Abstract
Mast cell activation syndrome is thought to be a common, yet under-recognized, chronic multi-system disorder caused by inappropriate mast cell activation. Gastrointestinal symptoms are frequently reported by these patients and are often mistaken by physicians as functional gastrointestinal disorders. This syndrome can be diagnosed by the medical history and measurable biomarkers. Gastroenterologists manage diseases associated with active inflammatory cells including neutrophils, lymphocytes, macrophages, and eosinophils. The mast cell has only recently been recognized as a major player in our specialty. Gastrointestinal disorders from mast cell mediators often present with apparent irritable bowel syndrome, dyspepsia, chronic or cyclical nausea, and heartburn. Individuals with mast cell activation syndrome experience significant delays in diagnosis. The gastrointestinal symptoms are often refractory to symptom-targeted prescription medications. Beyond avoiding triggers, the best therapy is directed at modulating mast cell activation and the effects of the mediators. Many of these therapies are simple over-the-counter medications. In this article, we review mast cell function and dysfunction and the gastrointestinal symptoms, comorbid conditions, diagnosis, and management of mast cell activation syndrome. Gastroenterologists who become aware of this syndrome can dramatically improve the quality of life for their patients who previously have been labeled with a functional gastrointestinal disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mast cell activation syndrome (MCAS) is a chronic multi-system disease of abnormal mast cell (MC) activation leading to inflammatory and allergic symptoms [1,2,3]. Gastrointestinal (GI) manifestations are common, with nausea, heartburn, abdominal pain, and altered bowel habits being the most frequently reported (Table 1) [2,3,4]. Unrecognized and untreated MCAS may account for refractory GI symptoms which may be attributed to functional GI disorders including irritable bowel syndrome (IBS) [5, 6]. Presently, no one has studied a large group of patients with IBS to determine prevalence of MCAS and/or MC activation as the primary etiology. In a study of 20 refractory IBS patients, 19 had MC symptoms and 11 of 12 studied for MC mediators had positive results [5]. In a study in Germans, the prevalence of MCAS was estimated to be 17% of the population [7]. In these patients, 74% also reported similar symptoms in one or more first-degree relatives. Indirect prevalence estimates for MCAS in Americans are 1%.
Symptoms of MCAS can be numerous and involve multiple organs and systems, which further complicates the clinical presentation (Table 2) [2, 6]. To entertain the diagnosis of MCAS in the “difficult GI patient,” one needs to be prepared to consider their entire symptomatology as “real” as opposed to a case of IBS with somatization syndrome [8]. Many of the MCAS patients will have symptoms checked off in virtually every section of the review of systems—some will seem to be inexplicable or even bizarre to the practitioner new to MCAS. Conversely, it is known that IBS is associated with other syndromes and symptoms and these also occur in MCAS patients [9, 10]. MCAS is often associated with hypermobile Ehlers–Danlos syndrome (hEDS) and postural orthostatic tachycardia syndrome (POTS), both of which also have extensive GI system involvement [11, 12]. MCAS, both alone and in association with these other disorders, results in significant GI morbidity [13,14,15,16,17].
MCAS patients pose a considerable management challenge due to their pathophysiological heterogeneity, numerous systemic symptoms and triggers, comorbid conditions, and varied responses to therapy. Triggers for MC activation include stress, food, alcohol, excipients in medications, infections, altered microbiome, and environmental stimuli including heat, chemical, and mold exposure [2, 3, 6, 18,19,20,21]. A multidisciplinary approach is optimal for diagnosis and management. The primary purpose of this review is to increase awareness of MCAS in the hopes of increasing diagnostic rates, decreasing time to diagnosis, and enhancing clinical care [22].
Mast Cell Activation Disease
There is some controversy surrounding MCAS due to confusing terminology of MC disease, evolving clinical diagnostic criteria, misperception that an increased serum tryptase level is prevalent, and that anaphylaxis and an increase of tryptase from baseline during an attack is required to make the diagnosis of MCAS [23, 24].
Mast cell activation disease (MCAD) can be classified simply into two main categories, systemic mastocytosis (SM) and MCAS, both of which can have systemic manifestations of aberrant MC activation. A more extensive and comprehensive classification scheme has been proposed [25]. SM and its subclass mast cell leukemia [26] are two rare diseases that are associated, respectively, with the risk of malignancy and malignant disease per se. The risk of malignant MC transformation in MCAS is not yet known, although MCAS itself has recently been reported to have an increased risk of melanoma and cancers of the thyroid, breast, cervix, and ovary [27].
We review normal and abnormal MC function and the GI involvement, comorbid conditions, and algorithms for diagnosis and management of MCAS.
