Abstract
Pancreatic cancer is a tumor with a high degree of malignancy, morbidity, and mortality. Immunotherapy is another important treatment for pancreatic cancer in addition to surgery and chemotherapy, but its application in pancreatic cancer is very limited, which is related to the unique biological behavior of pancreatic cancer and the tumor microenvironment. The immunosuppressive microenvironment of pancreatic cancer is highly heterogeneous and presents challenges for immunotherapy. The transformation of tumor immunosuppressive microenvironment contributes to the response to tumor immunotherapy, such that the tumor undergoes functional reprogramming to change from immunologically “cold” to immunologically “hot.” In this review, we summarized the research and progress in immunotherapy for pancreatic cancer, including immune checkpoint inhibitors, vaccines, adoptive T cell therapy, oncolytic viruses, and immunomodulators, and suggest that individualized, combination, and precise therapy should be the main direction of future immunotherapy in pancreatic cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is a tumor with a high degree of malignancy. Its mortality rate ranks fourth among malignant tumors, and the 5-year survival rate is only 8% [1]. The onset of pancreatic cancer is occult, with rapid progress and early metastasis. Although radical surgery is currently the most likely way to treat patients with pancreatic cancer, most patients have missed the opportunity for surgery when diagnosed [2]. Therefore, the treatment response and prognosis are very poor. At present, unresectable pancreatic cancer is mainly treated with chemotherapy, supplemented by local treatment to improve symptoms. The FOLFIRINOX (fluorouracil, folinic acid, irinotecan, and oxaliplatin) and gemcitabine plus nanoparticle albumin-bound paclitaxel (GEM + Nab-p), along with the classic single-drug gemcitabine (GEM), are recommended by the National Comprehensive Cancer Network (NCCN) guidelines as first-line chemotherapy for patients with unresectable pancreatic cancer in good physical condition [3]. However, the response to FOLFIRINOX is relatively low while the toxicity is significant [4]. The effect of chemoradiation therapy for locally advanced pancreatic cancer is controversial. Traditional therapies of pancreatic cancer include surgery, chemotherapy, radiotherapy, and palliative care. The survival rate of patients with pancreatic cancer remains low [5]. The main challenges in the treatment of pancreatic cancer are as follows: The disease is highly invasive; patients are diagnosed at a late stage; there are complex local structures around the pancreas; and pancreatic cancer has a unique tumor microenvironment (TME) that lacks effective targeted therapeutics. Confrontation between the human immune system and tumor cells mainly undergoes three phases: the elimination, equilibrium, and escape phase. In the escape phase, tumor cells recruit immunosuppressive cells to form an immunosuppressive tumor microenvironment and promote tumor development [6, 7].
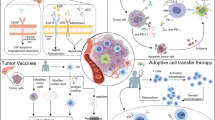
In recent years, immunotherapy has been shown to be another important anti-tumor method in addition to surgery and chemotherapy [8]. Immunotherapy is a therapeutic method to fight against tumors by activating the human immune system. It can kill tumor cells and control the development of tumors by stimulating and enhancing the immune response of patients. The best time for immunotherapy of pancreatic cancer is when the tumor is in the initial stage and immune depletion has not occurred. At present, immunotherapy for pancreatic cancer mainly includes immune checkpoint inhibitors, vaccines, adoptive T cell therapy, oncolytic viruses, specific immunomodulators, and other treatment methods (Fig. 1). This review summarizes the dilemmas and challenges of immunotherapy for pancreatic cancer and presents directions for future research.
Pancreatic Cancer Immunosuppressive Microenvironment
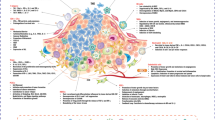
The TME refers to a complex internal environment formed by the interaction of tumor cells and their surrounding tissue components, which is beneficial to the biological behavior of tumor cells, including matrix components, cell components, and soluble factors [9]. The unique TME of pancreatic cancer includes a large number of tight matrix components, such as cancer-associated fibroblasts (CAFs), collagen deposits, hyaluronic acid and other extracellular matrices, various types of immune cells, and a large number of soluble immunosuppressive factors [10] (Fig. 2). The tight matrix components act as a physical barrier to prevent effector cells such as T cells and natural killer cells (NK cells) from infiltrating into the tumor, enabling pancreatic cancer cells to evade immune surveillance [11].
