiovascular disease, 15.9% had ESRD, and 23.2% had liver cirrhosis. The rates of these comorbidities were similar in the DEP and VG groups. Among the 19 patients with liver cirrhosis, 63.2% (N = 12) had a history of hepatic decompensation and 57.9% (N = 11) had upper GI manifestations of portal hypertension (esophageal or gastric varices (5); portal hypertensive gastropathy (5), varices and gastropathy (1)). 48.7% of patients were taking a high-risk bleeding medication. 45.1% of patients had a history of prior GI bleeding, including 63.2% in the DEP group and 39.7% in the VG group (p = 0.071). Most prior GI bleeds were obscure; however, peptic ulcer disease was the most commonly identified lesion in those with definitive diagnoses (10.5% DEP; 22.2% VG).
Clinical Presentation of Bleeding
Bleeding occurred in the inpatient setting after hospitalization for a non-bleeding diagnosis in 40.2% (N = 33) (Table 2). The most common bleeding sign at presentation was melena (N = 59; 72.0%). A greater percentage of patients in the VG cohort presented with hematemesis (41.3% vs. 10.5% in DEP cohort; p = 0.013). Mean Glasgow–Blatchford Scores were similar between the two groups.
The mean hemoglobin at presentation was 7.6 d/L with a mean decrease of 3.4 g/dL from the baseline (pre-bleeding) value. Patients received a mean of 4.2 units of red blood cells (RBCs) for resuscitation prior to endoscopy. 18.3% (N = 15) of patients required multiple endoscopies before the diagnosis of DL was made.
The location and stigmata of the UGI DL are described in Table 2. The most common location of DL was the gastric fundus (N = 29). Active arterial bleeding was the most frequent endoscopic bleeding stigmata (N = 46). Advanced maneuvers including gastric lavage, Biovac suctioning, and right-side-down repositioning were required to make the diagnosis in 11.0% (N = 9) of patients.
Endoscopic Treatments
Sixty-three patients in the cohort underwent visually guided treatment (VG): 32 with thermal modalities, 28 with mechanical (standard HC) and 3 with mechanical/thermal combination therapy. Nineteen patients underwent DEP-guided treatment. Prior to DEP-guided treatment, the blood flow detected underneath the DL in all patients was arterial and none was venous. This is similar to peptic ulcers as previously described [1, 14]. Among the 19 patients in the DEP group, two were treated with mechanical/thermal combination therapy and 17 were treated with mechanical therapy (13 standard HC and 4 OTSC). Patients in the DEP cohort were more likely to receive treatment with mechanical modalities as compared to VG patients (100% vs. 49.2%; p < 0.001).
30-Day Outcomes: Entire Cohort
At 30 days, 28.0% of patients (N = 23) rebled from DL (Table 3). Rebleeding occurred in 10.5% (2/19) of DEP patients and 33.3% (21/63) of VG patients (NNT 4.4; p = 0.051) (Fig. 2a).
a Kaplan–Meier plot of time to rebleeding up to 30 days for DEP-guided versus visually guided endoscopic treatments. By log rank p = 0.051. b Kaplan–Meier plot of time to poor outcome (rebleeding, surgery or angiography for rebleeding; severe complications related to rebleeding; or death) up to 30 days for DEP-guided versus visually guided endoscopic treatments. By log rank p = 0.017. c Kaplan–Meier plot of time to rebleeding up to 30 days for RCT patients treated with DEP-guided versus visually guided endoscopic treatments. By log rank p = 0.158. d Kaplan–Meier plot of time to poor outcome (rebleeding, surgery or angiography for rebleeding; severe complications related to rebleeding; or death) up to 30 days for RCT patients treated with DEP-guided versus visually guided endoscopic treatments. By log rank p = 0.063
Therapeutic angiography was utilized in two patients who rebled after endoscopic treatment (1 DEP, 1 VG). Five patients required surgery for rebleeding within 30-days of their index endoscopy. All five were in the VG cohort, and all had primary treatment with a thermal modality.