Mast Cell Function and Pathophysiology
Mast cells (MCs) are multifunctional immune cells which play crucial roles in innate and adaptive immunity [28, 29]. MCs participate in host defense, tissue repair, wound healing, and angiogenesis [30]. MCs react to allergens, tissue trauma, and infection and quickly respond by releasing biologically active mediators from both intracellular stores and by delayed de novo synthesis [28, 31]. Over 200 MC mediators have been identified, including biogenic amines (e.g., histamine), proteases (e.g., tryptase and chymase), cytokines (e.g., interleukins and TNF-α), eicosanoids (e.g., prostaglandins and leukotrienes), heparin, and growth factors [28,29,30,31]. The specific profile of MC mediators expressed by a particular MC varies according to the MC subtype and surrounding microenvironment. MCs are known to originate from myeloid progenitors in bone marrow and adipose tissue and then mature in peripheral tissues [32, 33].
MCs distribute into all vascularized tissues, preferentially residing at environmental interfaces, such as mucus membranes and mucosal layers of the GI, respiratory, and urinary tracts [29, 31]. Via their released mediators, MCs also help orchestrate growth and development in all tissues. MCs located within the GI mucosa act as an important interface between the human host and the environment (i.e., the microbiome and food antigens) [31].
Mast Cell Pathology
The pathologic behavior of MCs in MCAS is due to constitutive and reactive abnormal activation and release of mediators, leading to harmful local and distant effects [34, 35]. This may occur due to mutation of the MC regulatory genes [36, 37]. Through interactions with specific receptors on other cells, both locally and distantly from the source MC, mediators have broad effects and can cause tachycardia, urticaria, and many other symptoms [2, 6]. MCAS, in its myriad of clinical presentations, features inappropriate MC activation with relatively modest MC proliferation (in contrast to mastocytosis). In MCAS, it is not unusual to see up to > 50 MC per high-power field as in Fig. 1 yet the histologically normal ovoid MCs are scattered as opposed to the presence of sheets and clusters of spindle-shaped MCs in mastocytosis. Furthermore, the bone marrow is not involved in MCAS in contrast to SM. In both MCAS and mastocytosis, the circulating MC count is normal (i.e., undetectable by routine blood cell counting) as opposed to the rare cases of MCL [26]. In SM, MCs are increased in density in tissues focally with abnormal morphology (spindle-shaped), often occupy > 35% of the bone marrow, and always have a genetic variance which usually features the somatic mutation KITD816X and/or express CD25 (or CD2) antigens [38]. In SM, the tryptase level is usually elevated (> 20 ng/ml). Patients with SM have symptoms principally due to aberrant release of MC mediators, though in more advanced cases symptoms can come about from the mechanical and metabolic effects of neoplastic proliferation, just as with any cancer.
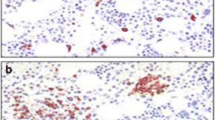
Duodenal biopsy from a patient with MCAS. a The hematoxylin and eosin stain is normal without evidence of inflammatory cells. b Immunohistochemical stain demonstrates increased CD-117-positive mast cells with > 50 mast cells per high-power field. c In comparison, a duodenal biopsy from a Lynch syndrome patient without MC activation symptoms stained with CD-117 demonstrated 10 MCs per high-power field. All three images are shown at 10x/0.65 objective magnification
Mast Cells in the Gastrointestinal Tract
Within the literature, there exists debate regarding normal MC counts from GI mucosal samples at different sites within the GI tract. The Jakate study identified a mean of 13 MCs/HPF in "healthy tissue" with a standard deviation of 3.5, such that per this study 20 MCs/HPF (i.e., two standard deviations above the mean) is the threshold distinguishing normal from abnormal mast cell counts [39]. Other studies question the validity of the Jakate study but are flawed owing to a small number of MCAS patients and uncertainty whether they used truly healthy controls [40]. We utilize CD-117 immunohistochemical staining to detect MCs within the GI tract mucosa obtained via endoscopic biopsy (Fig. 1). Our general protocol is to obtain eight specimens from the second part of the duodenum. Biopsies are placed in formalin for staining. Tissue from old cell blocks or unstained slides from the past can be utilized. At our institutions, the pathologists report the average MC count for 10 high-power field (HPF) per region sampled. In most MCAS patients, we commonly detect ≥ 20 MCs per HPF from the duodenum and ileum. The stomach and colon have less MCs, and the esophagus has the least. Stains targeting MC mediators or granules, such as tryptase or Giemsa, may not be as reliable and sensitive as CD117 since they may only detect MCs with secretory granules [41].
Gastrointestinal Involvement in MCAS
Symptoms can include tingling or burning, aphthous ulcers, globus, heartburn, dysphagia, chest pain, nausea, altered bowels, bloating, and abdominal pain [1,2,3,4, 42]. Dyspepsia may be due to mediator-induced nociception [43].
Gastritis in the absence of Helicobacter pylori and/or nonsteroidal anti-inflammatory medications could be explained by inflammation caused by MC mediators [29]. Chronic and acute peritoneal pain has been reported in the setting of epiploic appendagitis where local increased MC deposition was identified [44].