The specific TME in pancreatic cancer poses challenges for immunotherapy [12]. Pancreatic cancer cells promote the activation of surrounding stromal cells and immunosuppressive cells, including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), and their recruitment to tumor sites by secreting a variety of cytokines and chemokines. The activated stromal cells produce a large amount of extracellular matrix, forming a fibrous matrix layer around the pancreatic cancer cells, which hinders the infiltration of effector T cells and NK cells into the tumor. Immunosuppressive cells secrete immunosuppressive factors and express ligands such as programmed death ligand-1 (PD-L1) and B7-1/2 that inhibit effector T cells and NK cells, leading to an imbalance of immunosuppressive cells and forming a unique immunosuppressive microenvironment in pancreatic cancer, which plays an important role in the occurrence, development, invasion, metastasis, and drug resistance in this disease [13, 14]. Macrophages, MDSCs, and Tregs are the three major leukocyte subtypes found in the early pancreatic intraepithelial neoplasia (PanIN) stage. NK cells, FoxP3+ T cells, and CD8+ T cells are positively correlated with the survival of patients with pancreatic cancer, which is the theoretical basis for the application of immunotherapy in pancreatic cancer [15]. Dendritic cells (DCs), as the most functional antigen-presenting cells (APC), can induce the formation of specific cytotoxic T lymphocyte (CTL). In the pancreatic cancer TME, DCs are mostly immature phenotypes and have poor viability, which cannot present tumor antigens to effector T cells and initiate anti-tumor immune responses [16]. The immunosuppressive microenvironment of pancreatic cancer is highly heterogeneous and can affect the effectiveness of immunotherapy [17]. Studies have shown that GEM + Nab-p can effectively inhibit activated tumor-related fibroblasts and increase the immunogenicity of tumors, so as to play a synergistic effect in combination with PD-1/PD-L1 inhibitor [18, 19]. The remodeling of the tumor immunosuppressive microenvironment is helpful for tumor immunotherapy and enables the transformation of tumor cells from “cold” to “hot.” The ideal therapeutic antibody can kill tumor cells and reshape the tumor immune microenvironment, which has both anti-tumor and immune-enhancing effects.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors are monoclonal antibody drugs targeted at corresponding immune checkpoints. They block the interaction of inhibitory receptors expressed by T cells and related ligands, and regulate the activity of normal immune cells to improve their anti-tumor effects. Immune checkpoint inhibitors play an anti-tumor role by inhibiting the immune checkpoint activity of tumor cells and reactivating the immune activity of T cells against tumor cells [20, 21].
Common immune checkpoint inhibitors, such as those targeting PD1 (nivolumab, pembrolizumab, and pidilizumab), PD-L1 (atezolizumab), and Cytotoxic T lymphocyte associate protein-4 (CTLA-4) (ipilimumab and tremelimumab), have been widely used in the immunotherapy of metastatic melanoma, lung cancer, head and neck cancer, renal cell cancer, urothelial carcinoma, Hodgkin’s lymphoma, cervical cancer, and other cancers [22, 23]. The PD-1 ligand PD-L1 is highly expressed in tumor cells, which leads to the continuous activation of the PD-1 pathway in the TME. PD-1/PD-L1 inhibitors block the binding of PD-1 to PD-L1, thereby blocking the negative regulatory signals and enhancing the immune response of T cells. After treatment with PD-1 antibody, DCs need to express Interleukin-12 (IL-12) to license T cells to play an anti-tumor role [24]. Fibrinogen-like protein 1 (FGL1) is an important functional ligand of lymphocyte-activation gene 3 (LAG-3), and the interaction between FGL1 and LAG-3 is another tumor immune escape pathway independent of the B7-H1-PD-1 pathway; blocking this pathway can synergize with anti-PD-1 therapy [25]. CTLA-4 is expressed in activated CD4+ and CD8+ T cells. After binding with its ligand B7, CTLA-4 inhibits the activation of T cells, and blocking CTLA-4 can stimulate the activation and proliferation of immune cells, thus enhancing the anti-tumor immune response [26]. Utilizing the anti-tumor immune response is the basic strategy of immunotherapy. Anti-PD-1 therapy selectively recovers tumor-induced immune deficiency in the TME, namely it causes “immune normalization” and reduces immune-related adverse events (irAEs) [27]. Studies have shown that CD58/CD2 can also act as a co-stimulatory signal for CD28−CD8+ T cells. CD28 co-stimulation leads to the proliferation of CD8+ T cells, and the CD28/B7 pathway has a synergistic effect in the treatment of PD-1 [28]. In solid tumors, low tumor immunogenicity and a strong immunosuppressive tumor microenvironment result in significant intrinsic resistance to immune checkpoint blocking therapies [29]. Although immune checkpoint inhibitors can block the inhibitory effect on effector T cells from the cell contact-dependent protein pathway, there are still many soluble immunosuppressive factors that inhibit effector T cell function in the TME of pancreatic cancer. Pancreatic cancer, which contains a large number of dense stromal cells and few tumor-infiltrating lymphocytes (TILs), is a typical cold tumor, leading to the failure of some immune checkpoint inhibitors. Pancreatic cancer cells are surrounded by a dense fibrotic matrix that acts as a mechanical and functional barrier around the tumor, limiting drug delivery [30]. The decrease in effector T cells in TME and the increase in immunosuppressive cells lead to the formation of immunosuppressive microenvironment for pancreatic cancer. Therefore, the treatment with immune checkpoint inhibitors alone is not effective [12]. The fibrotic matrix in TME may prevent TILs from entering the tumor, and high expression of inhibitory receptor or ligand molecules may cause T cells to be depleted. Infiltrating immunosuppressive cells can directly or indirectly affect the activity of CD8+ T cells through contact-dependent or paracrine methods, eventually leading to resistance to immune checkpoint inhibitors [31]. Only patients with low neoantigen heterogeneity and high number of cloned neoantigens are more sensitive to immune checkpoint inhibitors. However, pancreatic cancer is a tumor with low mutation burden or low expression of neoantigens, so the anti-tumor response to immune checkpoint inhibitors is poor [32].