Four patients died within 30-days of their index bleed. None of the deaths were due to DL-related bleeding. Three patients in the VG cohort died due to unrelated severe comorbid medical conditions. One patient in the VG cohort with a gastric fundus DL that was treated with MPEC died from hemorrhagic shock due to an aortoduodenal fistula which was identified at autopsy. There was no evidence of bleeding in the small bowel at the time of index endoscopy.
Three patients had severe bleeding-related complications including stroke (1), congestive heart failure exacerbation (unrelated to resuscitation) (1), and acute coronary syndrome (1). These patients were all in the VG group.
34.1% (28/82) of the full cohort experienced a composite 30-day adverse outcome (rebleeding, DL-related surgery, angiography, severe complication, or death) (Fig. 2b). There was a significant difference according to endoscopic treatments with 10.5% (2/19) of DEP patients and 41.3% (26/63) of VG patients having a composite outcome (NNT 3.2; p = 0.017).
Patients in the DEP group remained in the hospital for a median of 4.0 days post-treatment (range 1–60), as compared to 9.0 days in the standard therapy group (range 0–365) (p = 0.032). There was a numerical difference between the treatment groups in terms of ICU length of stay (Table 3).
Thirty-day outcomes from 77 patients treated with mechanical or thermal modalities (regardless of the use of DEP) are compared in Table 4. There were no significant differences in 30-day rebleeding or 30-day composite outcomes based on treatment modality. DL-related surgery occurred more frequently in patients treated with thermal modalities as compared to those that received mechanical treatment (16.1% vs. 0%; p = 0.005).
Propensity Score Adjusted Results
After propensity score adjustment for the full cohort, 30-day rebleeding and 30-day composite outcomes occurred more frequently in patients treated with VG as compared to those treated with DEP. The PS adjusted 30-day rebleeding was 25.3% in VG compared to 2.6% in DEP (p < 0.001). The PS adjusted 30 day composite outcome was 39.0% in VG versus 2.6% in DEP (p < 0.001).
Randomized Patients
Twenty-five patients were randomized to either VG or DEP as participants in two different recent RCTs. Nine patients were randomized to DEP and 16 to VG. Among the 9 DEP patients, 5 were treated with HC, 3 with OTSC, and 1 with thermal/HC combination therapy. These treatments resulted in obliteration of arterial blood flow in all RCT DEP patients. The VG group of 16 patients included 12 treated with HC, 1 with thermal, and 3 with thermal/HC combination therapy.
Among RCT participants, 30-day rebleeding occurred in 37.5% (6/16) and 11.1% (1/9) of patients treated with VG and DEP, respectively (NNT 3.8; p = 0.158) (Table 5; Fig. 2c). 50% (8/16) of VG versus 11.1% (1/9) of DEP-treated patients experienced a 30-day composite outcome (NNT 2.6; p = 0.063) (Fig. 2d).
Discussion
Dieulafoy’s lesion is a rare but increasingly recognized cause of SUGIH. At our tertiary academic medical centers more than 10% of patients in recent RCTs of severe non-variceal upper GI hemorrhage had DLs [2]. In our entire cohort of 82 DL patients with SUGIH, many (40.2%) were inpatients already hospitalized for a non-bleeding indication. They had significant comorbidities including ESRD, liver cirrhosis, and cardiovascular disease.
In our full cohort, rebleeding occurred at 30-days in 10.5% (2/19) of patients with a negative post-treatment DEP signal compared to 33.3% (21/63) of patients treated with VG. The NNT was 4.4 with this 22.8% reduction in rebleeding. When a composite outcome was assessed, 10.5% of DEP patients compared to 41.3% of VG patients had poor clinical outcomes (NNT 3.2). This difference was largely driven by a reduction in rebleeding rates. After propensity score adjustment, both rebleeding and composite outcomes were significantly more likely in patients treated with VG as compared to DEP. This reduction in rebleeding and composite outcomes may help to explain the shorter length of hospital stay and trend toward shorter length of ICU stay in patients treated with DEP as compared to VG.