In studies of IBS, MC density is increased and the tissue concentration of tryptase and histamine is correlated with pain [45, 46]. Studies that demonstrate success with MC-directed therapy in IBS are also suggestive for a pathophysiological role in visceral hypersensitivity [5, 47,48,49]. Histamine release by aberrant MCs or by normal MCs that are activated by mediators from the unregulated MC could also explain visceral hypersensitivity in IBS through potentiation of transient receptor potential signaling in the submucosal neurons [50].
Small intestinal bacterial overgrowth (SIBO) was shown to be common in MCAS in a recent study [51]. Bacterial overgrowth was present in 30.9% of 139 MCAS subjects vs. 10.0% of 30 controls. It is possible that SIBO may be caused by altered motility from local MC mediators in paraneuronal tissue or damage to glial cells affecting the migrating motor complex or by abnormal immunity. SIBO could explain diarrhea and bloating in some MCAS patients. The effect of mediators on the GI tract likely explains the remainder.
Constipation is a common problem for MCAS patients. When MCs are located within the muscular layers of the GI tract, they can contribute to the development of GI dysmotility [52]. In a histopathology study of colons removed for severe constipation compared to controls who had resections for a different reason, investigators demonstrated that those with severe constipation had significantly higher number of MCs and there were degranulating MCs close to enteric glial cells and filaments in patients [52].
Changes in the microbiome and intestinal permeability lead to the accumulation and activity of MCs and lymphocytes within the GI tract [20]. Dysbiosis and small intestinal bacterial overgrowth act as stimuli to the MCs which release mediators that activate lymphocytes. In turn, the T-lymphocytes secrete microparticles which further activate MCs [53]. The activated MCs and T-cells secrete cytokines which increase intestinal permeability. This furthers the vicious cycle whereby bacterial and lipopolysaccharide translocation causes inflammation and increased intestinal permeability. The clinical impact is that MC degranulation is increased and the overall inflammatory state is increased. When the aberrant MCs of MCAS are present, this magnifies this condition.
Finally, hepatic involvement by MCAS may be present: 44% of MCAS patients had mildly increased liver chemistries, especially during a symptom flare in a study of 56 patients [54].
Comorbidities in MCAS
Postural orthostatic tachycardia syndrome (POTS) is a common disorder of the autonomic nervous system (ANS), affecting 1–3 million Americans [12]. POTS is diagnosed by detecting an abnormal increase in heart rate upon assuming the upright position without a concomitant decrease in blood pressure. POTS can affect individuals with nausea, heartburn, abdominal pain, bloating, constipation, and diarrhea [12, 16]. Studies have also shown evidence of SIBO which may explain some of the GI symptoms [55, 56].
Connective tissue MCs within the GI tract interact with other immune cells and fibroblasts. They are also in close proximity to nerve fibers from the ANS and small blood vessels [15]. When the MCs are activated, their mediators can interact locally with components of the ANS and blood vessels, resulting quickly in a systemic response. The ANS controls vascular permeability, and during periods of ANS dysfunction, there are increased vascular permeability, tissue edema, and translocation of immune cells into the tissues [17, 57].
Ehlers–Danlos syndrome (EDS) is a group of heterogeneous connective tissue disorders [58]. Individuals with EDS, particularly the hypermobile form of EDS (hEDS), have high rates of comorbid MCAS and POTS. Hypermobile EDS is a common disorder characterized by hypermobile joints. GI symptoms are commonly reported in patients with hEDS [11]. Small intestinal bacterial overgrowth due to small bowel stasis may be associated with hEDS due to enteroptosis, defective collagen synthesis, α-actin deficiency, and/or autonomic dysfunction [59, 60]. In studies involving secondary and tertiary care patients, 50% of patients with a diagnosis of functional dyspepsia were found to have hEDS and 40% of patients with IBS were found to have hEDS [61, 62].
The number of studies examining the prevalence of POTS and/or EDS in MCAS is limited. In a recent prospective study, 139 MCAS patients with refractory GI symptoms had comorbid POTS in 25.2% and EDS in 23.7%. Both syndromes were present in 15.1% (51%) [51]. In a retrospective chart review, patients with hypermobile EDS and/or POTS were evaluated for symptoms suggestive of MC activation [63]. Thirty-one patients were diagnosed with POTS and 38 patients with hEDS; of them, 23 patients had both POTS and hEDS. In 100% of all groups, all patients had both cutaneous and gastrointestinal involvement. Next in frequency were naso-ocular symptoms in 45–61%, cardiovascular 52–58%, respiratory 43–47%, and central nervous system 3–5%. Over 95% of patients in all groups reported a significant response to histamine-1 receptor blockers, over 89% to histamine-2 receptor blockers, and 80% to MC stabilizers. Mediator testing was not measured, and thus, an actual percent of MCAS patients could not be stated.