In the treatment of pancreatic cancer, a variety of anti-PD-1 drugs are undergoing clinical trials [33]. PD-1 is expressed in infiltrating DCs and macrophages on solid tumors, and the expression of PD-L1 in pancreatic cancer is used to evaluate tumor proliferation and to determine whether the tumor is highly invasive [34]. Blocking PD-L1 can significantly enhance the progress of the immune response, enhance the activation of T cells, and block the PD-1-PD-L1 signaling pathway. The increased expression of PD-L1 and PD-L2 in tumors is significantly correlated with poor prognosis in pancreatic cancer. Knocking down PD-L1 can inhibit the proliferation of pancreatic cancer cells. Two proteins, dectin-1 and galectin-9, were found to be abnormally high in pancreatic cancer samples, and their interactions can help pancreatic cancer cells escape immune attack. Galectin-9 is a β-galactoside binding protein that binds to the T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) of T cells and leads to the inactivation and apoptosis of T effector cells, while its binding to CD44 promotes the differentiation and function of induced Tregs and inhibits the metastasis of tumor cells. Pancreatic cancer is insensitive to anti-PD-1 antibodies, but a synergistic effect was found when mice were given both anti-PD-1 and anti-galectin-9 antibodies, and the tumor size in the mice was more than half that of the tumors in mice treated with either antibody alone [35]. Some CTLA-4 antibodies have been tested in clinical trials. Ipilimumab binds to CTLA-4, blocking the inhibition of T cells and producing cytotoxic T lymphocytes to enhance the anticancer immune response. Mouse models of latent pancreatic cancer have shown that when CTLA-4 is inhibited, it can control tumor growth and shrink tumors. Preliminary clinical data have shown that ipilimumab combined with a granulocyte macrophage colony-stimulating factor vaccine (GVAX) could produce a synergistic effect. This combination therapy is worthy of further study in pancreatic cancer. A large number of phase I and phase II clinical trials of tremelimumab are currently underway in locally advanced and metastatic pancreatic cancer. In a phase II clinical trial of ipilimumab, 20 patients with metastatic pancreatic cancer and 7 patients with locally advanced pancreatic cancer received ipilimumab without disease remission, but 1 patient underwent delayed tumor regression, suggesting that ipilimumab alone was ineffective in the treatment of advanced pancreatic cancer [36].
Tumor Therapeutic Vaccines
Tumor therapeutic vaccines amplify the anti-tumor immune response of tumor patients through active immunity, which plays an important role in tumor immunotherapy [37]. The basic principle of tumor therapeutic vaccines is to introduce tumor antigens in various forms such as tumor cells, tumor-related proteins or peptides, genes expressing tumor antigens into patients to overcome the immunosuppressive state caused by tumors, enhance the immunogenicity, and induce the human immune response, so as to control or remove the tumor. The antigens are given in the form of DNA, peptides, whole tumor cells, DCs that contain antigens, etc. Tumor therapeutic vaccines mainly include whole tumor vaccines, telomerase peptide vaccines, GVAX vaccines, and Wilms tumor 1 (WT1) vaccines. Although tumor vaccines can induce the activation of effector T cells, the activation degree is very limited, and only a few effector T cells and NK cells exist in the TME and peripheral blood of pancreatic cancer patients. Effector T cells often become incapacitated or fatigued under the action of the TME, rendering the tumor vaccines less effective. Currently, a large number of vaccines have been used in pancreatic cancer, such as vaccines targeting KRAS, MUC-1/CEA, WT1, heat shock protein, and vascular endothelial growth factor 2 (VEGF2), as well as polypeptide vaccines [38, 39]. GVAX is a secreted granulocyte–macrophage colony-stimulating factor (GM-CSF) secreting vaccine consisting of two irradiated pancreatic cancer cell lines that secrete GM-CSF. The expression level of PD-L1 in pancreatic cancer cells was found to be low in untreated pancreatic cancer models in animal experiments, but the expression level of PD-L1 was upregulated in patients receiving GVAX [40]. In addition, the combination of GVAX and PD-1 antibody inhibitors significantly improved