We also observed arithmetic differences in rates of rebleeding and composite outcomes in our subset of RCT patients. In this cohort of 25 patients, rebleeding occurred in 37.5% and 11.1% of patients treated with VG and DEP, respectively (NNT 3.8). There was a 38.9% reduction in composite outcome with DEP compared to VG (NNT 2.6) which was largely driven by a reduction in rebleeding rates.
Our findings suggest that using DEP to guide treatment and subsequently confirm elimination of lesion blood flow reduces rebleeding and improves other clinically important outcomes. Although differences in the primary outcomes did not reach statistical significance in the RCT subgroup, we believe that this is a Type 2 error and predict that an increase in sample size would make this difference significant.
Prospective studies comparing treatment modalities for DL are limited. Two out of three studies comparing mechanical therapy (HC or EBL) to injection monotherapy reported a significant advantage for mechanical treatment in terms of rebleeding [7, 9, 13]. Two studies comparing band ligation to mechanical or thermal treatment found no difference in rates of recurrent bleeding [8, 19]. These two studies were included in a meta-analysis of 162 patients undergoing treatment with EBL versus HC in which a non-significant trend in favor of band ligation was identified (RR for rebleeding: 0.37; 95% CI 0.12-1.09) [20]. Thus, there is little consensus about a preferred approach to endoscopic treatment.
Because DLs have underlying large arteries that course below a mucosal defect, eliminating blood flow into and out of the DL is crucial for definitive hemostasis. A recent CURE RCT of severe non-variceal UGI hemorrhage reported significantly lower rates of 30-day rebleeding (26.3% vs. 11.1%, p = 0.02) among patients treated with endoscopic hemostasis assisted by DEP monitoring of blood flow under the stigmata as opposed to standard, visually guided hemostasis. Most of the patients in that study had bleeding from peptic ulcers, although DLs were also included. To our knowledge, the literature describing the use of DEP to assist in endoscopic treatment of DL is limited to this RCT and single case reports [21].
Although the use of DEP for treatment of DL has not been widely reported, standard endoscopic ultrasound (EUS) has been advocated to assist with the diagnosis and treatment of DL and serves to illustrate the importance of understanding the submucosal vascular anatomy of DLs [22,23,24,25,26,27]. DEP has several advantages over EUS. It can be used through the working channel of a standard endoscope and does not require changing to an echoendoscope. The Doppler probe can be easily used pre- and post-treatment to assess blood flow without having to change the endoscope. Perhaps most importantly, DEP relies only on a straightforward acoustic evaluation and does not require additional specialized training or interpretation of ultrasonographic images. It is easy to learn how to use and inexpensive, as detailed in our recent reports [2, 14].
This study is the largest prospectively studied cohort of DL in the published literature. The overall rebleeding rate of 28.0% was higher than other cohorts of patients with bleeding from DL. This may be related to the severe comorbidity of our patients and the fact that only lesions without surrounding mucosal ulcerations (and not small ulcers) were included as DLs. Lim et al. [28] noted rebleeding in 17.9% of patients with initially successful hemostasis after treatment with EBL, injection, or HC in 39 patients with non-variceal upper GI hemorrhage. Park and colleagues found rebleeding in 8.5% of 117 Korean patients treated with epinephrine injection, HC, EBL, APC or combination therapy [29]. A Japanese group reported that rebleeding occurred in just 1 of 61 patients treated with either injection or HC over a mean follow-up period of 47 months [30]. Notably, more than 30% of DL in our cohort were in the fundus, which is higher than in the above cited studies. This may help to explain our higher overall rebleeding rate, since lesions in the cardia and fundus may be more difficult to treat effectively and often require either retroflexion, tangential treatment, or other maneuvers such as patient repositioning to the right side down.
Our high DL rebleeding rate may also be attributable to treatment practices at our institution, in which EBL is not routinely used to treat DL. EBL may be especially helpful for treating lesions in the proximal stomach where a band can be applied tangentially more easily than HC. [5,6,7,8, 19, 30,31,32,33]. However, EBL is associated with complications including rebleeding due to post-banding ulceration or incomplete obliteration of the vessel, as well as gastric perforation in the thin-walled proximal stomach [5, 6, 8, 34]. DEP monitoring is especially useful for confirming complete vessel obliteration and may be a useful adjunct when treating DL with either band ligation or OTSC. However, because OTSC usually obliterates underlying blood flow in DLs and the large clips are not sloughed off early (as rubber bands are), OTSC should be safer than band ligation and as effective.