In our clinical experience, individuals with comorbid MCAS, POTS, and hEDS remain some of the most difficult-to-manage patients. This may be due to the complex interactions among the three disorders. For example, with release of chymases from MCs, excess extracellular matrix remodeling can occur, which may increase tissue laxity in individuals with preexisting tissue laxity from their underlying EDS. This could explain connective tissue disturbances within the mesentery which lead to enteroptosis as demonstrated by a standing small-bowel follow-through image [59]. Dysfunction of the ANS can alter both GI permeability and vascular permeability, which can independently lead to aberrant MC activation. Furthermore, MC mediator release in close proximity to nerves of the ANS could lead to worsening of POTS [15, 17]. These interactions can lead to a vicious cycle which is difficult to break without aggressive intervention which addresses all comorbid conditions simultaneously.
Diagnosis
After excluding of mimickers or alternative explanations for the symptoms, criteria for diagnosis of MCAS include the major criteria of characteristic MC activation symptoms in two or more systems (Table 2) plus one or more minor criteria [27]. Minor criteria include (1) elevation in the blood and/or urine of mediators relatively specific to the MC, (2) clinical improvement using MC-directed medical therapy, and (3) ≥ 20 MCs per HPF in extracutaneous tissue (luminal GI tract or bladder biopsies). Two prior minor criteria are no longer advised: (1) The KITD816V mutation almost always found in SM is usually not seen in MCAS and tests for other MC-activating mutations are not yet available in commercial laboratories; and (2) MC counts with > 25% spindle-shaped cells and CD25 expression are generally specific to SM, not MCAS. In one of the two published criteria for MCAS [23], anaphylaxis is required as part of the clinical criteria yet most of our patients do not have this problem. The original criteria by Molderings et al. [64] allow for the diagnosis of a larger group of patients who would not be given a diagnosis by Valent et al. and hence not be treated for this mast cell activation disease [24].
Use of the validated Mast Cell Mediator Release Syndrome (MCMRS) questionnaire can help lead to diagnosis of a MC activation disorder (Appendix 1) [7]. This document takes into account characteristic MC symptoms, pertinent medical history, laboratory assessment, radiographic changes, and biopsy results, and it provides a differential diagnosis for other mimicking diseases. The questionnaire brings out the most common symptoms of MC activation which can occur in a variety of MC diseases and is not specific for one versus another. For instance, splenomegaly may be found in SM but not MCAS.
Mast Cell Mediator Measurements
Of the > 200 MC mediators, only a small number of mediators are measurable in clinical laboratories at present, and even fewer are relatively specific to MCs. A reasonable diagnostic MC mediator panel includes: (1) plasma prostaglandin D2 and histamine, (2) serum tryptase and chromogranin A, and (3) 24-h and/or random urine N-methylhistamine, leukotriene E4, and 2,3-dinor-11-ß-prostaglandin-F2-α.
Tryptase is the most well-recognized MC mediator owing to elevated blood levels (> 20 ng/ml) in almost all SM patients. It, however, is elevated in only 15% of MCAS patients [2, 64]. This may be reflective of the presence of the KITD816X mutation in mast cells in SM versus MCAS. High tryptase levels along with MC activation symptoms are also seen in a recently described condition called hereditary alpha tryptasemia where individuals have extra copies of the TPSAB1 gene which encodes for the alpha form of tryptase [65]. Mild elevations in tryptase can be helpful in diagnosing MCAS [64]; however, a normal tryptase level does not exclude MCAS [2, 64]. Although it has been proposed that an increase in tryptase from baseline within 1 to 4 h of having a reaction is important in identifying abnormal MC activation [23], limited data to substantiate the frequency of this laboratory change have been published [66].
Since tryptase is a poor biomarker for MCAS, one needs to consider other specific mediators of MC activation: leukotriene E4 (urine) and heparin (plasma). The problem with measurement of heparin in the USA is that commercial laboratories do not have sensitive assays for endogenous heparin [67]. Prostaglandin D2 (plasma) appears to be a sensitive and relatively specific mediator in MCAS [2]. Chromogranin A is another commonly elevated mediator and is easily measured in serum [2]. However, increased chromogranin A levels can be seen in heart, kidney, or liver failure, active or recent proton pump inhibitor use, chronic atrophic gastritis, and the rare neuroendocrine malignancies. Mediators can all be tested at baseline, i.e., in the absence of an acute MC reaction. If they are found to be normal at baseline, and the clinical suspicion for a MCAS remains high, repeat testing within 1—6 h of an acute MC activation attack is helpful. Failure to discover increased levels of MC-specific mediators does not argue against the potential presence of MCAS due to the disease’s heterogeneity vis-à-vis the fact that only a few of the MC’s many mediators are tested at present. Thus, while elevated mediator levels help substantiate a diagnosis of MCAS, negative results do not necessarily exclude this diagnosis (Table 3).
Plasma and urine MC mediators are unstable at room temperature or higher, thus requiring stringent specimen collection and handling protocols (i.e., chilled centrifugation and frozen storage and transportation). Urine collection is generally performed for 24 h, and the collection container(s) should be kept either on ice or in a refrigerator, then transported on ice to the laboratory, and frozen for transportation to the testing laboratory.