survival in mice with pancreatic cancer. PD-1/PD-L1 inhibitors combined with chemotherapy and vaccines represent a future research direction in the treatment of pancreatic cancer. CRS-207 is a recombinant, double-deletion, live, attenuated form of Listeria monocytogenes that infects antigen-presenting cells and secretes mesothelin into cells. Mesothelin is expressed on most pancreatic cancer cells, and antigen-presenting cells express mesothelin and present it to T cells, producing an immune response against mesothelin. In a clinical trial, 30 patients with advanced pancreatic cancer received ipilimumab plus GVAX or ipilimumab monotherapy, and there was no significant difference in overall survival (OS) (5.7 versus 3.6 months) [41]. In a phase II clinical trial of 90 patients with metastatic pancreatic cancer, GVAX was combined with CRS-207 versus GVAX alone, and the median OS (6.1 versus 3.9 months) showed a significant survival advantage in the combination arm. However, in a phase IIb clinical trial of 303 patients with advanced pancreatic cancer, the median OS of GVAX combined with CRS-207 was 3.8 months, while CRS-207 alone had an OS of 5.4 months, and the standard chemotherapy group had an OS of 4.6 months; satisfactory results were not achieved [42]. In a recent phase IIb trial, the Cy/GVAX + CRS-207 combination did not improve survival compared to chemotherapy [43]. In a mouse model of pancreatic cancer, it was demonstrated that Listeria-based ANXA2-targeted cancer immunotherapy (Lm-ANXA2) induced the production of tumor antigens and specific T cell responses in the TME of “cold” tumors and sensitized tumors to checkpoint inhibitor therapy, supporting the use of Lm-ANXA2 in combination with anti-PD-1 antibodies for pancreatic cancer therapy [44]. The pancreatic cancer vaccine algenpantucel-L is made from a pancreatic cancer cell line transfected with mouse α-1,3-galactosyltransferase, which induces hyperacute immune rejection and exerts anti-tumor effects through specific immunity. It failed to significantly improve survival in patients with pancreatic cancer in recent phase III clinical trials [45]. DCs are professional antigen-presenting cells with the strongest antigen-presenting ability in the body, and they induce the generation of cytotoxic T cells and mediate specific anti-tumor cell immunity. In a clinical trial of 32 patients with advanced pancreatic cancer treated with the WT1 peptide DC vaccine combined with gemcitabine, the median OS was 8.1 months, showing no significant advantage over previous results [46]. In a phase I clinical trial, the WT1-DC vaccine combined with radiotherapy was significantly effective in patients with pancreatic cancer after surgery, increasing WT1-specific cytotoxic T lymphocytes, which play an important role in tumor immunity [47].
Adoptive T Cell Therapy
Chimeric antigen receptor (CAR) T cell therapy is a type of adoptive cell therapy. T cells are collected from patients or donors by apheresis, then amplified and genetically modified to express CAR that recognizes tumor cells. Finally, CAR-T cells are injected into the patient to target and kill tumor cells [48]. CAR-T can recognize antigens on the surface of tumor cells directly without being restricted by HLA molecules. Target-specific CAR-T cells are designed to target the highly expressed tumor-associated antigens of pancreatic cancer, making the treatment more specific. CAR-T cells have difficulty in treating solid tumors. Due to the complex tumor immune microenvironment in pancreatic cancer, various suppressive immune cells are the main obstacle to the therapeutic effect of CAR-T cells [49, 50]. In adoptive cell transfer therapy, autologous or allogeneic immune cells are used, most of which are T cells that produce an immune response. T cells around the tumor show specific T cell receptors, such as p53 or telomerase, associated with pancreatic cancer. These cytotoxic T cells maintain tumor reactivity, and their presence is closely related to improved survival [51, 52]. Most immunotherapies in preclinical trials of pancreatic cancer rely almost exclusively on improving T cell function to improve outcomes [53]. For CAR-T treatment, in addition to being influenced by immunosuppressive factors in the TME, the fibrous stroma layer around pancreatic cancer cells can prevent the infiltration of CAR-T into the tumor and affect the efficacy.