Two randomized trials have demonstrated that OTSC is superior to standard therapy at preventing further bleeding in patients with peptic ulcer hemorrhage [11, 12]. It has been used with success to treat DL in small published case reports [11, 35, 36]. Because the OTSC grasps a much larger volume of tissue than the traditional hemoclip, attaches deeper, and has the capability of sealing larger arteries and obliterating underlying arterial blood flow, it should be more effective than standard therapy, including either thermal coagulation and HC. Four patients in our DEP cohort were treated with OTSC and none experienced rebleeding at 30-days.
Our study has several strengths. First, it is a large cohort of prospectively collected data from a diverse population in both a tertiary academic medical center and a large, university-affiliated VA. Second, the same CURE GI Hemostasis group treated all the patients in this study, so there was no difference in expertise or approach to endoscopic hemostasis. Third, to our knowledge this is the first study to compare visually guided treatment for DL with DEP-guided treatment. Fourth, our treatment groups were well matched in demographic, endoscopic, and laboratory parameters. Fifth, as advocated by some experts in gastroenterology and similar to other medical specialties such as cardiology, we reported a composite outcome [16]. Sixth, we performed propensity score matching for several variables to account for possible differences between the VG and DEP patients. After this adjustment, we identified significant differences in rebleeding and composite outcomes between the two groups. Last, our report includes RCT results for DL patients who were randomized to DEP-assisted treatment or current standard of care visually guided endoscopic hemostasis.
Our study also has several limitations. First, it is a modest sized study. Second, we did not include EBL as a treatment modality. We also did not use APC, which has been described as an effective treatment [4]. However, APC without mucosal contact is superficial with ≤ 1 mm coagulation depth. With mucosal contact, it becomes monopolar coagulation with potentially deep coagulation (and the possibility of perforation). Third, while almost 1/3 of the patients in our study were randomized, approximately 2/3 were not assigned to treatments in a randomized fashion. The RCT patients were enrolled after 2009, which introduces the possibility of treatment selection bias in the earlier cohort study patients. However, especially in the composite outcome, RCT patients had similar results to the overall cohort. Moreover, we included time as a variable in our propensity score adjusted analysis. Fourth, there was a difference in the endoscopic therapies that were used in the DEP and VG groups. However, the primary outcomes between mechanical and thermal treatment were not statistically different. Finally, our RCT cohort was inadequately powered to demonstrate differences in rebleeding rates between the VG and DEP groups. A larger multicenter RCT of DL hemorrhage would be needed to assess these differences. While that is the next logical recommendation, this may be difficult and expensive due to the rarity of DLs and the difficulty of standardizing the endoscopic classification (vs. small ulcers with a visible vessel).
Our conclusions are: 1. DL patients with SUGIH were frequently inpatients and had severe comorbidities and recurrent bleeding. 2. DEP monitored treatments are promising for obliteration of residual arterial blood flow and for definitive endoscopic hemostasis in patients with SUGIH from DL. 3. 30-day composite outcomes and 30-day rebleeding occurred significantly less often in patients treated with DEP compared to VG after propensity score adjustment.
Abbreviations
- APC:
-
Argon plasma coagulation
- ASA:
-
American Society of Anesthesiology
- DEP:
-
Doppler endoscopic probe
- DL:
-
Dieulafoy’s lesion
- EBL:
-
Endoscopic band ligation
- HC:
-
Standard (through-the-scope) hemoclip
- HP:
-
Heater probe
- MPEC:
-
Multipolar electrocautery
- OTSC:
-
Over-the-scope-clip
- PS:
-
Propensity Score
- SUGIH:
-
Severe upper gastrointestinal hemorrhage
- RBC:
-
Red blood cells
- RCT:
-
Randomized controlled trial
- UGI:
-
Upper gastrointestinal (tract)
- VG:
-
Visually guided endoscopic treatment
References
Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade: a nationwide analysis. Dig Dis Sci.. 2018;63:1286–1293. https://doi.org/10.1007/s10620-017-4882-6.