Management
Treatment of MCAS invariably involves trigger identification and avoidance along with control of MC mediator production and action (Table 4) [3, 68, 69]. Patients are often able to offer clues to many of their triggers, which can include particular or combination of foods, temperature, medications, and other physiologic and emotional stressors. Data regarding specific dietary interventions in the treatment of MCAS are lacking, and the most effective dietary intervention remains the identification and avoidance of triggers. Histamine, gluten, and dairy-protein-free diets have been recommended on the basis of clinical experience. Elimination diets play an important role in MCAS therapy [69]. In our experience and in the MCMRS (Appendix 1), high-histamine foods can activate MCs in the gut causing direct and systemic symptoms. The aberrant MCs and normal MCs not only release histamine, but the MCs have receptors for histamine which then activate other MCs and other cells in the body. High-histamine foods can release histamine which binds to mucosal MC receptors on both aberrant and normal MCs. Gluten (and dairy products) are listed as high-FODMAP foods. Investigations of the effect of a low-FODMAP diet in IBS-d patients have shown reduction of plasma histamine levels [70]. Furthermore, high-FODMAP diet in mice results in increased visceral hypersensitivity and increased MC density in the colon [71].
Pharmacologic therapy is offered in stepwise fashion, often trying one medication at a time to look for benefit and risk since reactions to excipients (e.g., fillers, dyes, preservatives) are seen in MCAS patients (Table 4) [19]. First-line therapy includes a non-sedating H1 histamine receptor antagonist once to twice daily and a H2 histamine receptor antagonist once to twice daily [3]. Histamine receptor antagonists block receptors not only on MCs but on many other types of effector cells throughout the body which are responsible for symptoms. Finding the best combination of antihistamines for each patient is important since histamine release by MCs causes not only allergic symptoms but also pain and visceral hypersensitivity [43]. Sustained release vitamin C 500 mg daily, vitamin D daily, and quercetin 500 mg to 1000 mg once to twice daily are other over-the-counter interventions sometimes found helpful in MCAS [69]. Vitamin C stabilizes MCs by reducing histamine formation and chemical degradation of released histamine [72]. Quercetin is a plant-based flavonoid that inhibits cyclooxygenase and lipoxygenase activity, thereby reducing production of inflammatory mediators such as prostaglandins [73]. Supplementation with vitamin D is dose adjusted according to the serum level and may play a role owing to downregulation of MC receptors [74]. Second-line pharmacologic therapy can include montelukast, a leukotriene receptor antagonist (in our experience, twice daily dosing usually helps MCAS patients more than once daily), and/or oral cromolyn sodium, a MC stabilizer. Cromolyn sodium can be introduced earlier into the regimen in patients with severe GI symptoms. Oral cromolyn is started at 100 mg per dose (or even more cautiously when GI symptoms are severe) with a goal of reaching, in stepwise fashion, a dose of up to 200 mg four times per day (30 min before meals and at bedtime). Tachyphylactic worsening of symptoms sometimes occurs in the first few days of cromolyn use, but ongoing exacerbation may signal reactivity to a contaminating excipient (such as microparticulate plastic residue from the drug’s vial), calling for trials of different formulations. Third-line pharmacologic therapy can include ketotifen, a second-generation H1 antagonist with anti-inflammatory effects (not currently approved by the U.S. Food and Drug Administration for oral use). This is available through compounding pharmacies at doses of 1 mg to 4 mg once to twice daily. Fourth-line pharmacologic therapy can include omalizumab, a humanized murine IgG monoclonal antibody which inhibits IgE binding to high-affinity IgE receptors that are present on MCs and basophils [75]. Omalizumab should be administered by allergists and others familiar with MCAS and in the absence of concomitant steroids.
Prognosis
MCAS is caused by probably epigenetically induced somatic genetic mutations which cannot be cured per se. The course is at best constant, but frequently the intensity of symptoms is progressive. MCAS is thus generally regarded as incurable because of its likely genetic causes, yet it is treatable and the majority of patients ultimately achieve symptomatic improvement [2]. Furthermore, treatment of common comorbid conditions is essential for overall improvement, and working with other specialists in allergy, cardiology, neurology, and physical therapy is important. In many of these patients, their pharmacologic regimen may be decreased and simplified over time. However, even in patients who achieve significant symptomatic improvement, acute decompensating events can be experienced. During these events, they will require increased doses of their MCAS medications or a more aggressive treatment regimen (i.e., higher-dose H2 receptor blockers, budesonide, prednisone, and/or additional MC-directed medications). In an emergency involving a patient with MCAS, both patients and their family members should be educated to inform treating providers of the diagnosis of MCAS using available articles and information posted on www.TMSforacure.org. Use of sedatives and anesthesia for endoscopy or surgery can trigger MC activation in MCAS patients. This may be prevented by using preoperative intravenous diphenhydramine, famotidine, midazolam, and, in very reactive patients, solumedrol.