A study on the treatment of gastric cancer and pancreatic cancer with CAR-T cells found that claudin-18 was highly expressed in gastric cancer and pancreatic cancer; therefore, a CAR-T cell therapy (CAR-CLDN18.2) targeting claudin-18 was developed. A total of 12 patients with claudin-18-positive drug-resistant gastric cancer and pancreatic cancer were enrolled, receiving 1–5 cycles of CAR-T cell therapy, and the last 11 patients were evaluated for efficacy: 1 patient had a complete response (CR), 3 patients had a partial response (PR), 5 patients were stable, and 2 patients progressed. The objective response rate (ORR) was 33.3%, the disease control rate (DCR) was 75%, and the median progression-free survival (PFS) time was 130 days [54]. A variety of tumor-associated antigens (TAAs), such as mesothelin, CEA, MUC-1, and human epidermal growth factor receptor 2 (HER2), are expressed on pancreatic cancer cells, which provides natural conditions for the design of corresponding antibodies for treatment [55]. Antibodies against TAAs exert anti-tumor effects through at least the following pathways: ligand binding to block growth signals; antibody-dependent cellular cytotoxicity (ADCC); complement-mediated cytotoxicity; and antibody-dependent cellular phagocytosis. Finding tumor-specific antigens expressed on the surface of tumor cells is the most important direction for eliminating the “off-target” adverse reactions of CAR-T cells. The CAR-T cell target antigens include mesothelin, prostate stem cell antigen (PSCA), CEA, HER2, MUC-1, and CD133 [56, 57]. In previous studies, autologous mesothelin-specific T lymphocytes were used for the treatment of metastatic pancreatic cancer. Among the 6 patients tested, 1 patient had complete remission of all liver metastases, and 2 patients were stable and had PFS values of 3.8 months and 5.4 months [58]. CAR-T cell therapy targeting MUC1 has been shown to be effective in mouse xenograft models of pancreatic cancer and leukemia. When selecting therapeutic targets, new antigens may be discovered when protein structures and modifications are considered [59]. CD47-CAR-T cells not only effectively inhibited the growth of pancreatic cancer cell lines but also significantly blocked tumor growth in a mouse model of pancreatic cancer xenografts, indicating the potential anti-pancreatic cancer activity of these CAR-T cells [60]. Studies have established concentration-dependent CAR-T cells, and their immunogenicity is not significantly different from that of traditional CAR-T cells. Animal experiments have confirmed that they have a specific killing effect on pancreatic cancer cells with high expression of HER2 but no obvious toxic side effects on normal tissues with low expression of HER2 [61]. CAR-T cells were able to simultaneously recognize PSCA, transforming growth factor-beta (TGF-β), and interleukin-4 (IL-4). These cells were able to transmit activation and co-stimulation signals more efficiently and had improved cytokine secretion, enabling them to expand and survive for a longer period with stronger anti-tumor effects [62].
Oncolytic Virus
Oncolytic viruses (OVs) are modified therapeutic drugs that selectively infect and self-replicate in tumor cells and have a tumor-dissolving effect. OVs have the advantages of specificity, low toxicity, and low drug resistance. They can induce inflammatory cascades and participate in adaptive immune responses [63]. The anti-tumor effect of OVs not only depends on oncolysis, but also correlates with virus-induced anti-tumor immunity. OVs can release tumor antigens and upregulate chemokines after they act on tumors, thereby recruiting lymphocytes for infiltration [64]. OVs can affect the immunogenicity of TME in a variety of ways, changing the TME from an immunosuppressed state to an immune-activated state. OVs infection kills tumor cells and causes TAA to be released into the TME, causing it to undergo immunogenic cell death. It can also change the immune state of the TME by regulating the release of cytokines.
At present, a variety of oncolytic virus products have been used in clinical research in pancreatic cancer [65]. In a phase II clinical trial, 76 patients were randomized to receive the oncolytic virus pelareorep combined with chemotherapy (carboplatin and paclitaxel) or chemotherapy alone; there was no significant difference in median OS (7.3 versus 8.8 months) [66]. Animal experiments have shown that oncolytic viruses can enhance the anti-tumor immune response in pancreatic cancer and significantly reduce tumor load. Unfortunately, follow-up clinical trials did not produce positive results. Pancreatic cancer contains a large number of dense stromal cells and scarce TILs, leading to the failure of some immune checkpoint inhibitors. OVs can release tumor antigens and upregulate chemokines after acting on tumors to recruit infiltrating lymphocytes. The combination of OVs with immune checkpoint inhibitors or adoptive cell therapy may improve the therapeutic effect [67]. The combination of a tumor necrosis factor-α (TNF-α) and IL-2 oncolytic adenovirus with mesothelin-targeted CAR-T cells in immunodeficient mice with human pancreatic cancer xenografts was found to significantly inhibit tumor lung metastasis and to reduce tumor volume. This may be due to the increase in TILs and the enhancement of T cell function, which exert anti-tumor effects [68]. In summary, oncolytic viruses can change the immune status of the host tumor by changing the TME and improve the therapeutic effect of immune checkpoint and adoptive cell therapies.
Immunomodulators
In pancreatic cancer, tumor cells express immunosuppressive cytokines, which regulate the TME and act on tumor cells to help tumors escape. Immunomodulators are a class of non-specific biological products that enhance, promote and regulate immune function. Currently, immunomodulators for treating pancreatic cancer include interferon (IFN), IL, and other related molecules. Studies have shown that combination of IL-10 can enhance the immunological activity of a vaccinia virus-based oncolytic virus-targeting tumor cells in pancreatic cancer cells. IL-10 inhibits the secretion of IFN-γ and granzyme B, thereby reducing the anti-tumor activity of CAR-T cells. After depletion of IL-10 in the microenvironment, the activity of CAR-T cells was significantly restored [69]. IL-10 can promote memory T cell formation, and the combination of PEG-IL-10 (pegilodecakin) and anti-PD-1 can promote PD-1+LAG3+CD8+ T cell expansion. Pegilodecakin combined with anti-PD-1 treatment can enhance the immune response [70, 71]. Combination of IL-6 inhibitors and anti-PD-1 can lead to an increase in the number of CD8+ T cells and enhanced anti-tumor activity in tumors compared to the use of immune checkpoint inhibitors alone [72]. In addition, TGF-β can induce T cells to take on a regulatory phenotype. Inhibition of TGF-β signaling can inhibit the regulation of regulatory T cells in pancreatic cancer tissues and promote the production of anti-tumor immunity [73]. Indoleamine 2,3-dioxygenase (IDO) inhibits T cell function, induces tumor immune tolerance, and leads to chemoresistance and immunotherapy resistance, and IDO inhibitors also represent a research direction in pancreatic cancer immunotherapy [74]. IDO1 inhibitors enhance the anti-tumor efficacy of GVAX in the PDAC mouse model. The combination of the two enhances the infiltration and function of T cells in tumors, but an anti-PD-L1 antibody did not play a synergistic role. Therefore, IDO1 inhibitors can be combined with vaccine therapy [75].