Jensen DM, Kovacs TOG, Ohning GV, et al. Doppler endoscopic probe monitoring of blood flow improves risk stratification and outcomes of patients with severe nonvariceal upper gastrointestinal hemorrhage. Gastroenterology.. 2017;152:1310–1318.
Alshumrani G, Almuaikeel M. Angiographic findings and endovascular embolization in Dieulafoy disease: a case report and literature review. Diagn Interv Radiol.. 2006;12:151–154.
Iacopini F, Petruzziello L, Marchese M, et al. Hemostasis of Dieulafoy’s lesions by argon plasma coagulation. Gastrointest Endosc.. 2007;66:20–26.
Ahn D-W, Lee SH, Park YS, et al. Hemostatic efficacy and clinical outcome of endoscopic treatment of Dieulafoy’s lesions: comparison of endoscopic hemoclip placement and endoscopic band ligation. Gastrointest Endosc.. 2012;75:32–38.
Ji J-S, Kim H-K, Kim SS, et al. Clinical outcome of endoscopic management of duodenal Dieulafoy’s lesions: endoscopic band ligation versus endoscopic hemoclip placement. Surg Endosc.. 2016;30:3526–3531.
Alis H, Oner OZ, Kalayci MU, et al. Is endoscopic band ligation superior to injection therapy for Dieulafoy lesion? Surg Endosc.. 2009;23:1465–1469.
Park CH, Joo YE, Kim HS, et al. A prospective, randomized trial of endoscopic band ligation versus endoscopic hemoclip placement for bleeding gastric Dieulafoy’s lesions. Endoscopy.. 2004;36:677–681.
Chung IK, Kim EJ, Lee MS, et al. Bleeding Dieulafoy’s lesions and the choice of endoscopic method: comparing the hemostatic efficacy of mechanical and injection methods. Gastrointest Endosc.. 2000;52:721–724.
Schmidt A, Gölder S, Goetz M, et al. Over-the-scope clips are more effective than standard endoscopic therapy for patients with recurrent bleeding of peptic ulcers. Gastroenterology.. 2018;155:674–686.
Skinner M, Gutierrez JP, Neumann H, et al. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open.. 2014;2:E37–E40.
Jensen DM, Kovacs TOG, Ghassemi K. Randomized controlled trial (RCT) of over-the-scope clip (OTSC) as initial endoscopic treatment of severe non-variceal upper gastrointestinal bleeding (NVUGIB). Clin Gastroenterol Hepatol. 2020. In press.
Park CH, Sohn YH, Lee WS, et al. The usefulness of endoscopic hemoclipping for bleeding Dieulafoy lesions. Endoscopy.. 2003;35:388–392.
Jensen DM, Ohning GV, Kovacs TOG, et al. Doppler endoscopic probe as a guide to risk stratification and definitive hemostasis of peptic ulcer bleeding. Gastrointest Endosc.. 2016;83:129–136.
Jensen DM, Kovacs TOG, Jutabha R, et al. Randomized, controlled trial of medical therapy compared to endoscopic therapy for prevention of recurrent ulcer hemorrhage in patients with non-bleeding adherent clots. Gastroenterology.. 2002;123:407–413.
Laine L, Spiegel B, Rostom A, et al. Methodology for randomized trials of patients with nonvariceal upper gastrointestinal bleeding: recommendations from an international consensus conference. Am J Gastroenterol.. 2010;105:540–550.
Li F, Thomas LE. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol.. 2018;188:250–257.
Rao JNK, Scott AJ. On simple adjustments to Chi square tests with sample survey data. Ann. Stat.. 1987;15:385–397.
Matsui S, Kamisako T, Kudo M, Inoue R. Endoscopic band ligation for control of nonvariceal upper GI hemorrhage: comparison with bipolar electrocoagulation. Gastrointest Endosc.. 2002;55:214–218.
Barakat M, Hamed A, Shady A, et al. Endoscopic band ligation versus endoscopic hemoclip placement for Dieulafoy’s lesion: a meta-analysis. Eur J Gastroenterol Hepatol.. 2018;30:995–996.