Future Directions
Studies are needed to assess the prevalence and burden of MCAS in general GI and GI motility practices. More research is indicated to see how MCAS fits into the differential diagnosis, testing, and therapy of apparently functional GI disorders and GI motility disorders. IBS and MCAS patients often have chronic syndromes with poor quality of life (e.g., restless legs syndrome, chronic fatigue syndrome, fibromyalgia syndrome, and chronic pelvic pain syndromes) [76,77,78,79,80]. Explorations into possible inflammatory and immune links could lead to new understandings of the pathophysiology of each disorder and to common, previously unimagined, therapeutic approaches. Treatment with probiotics that reduce histamine output or new medicines that inhibit MCs are other avenues to explore in the therapy of MCAS.
Conclusion
The recent recognition of MCAS provides new answers and treatment approaches for the gastroenterologist who is managing patients with “idiopathic” GI disorders which range in severity from bothersome to completely disabling.
References
Hamilton MJ, Hornick JL, Akin C, et al. Mast cell activation syndrome: a newly recognized disorder with systemic clinical manifestations. J Allergy Clin Immunol. 2011;128:147-152
Afrin LB, Self S, Menk J, et al. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353:207-215.
Hamilton MJ. Nonclonal mast cell activation syndrome: a growing body of evidence. Immunol Allergy Clin North Am. 2018;38:469-481.
Hsieh FH. Gastrointestinal involvement in mast cell activation disorders. Immunol Allergy Clin North Am. 2018;38:429-441.
Frieling T, Meis K, Kolck UW, et al. Evidence for mast cell activation in patients with therapy-resistant irritable bowel syndrome. Z Gastroenterol. 2011;49:191-194.
Afrin LB, Butterfield JH, Raithel M, et al. Often seen, rarely recognized: mast cell activation disease--a guide to diagnosis and therapeutic options. Ann Med. 2016;48:190-201.
Molderings GJ, Haenisch B, Bogdanow M, Fimmers R, Nöthen MM. Familial Occurrence of Systemic Mast Cell Activation Disease. PLoS One. 2013;8:e76241. doi: 10.1371/journal.pone.0076241.
Riedl A, Schmidtmann M, Stengel A, Goebel M, Wisser AS, Klapp BF, Mönnikes H. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. 2008;64:573-582.
Shen TC, Lin CL, Wei CC, et al. Bidirectional Association between Asthma and Irritable Bowel Syndrome: Two Population-Based Retrospective Cohort Studies. PLoS One. 2016;11:e0153911. doi: 10.1371/journal.pone.0153911.eCollection 2016.
Cole JA, Rothman KJ, Cabral HJ, Zhang Y, Farraye FA. Migraine, fibromyalgia, and depression among people with IBS: a prevalence study. BMC Gastroenterol. 2006;6:26. DOI: 10.1186/1471-230X-6-26
Beckers AB, Keszthelyi D, Fikree A, et al. Gastrointestinal disorders in joint hypermobility syndrome/Ehlers‐Danlos syndrome hypermobility type: A review for the gastroenterologist. Neurogastroenterol Motil. 2017;29:e13013. doi: 10.1111/nmo.13013.
DiBaise JK, Harris LA, Goodman B. Postural tachycardia syndrome (POTS) and the GI tract: a primer for the gastroenterologist. Am J Gastroenterol. 2018;113:1458-1467.
Seneviratne SL, Maitland A, Afrin L. Mast cell disorders in Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2017;175:226-236.
Wallman D, Weinberg J, Hohler AD. Ehlers-Danlos syndrome and postural tachycardia syndrome: a relationship study. J Neurolog Sci. 2014;340:99-102.
Shibao C, Arzubiaga C, Roberts LJ II, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45:385-390.
Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep. 2015;15:60. doi: 10.1007/s11910-015-0583-8.
Doherty TA, White AA. Postural orthostatic tachycardia syndrome and the potential role of mast cell activation. Auton Neurosci. 2018;215:83-88.
Jennings S, Russell N, Jennings B, et al. The Mastocytosis Society survey on mast cell disorders: patient experiences and perceptions. J Allergy Clin Immunol Pract. 2014;2:70-76.
Schofield JR, Afrin LB. Recognition and Management of Medication Excipient Reactivity in Patients With Mast Cell Activation Syndrome. Am J Med Sci. 2019;357:507-511.
Afrin LB, Khoruts A. Mast Cell Activation Disease and Microbiotic Interactions. Clin Ther. 2015;37:941-953.
Ratnaseelan AM, Tsilioni I, Theoharides TC. Effects of Mycotoxins on Neuropsychiatric Symptoms and Immune Processes. Clin Ther. 2018;40:903-917.
Weinstock LB, Rezaie A, Afrin LB. The significance of mast cell activation in the era of precision medicine. Am J Gastroenterol. 2018;113:1725-1726.
Valent P, Akin C, Bonadonna P, et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J Allergy Clin Immunol Pract. 2019;7:1125-1133.