MSI or MMR Pancreatic Cancer Immunotherapy
Although immune checkpoint inhibitors are not effective in most patients with pancreatic cancer, patients with higher microsatellite instability (MSI) can achieve better results. In these patients, the mismatch repair (MMR) defect leads to MSI accumulation during DNA replication, and a large number of mutations lead to the production of tumor-related neoantigens [76]. Unfortunately, the MSI-H group makes up only approximately 1% of pancreatic cancer patients, and pancreatic cancer is a tumor with a very low mutation load [77]. Single immunosuppressive agents are not effective for pancreatic cancer, and pancreatic cancer may be resistant to various factors due to the presence of innate or adaptive immune effects [78, 79]. In addition, the TME and stroma of pancreatic cancer are very complex; as such, the immunotherapy of pancreatic cancer must select specific antigens according to the heterogeneous characteristics of pancreatic cancer and then cellular immunotherapy must be administered in vivo, including a combination of cytokines, immune checkpoint inhibitors and other therapies, in addition to radiotherapy and chemotherapy when necessary. The NCCN guidelines recommend PD-1 and PD-L1 inhibitors for patients with MSI-H or dMMR unresectable pancreatic cancer, and immunotherapy exists as a second-line standard. MSI detection was performed for locally advanced and metastatic pancreatic cancer, and MMR and MSI in pancreatic cancer were evaluated by IHC and PCR, leading to patient classification as MSI-H, MSI-L or microsatellite stable [80, 81]. In a prospective study of pembrolizumab in the treatment of dMMR or MSI-H metastatic solid tumors, 2 of 8 patients with metastatic pancreatic cancer achieved complete remission, 3 patients achieved partial remission, and 1 patient had stable disease, and the disease control rate was 75% [82]. Studies found that in some patients with pancreatic cancer and MSI, patients with a high tumor epitope load saw efficacy with PD-1 mAb treatment [83]. However, recent studies found that patients with pancreatic cancer who had both activated T cells and detectable neoplastic epitopes saw no efficacy with PD-1 mAb treatment [84]. The combination of multiple immune checkpoint inhibitors may improve the clinical efficacy of pancreatic cancer.
Conclusions
Tumor immunotherapy is a hot topic at present. The research on immunotherapy for pancreatic cancer is very limited, which is related to the unique biological behavior and the TME in pancreatic cancer [85]. The microenvironment of pancreatic cancer often includes increased immunosuppressive cells, immune cell inactivation, and low tumor mutational load. The effect of immunotherapy on pancreatic cancer requires further clinical research and evaluation. How to carry out individualized, combination, and precise immunotherapy is a problem we still have to solve [86].
A single immunotherapy does not achieve the desired results, but in combination with other treatments, immunotherapy can significantly improve the effectiveness of treatment. Genome-wide analysis has shown that the heterogeneity between individuals in pancreatic cancer is very obvious; as such, individualized treatment programs can help improve the efficiency of immunotherapy. In view of the obvious individual differences in the response of different patients to immunotherapy, some studies have established “immunization scores” that combine the results of immunohistochemistry and gene expression to evaluate the infiltration of immune cells in tumors and to evaluate the efficacy of immunotherapy [87]. Screening for “immunogenic” subtypes that are more likely to benefit from immunotherapy is likely to achieve better results in patients with pancreatic cancer, as is the combination of immunotherapy with chemoradiotherapy [88]. The TME is involved in the metastasis of pancreatic cancer. According to the characteristics of the TME in pancreatic cancer, reasonable immunotherapy strategies can be designed to improve the efficacy of pancreatic cancer immunotherapy [9]. Future versions of immunotherapy for pancreatic cancer include the shift of immune checkpoint inhibitors from single to combination therapies; the combination of different immunotherapy methods; and the combination of immunotherapy with chemotherapy, radiotherapy, and targeted therapy [89, 90]. The key to the success of immunotherapy is the activation or inhibition of the immune system via therapy that targets tumor cells, which can be achieved by various immunotherapy methods [91]. Studies have used single-cell transcriptome sequencing technology to identify and analyze the cell types in pancreatic cancer patients and control pancreatic samples. The expression of proliferative ductal cell subsets in tumor tissues is negatively correlated with the activation of tumor-infiltrating T cells, suggesting that the presence of proliferative ductal cells and the loss of activated T cells may lead to poor prognosis in pancreatic cancer. The feasibility of identifying and precisely targeting tumor markers within subgroups of patients provides a new research direction for the precise treatment of pancreatic cancer [92]. Exosomes are involved in the remodeling of tumor stroma and play an important role in the tumor immunosuppressive microenvironment in pancreatic cancer. Recent studies suggest that exosomes can be used as carriers to enhance tumor immunotherapy in pancreatic cancer [93].