Satyavada S, Davitkov P, Akbar Ali M, et al. Endoscopic doppler probe in the diagnosis and management of upper gastrointestinal hemorrhage. ACG Case Rep J.. 2018;5:e68.
Pohle T, Helleberg M, Menzel J, et al. An extraordinary Dieulafoy’s lesion presenting as varices of the gastric fundus. Gastrointest Endosc.. 2001;54:776–779.
Fockens P, Meenan J, van Dullemen HM, et al. Dieulafoy’s disease: endosonographic detection and endosonography-guided treatment. Gastrointest Endosc.. 1996;44:437–442.
Nesje LB, Skarstein A, Matre K, et al. Dieulafoy’s vascular malformation: role of endoscopic ultrasonography in therapeutic decision-making. Scand J Gastroenterol.. 1998;33:104–108.
Jaspersen D. Dieulafoy’s disease controlled by Doppler ultrasound endoscopic treatment. Gut.. 1993;34:857–858.
Jaspersen D, Gaster CB, Koerner T, Hammar CH. Doppler-controlled injection treatment of Dieulafoy’s disease. J Gastroenterol Hepatol.. 1993;8:267–269.
Law R, Fujii-Lau L, Song LMWK, et al. Efficacy of endoscopic ultrasound-guided hemostatic interventions for resistant nonvariceal bleeding. Clin Gastroenterol Hepatol.. 2015;13:808–812.
Lim W, Kim TO, Park SB, et al. Endoscopic treatment of dieulafoy lesions and risk factors for rebleeding. Korean J Intern Med.. 2009;24(4):318–322.
Park S-H, Lee D-H, Park C-H, et al. Predictors of rebleeding in upper gastrointestinal dieulafoy lesions. Clin Endosc.. 2015;48:385–391.
Sone Y, Kumada T, Toyoda H, et al. Endoscopic management and follow up of Dieulafoy lesion in the upper gastrointestinal tract. Endoscopy.. 2005;37:449–453.
Nikolaidis N, Zezos P, Giouleme O, et al. Endoscopic band ligation of Dieulafoy-like lesions in the upper gastrointestinal tract. Endoscopy.. 2001;33:754–760.
Mumtaz R, Shaukat M, Ramirez FC. Outcomes of endoscopic treatment of gastroduodenal Dieulafoy’s lesion with rubber band ligation and thermal/injection therapy. J Clin Gastroenterol.. 2003;36:310–314.
Valera JM, Pino RQ, Poniachik J, et al. Endoscopic band ligation of bleeding dieulafoy lesions: the best therapeutic strategy. Endoscopy.. 2006;38:193–194.
Chen Y-Y, Su W-W, Soon M-S, Yen H-H. Delayed fatal hemorrhage after endoscopic band ligation for gastric Dieulafoy’s lesion. Gastrointest Endosc.. 2005;62:630–632.
Manta R, Mangiafico S, Zullo A, et al. First-line endoscopic treatment with over-the-scope clips in patients with either upper or lower gastrointestinal bleeding: a multicenter study. Endosc Int Open.. 2018;6:E1317–E1321.
Richter-Schrag H-J, Glatz T, Walker C, et al. First-line endoscopic treatment with over-the-scope clips significantly improves the primary failure and rebleeding rates in high-risk gastrointestinal bleeding: a single-center experience with 100 cases. World J Gastroenterol.. 2016;22:9162–9171.
Funding
This research was funded by the NIH CURE: Digestive Diseases Research Center – Human Studies Core (NIH NIDDK P30 DK41301), VA Clinical Merit Review Grant (CLIN-013-07F) and an American Society for Gastrointestinal Endoscopy Research Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the studies comprising this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nulsen, B., Jensen, D.M., Kovacs, T.O.G. et al. Outcomes in Severe Upper GI Hemorrhage from Dieulafoy’s Lesion with Monitoring of Arterial Blood Flow. Dig Dis Sci 66, 3495–3504 (2021). https://doi.org/10.1007/s10620-020-06679-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06679-4