Afrin LB, Ackerley MB, Bluestein LS, et al. Diagnosis of mast cell activation syndrome: A global “consensus-2.” Diagnosis. 2020; In press.
Theoharides TC, Tsilioni I, Ren H. Recent advances in our understanding of mast cell activation – or should it be mast cell mediator disorders? Expert Review Clin Immunol. 2019;15:639-656.
Georgin-Lavialle S, Lhermitte L, Dubreuil P, et al. Mast cell leukemia. Blood. 2013;121:1285-1295.
Molderings GJ, Zienkiewicz T, Homann J, et al. Risk of solid cancer in patients with mast cell activation syndrome: results from Germany and USA. F1000Res. 2017;6:1889. doi: 10.12688/f1000research.12730.1.
da Silva EZM, Jamur MC, et al. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014;62:698-738.
Vliagoftis H, Befus AD. Mast cells at mucosal frontiers. Curr Mol Med. 2005:573-589.
Albert-Bayo M et al. Intestinal Mucosal Mast cell: Key modulators barrier function and homeostasis. Cells. 2019;doi: 10.3390/cells8020135.
Rizzi A, Crivellato E, Benagiano V, et al. Mast cells in human digestive tube in normal and pathological conditions. Immunol Lett. 2016;177:16-21.
Poglio S, De Toni Costes F, Arnaud E, et al. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28:2065-2072.
Li Z, Liu S, Xu J, et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity. 2018;49:640-53.
Ravanbakhsh N, Kesavan A. The role of mast cells in pediatric GI disease. Clin Rev Allergy Immunol. 2019;32:338-345.
Frieri M. Mast cell activation syndrome. Clin Rev Allergy Immunol. 2018;54:353-365.
Molderings GJ, Kolck UW, Scheurlen C, et al. Multiple novel alterations in Kit tyrosine kinase in patients with gastrointestinally pronounced systemic mast cell activation disorder. Scand J Gastroenterol. 2007;42:1045-1053.
Molderings GJ. The genetic basis of mast cell activation disease - looking through a glass darkly. Crit Rev Oncol Hematol, 2015;93:75-89.
Scherber RM, Borate U. How we diagnose and treat systemic mastocytosis in adults. Brit J Haematol. 2018;180:11-23.
Jakate S, Demeo M, John R, Tobin M, Keshavarzian A. Mastocytic enterocolitis: increased mucosal mast cells in chronic intractable diarrhea. Arch Pathol Lab Med. 2006;130:362-367.
Doyle LA, Sepehr GJ, Hamilton MJ, et al. A clinicopathologic study of 24 cases of systemic mastocytosis involving the gastrointestinal tract and assessment of mucosal mast cell density in irritable bowel syndrome and asymptomatic patients. Am J Surg Pathol. 2014;38:832-843.
Atiakshin D, Buchwalow I, Samoilova V, Tiemann M. Tryptase as a polyfunctional component of mast cells. Histochem Cell Biol. 2018;149:461-477.
Lee H, Chung H, Park JC, et al. Heterogeneity of mucosal mast cell infiltration in subgroups of patients with esophageal chest pain. Neurogastroenterol Motil. 2014;26:786-793.
Aich A, Afrin LB, Gupta K. Mast cell-mediated mechanisms of nociception. Int J Molecular Sci. 2015;16:29069-29092.
Weinstock LB, Kaleem Z, Selby D, et al. Mast cell deposition and activation may be a new explanation for epiploic appendagitis. BMJ Case Rep. 2018;2018:bcr–2018–224689.
Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126: 693-702.
Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37.
Zhang L, Song J, Hou X. Mast Cells and Irritable Bowel Syndrome: From the Bench to the Bedside. J Neurogastroenterol Motil. 2016;22:181-92.
Lobo B, Ramos L, Martínez C. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study. United European Gastroenterol J. 2017;5:887-897.
yKlooker TK, Braak B, Koopman KE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 201;59:1213-21.
Balemans D, Aguilera-Lizarraga J, Florens MV, et al. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2019;316:G338-9.
Weinstock LW, Rezaie R, Brook JB, et al. Small intestinal bacterial overgrowth is common in mast cell activation syndrome. Am J Gastroenterol. 2019;114:pS670.
Bassotti, G, Villanacci V, Nascimbeni R, et al. Colonic mast cells in controls and slow transit constipation patients. Aliment Pharmacol Ther. 2011;34:92-9.
Shefler I, Salamon P, Reshef T, Mor A, Mekori YA. T Cell-Induced Mast Cell Activation: A Role for Microparticles Released from Activated T Cells. J Immunol. 2010;185:4206-12.
Alfter K, Kügelgen Von I, Haenisch B, et al. New aspects of liver abnormalities as part of the systemic mast cell activation syndrome. Liver Int. 2009;29:181-6.
Rehman Z, Rajumon M, Alam SB, et al. Prevalence and treatment of small intestinal bacterial overgrowth (SIBO) in patients with postural orthostatic tachycardia syndrome (POTS). Clin Auton Res. 2018;28:A489.
Weinstock LB, Brook JB, Myers TL, Goodman B. Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep. 2018;2018:bcr–2017–221405.
Plante GE. Vascular response to stress in health and disease. Metabol Clin Experiment. 2002;51:25-30.
Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:8-26.
Rezaie A, Raphaeal Y, Sukov R, Liu X. Ehlers-Danlos syndrome type III (EDS) and visceroptosis: getting to the bottom of this diagnosis. Am J Gastroenterolol. 2018;113:S270-1.
Weinstock LB, Myers TL, Walters AS, et al. Identification and treatment of new inflammatory triggers for complex regional pain syndrome: small intestinal bacterial overgrowth and obstructive sleep apnea. A&A Case Reports. 2016;6:272-276.
Fikree A, Chelimsky G, Collins H, et al. Gastrointestinal involvement in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175:181-187.
Fikree A, Grahame R, Aktar R, et al. A prospective evaluation of undiagnosed joint hypermobility syndrome in patients with gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2014;12:1680-1687.
Chang AR, Vadas P. Prevalence of Symptoms of Mast Cell Activation in Patients with Postural Orthostatic Tachycardia Syndrome and Hypermobile Ehlers-Danlos Syndrome. J Allergy Clin Immunol. 2019. AB182.
Molderings GJ, Brettner S, Homann J, et al. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. 2011 Mar 22;4:10. doi: 10.1186/1756-8722-4-10.
Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
Valent PA, Bonadonna PB, Hartmann KC, et al. Why the 20% + 2 tryptase formula is a diagnostic gold standard for severe systemic mast cell activation and mast cell activation syndrome. Int Arch Allergy Immunol. 2019;180:44-51.
Vysniauskaite M, Hertfelder H-J, Oldenburg J, et al. Determination of plasma heparin level improves identification of systemic mast cell activation disease. PLoS One. 2015;10(4):e0124912. doi: 10.1371/journal.pone.0124912.
Castells M, Butterfield J. Mast Cell Activation Syndrome and Mastocytosis: Initial Treatment Options and Long-Term Management. J Allergy Clin Immunol Pract. 2019;7:1097-1106.
Molderings GJ, Haenisch B, Brettner S, et al. Pharmacological treatment options for mast cell activation disease. Naunyn-Schmiedebergs Arch Pharmacol. 2016;389:671-694.
McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66:1241-51.
Kamphuis JBJ, Guiard B, Leveque M, et al. Lactose and Fructo-oligosaccharides Increase Visceral Sensitivity in Mice via Glycation Processes, Increasing Mast Cell Density in Colonic Mucosa. Gastroenterology. 2020;158:652-63.
Hagel AF, Layritz CM, Hagel WH, et al. Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn-Schmiedebergs Arch Pharmacol. 2013;386:789-793.
Theoharides TC, Bielory L. Mast cells and mast cell mediators as targets of dietary supplements. Ann Allergy Asthma Immunol. 2004;93:S24-34.
Liu Z-Q, Li X-X, Qiu S-Q, et al. Vitamin D contributes to mast cell stabilization. Allergy. 2017; 72:1184-1192.
Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693-704.
Weinstock LB, Walters AS. Brook JB, Kaleem Z, Afrin LB, Molderings GJ. Restless legs syndrome is associated with mast cell activation syndrome. J Clin Sleep Med. 2020; https://doi.org/10.5664/jcsm.8216.
Hamilton W, Gallagher A, Thomas J, White P. Risk markers for both chronic fatigue and irritable bowel syndromes: A prospective case-control study in primary care. Psychol Med. 2009;39:1913-21.
Sperber AD, Atzmon Y, Neumann L, et al. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94:3541-6.
Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urology. 2010;184:1358–63.
Pang X, Boucher W, Triadafilopoulos G, Sant GR, Theoharides TC. Mast cell and substance p-positive nerve involvement in a patient with both irritable bowel syndrome and interstitial cystitis. Urology. 1996;47:436-8.
Funding
This study was funded by LAP, Office of Research on Women’s Health of the NIH K12HD085852.
Author information
Authors and Affiliations
Contributions
LBW, MD, FACG, is the guarantor of the article. LBW and LAP performed the literature search and co-wrote the manuscript. AR, LBA, and GJM provided significant intellectual input, critical review, and revisions to the manuscript. The final draft was reviewed and approved by LBW, LAP, AR, LBA, and GJM.
Corresponding author
Ethics declarations
Conflict of interest
Drs. Weinstock, Pace, Rezaie, and Afrin do not have any conflicts of interest. Dr. Molderings is the Chief Medical Officer of the startup company MC Sciences, Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix








Rights and permissions
About this article
Cite this article
Weinstock, L.B., Pace, L.A., Rezaie, A. et al. Mast Cell Activation Syndrome: A Primer for the Gastroenterologist. Dig Dis Sci 66, 965–982 (2021). https://doi.org/10.1007/s10620-020-06264-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06264-9