Immunotherapy in pancreatic cancer was less effective than other solid tumors due to the tumor heterogeneity and individual patient differences in pancreatic cancer. Based on the results from other tumor immunotherapy strategies, immunotherapy combined with chemoradiotherapy and targeted therapy is expected to be superior to single drug therapy [94, 95]. Combination immunotherapy can transform immune cold tumors into hot tumors by reshaping the tumor immune microenvironment, thereby improving the survival of patients with advanced pancreatic cancer [96]. We need to explore more specific tumor biomarker molecules and develop new targeted drugs and tumor vaccines. Individualized, combination, and precise therapy is the main direction of future immunotherapy strategies in pancreatic cancer, which may offer new hope for the treatment of pancreatic cancer.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Witkowski ER, Smith JK, Tseng JF. Outcomes following resection of pancreatic cancer. J Surg Oncol. 2013;107:97–103.
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15:1028–1061.
Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810.
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85.
Banerjee K, Kumar S, Ross KA, et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018;417:35–46.
Liu Q, Liao Q, Zhao Y. Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 2017;17:68.
Xu JW, Wang L, Cheng YG, et al. Immunotherapy for pancreatic cancer: a long and hopeful journey. Cancer Lett. 2018;425:143–151.
Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108.
Osipov A, Saung MT, Zheng L, Murphy AG. Small molecule immunomodulation: the tumor microenvironment and overcoming immune escape. J Immunother Cancer. 2019;7:224.
Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868.
Sideras K, Braat H, Kwekkeboom J, et al. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014;40:513–522.
Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 2019;156:2056–2072.
Looi CK, Chung FF, Leong CO, Wong SF, Rosli R, Mai CW. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38:162.
Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, Olive D. Pancreatic ductal adenocarcinoma: a strong imbalance of good and bad immunological cops in the tumor microenvironment. Front Immunol. 2018;9:1044.
Granot T, Senda T, Carpenter DJ, et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity. 2017;46:504–515.
Smith JP, Wang S, Nadella S, Jablonski SA, Weiner LM. Cholecystokinin receptor antagonist alters pancreatic cancer microenvironment and increases efficacy of immune checkpoint antibody therapy in mice. Cancer Immunol Immunother. 2018;67:195–207.
Weiss GJ, Blaydorn L, Beck J, et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs. 2018;36:96–102.
Liang C, Shi S, Meng Q, et al. Do anti-stroma therapies improve extrinsic resistance to increase the efficacy of gemcitabine in pancreatic cancer? Cell Mol Life Sci. 2018;75:1001–1012.
Kabacaoglu D, Ciecielski KJ, Ruess DA, Algul H. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: current limitations and future options. Front Immunol.. 2018;9:1878.
Stone ML, Beatty GL. Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol Ther. 2019;201:202–213.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355.
Ruiz-Banobre J, Goel A. DNA mismatch repair deficiency and immune checkpoint inhibitors in gastrointestinal cancers. Gastroenterology. 2019;156:890–903.
Garris CS, Arlauckas SP, Kohler RH, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity. 2018;49:1148–1161.
Wang J, Sanmamed MF, Datar I, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176:334–347.
Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431.
Sanmamed MF, Chen L. A Paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326.
Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427.
Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Res. 2017;77:817–822.
Vennin C, Murphy KJ, Morton JP, Cox TR, Pajic M, Timpson P. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology. 2018;154:820–838.
Knudsen ES, Vail P, Balaji U, et al. Stratification of pancreatic ductal adenocarcinoma: combinatorial genetic, stromal, and immunologic markers. Clin Cancer Res. 2017;23:4429–4440.
Liu X, Jia Y, Stoopler MB, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34:794–802.
Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30.
Feng M, Xiong G, Cao Z, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65.
Daley D, Mani VR, Mohan N, et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med. 2017;23:556–567.
Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: randomized phase II study of PEGPH20 Plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36:359–366.
Kartikasari AER, Prakash MD, Cox M, et al. Therapeutic cancer vaccines-T cell responses and epigenetic modulation. Front Immunol. 2018;9:3109.
Deicher A, Andersson R, Tingstedt B, Lindell G, Bauden M, Ansari D. Targeting dendritic cells in pancreatic ductal adenocarcinoma. Cancer Cell Int. 2018;18:85.
Saung MT, Muth S, Ding D, et al. Targeting myeloid-inflamed tumor with anti-CSF-1R antibody expands CD137 + effector T-cells in the murine model of pancreatic cancer. J Immunother Cancer. 2018;6:118.
Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11.
Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389.
Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333.
Le DT, Picozzi VJ, Ko AH, et al. Results from a phase IIb, randomized, multicenter study of GVAX pancreas and CRS-207 compared with chemotherapy in adults with previously treated metastatic pancreatic adenocarcinoma (ECLIPSE study). Clin Cancer Res. 2019;25:5493–5502.
Kim VM, Blair AB, Lauer P, et al. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J Immunother Cancer. 2019;7:132.
Coveler AL, Rossi GR, Vahanian NN, Link C, Chiorean EG. Algenpantucel-L immunotherapy in pancreatic adenocarcinoma. Immunotherapy. 2016;8:117–125.
Nishida S, Koido S, Takeda Y, et al. Wilms tumor gene (WT1) peptide-based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother. 2014;37:105–114.
Yanagisawa R, Koizumi T, Koya T, et al. WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res. 2018;38:2217–2225.
Liu J, Zhong JF, Zhang X, Zhang C. Allogeneic CD19-CAR-T cell infusion after allogeneic hematopoietic stem cell transplantation in B cell malignancies. J Hematol Oncol. 2017;10:35.
Wu AA, Jaffee E, Lee V. Current status of immunotherapies for treating pancreatic cancer. Curr Oncol Rep. 2019;21:60.
Akce M, Zaidi MY, Waller EK, El-Rayes BF, Lesinski GB. The potential of CAR T cell therapy in pancreatic cancer. Front Immunol. 2018;9:2166.
Ali AI, Oliver AJ, Samiei T, Chan JD, Kershaw MH, Slaney CY. Genetic redirection of T cells for the treatment of pancreatic cancer. Front Oncol. 2019;9:56.
Jindal V, Arora E, Masab M, Gupta S. Chimeric antigen receptor T cell therapy in pancreatic cancer: from research to practice. Med Oncol. 2018;35:84.
Li T, Li H, Li S, et al. Research progress and design optimization of CAR-T therapy for pancreatic ductal adenocarcinoma. Cancer Med. 2019;8:5223–5231.
Jiang H, Shi Z, Wang P, et al. Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111:409–418.
Chen N, Li X, Chintala NK, Tano ZE, Adusumilli PS. Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Curr Opin Immunol. 2018;51:103–110.
DeSelm CJ, Tano ZE, Varghese AM, Adusumilli PS. CAR T-cell therapy for pancreatic cancer. J Surg Oncol. 2017;116:63–74.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365.
Beatty GL, O’Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32.
Posey AD Jr, Schwab RD, Boesteanu AC, et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity. 2016;44:1444–1454.
Golubovskaya V, Berahovich R, Zhou H, et al. CD47-CAR-T cells effectively kill target cancer cells and block pancreatic tumor growth. Cancers (Basel). 2017;9:139.
Raj D, Yang MH, Rodgers D, et al. Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut. 2019;68:1052–1064.
Sukumaran S, Watanabe N, Bajgain P, et al. Enhancing the potency and specificity of engineered T cells for cancer treatment. Cancer Discov. 2018;8:972–987.
Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2:295–300.
Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–567.
Rahal A, Musher B. Oncolytic viral therapy for pancreatic cancer. J Surg Oncol. 2017;116:94–103.
Noonan AM, Farren MR, Geyer SM, et al. Randomized phase 2 trial of the oncolytic virus pelareorep (reolysin) in upfront treatment of metastatic pancreatic adenocarcinoma. Mol Ther. 2016;24:1150–1158.
Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(1109–1119):e1110.
Watanabe K, Luo Y, Da T, et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3:99573.
Batchu RB, Gruzdyn OV, Mahmud EM, et al. Inhibition of Interleukin-10 in the tumor microenvironment can restore mesothelin chimeric antigen receptor T cell activity in pancreatic cancer in vitro. Surgery. 2018;163:627–632.
Laidlaw BJ, Cui W, Amezquita RA, et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol. 2015;16:871–879.
Autio K, Oft M. Pegylated interleukin-10: clinical development of an immunoregulatory cytokine for use in cancer therapeutics. Curr Oncol Rep. 2019;21:19.
Mace TA, Shakya R, Pitarresi JR, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67:320–332.
Pu N, Zhao G, Gao S, et al. Neutralizing TGF-beta promotes anti-tumor immunity of dendritic cells against pancreatic cancer by regulating T lymphocytes. Cent Eur J Immunol. 2018;43:123–131.
Lemos H, Mohamed E, Huang L, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76:2076–2081.
Blair AB, Kleponis J, Thomas DL 2nd, et al. IDO1 inhibition potentiates vaccine-induced immunity against pancreatic adenocarcinoma. J Clin Invest. 2019;129:1742–1755.
Humphris JL, Patch AM, Nones K, et al. Hypermutation in pancreatic cancer. Gastroenterology. 2017;152:68–74.
Kieler M, Unseld M, Bianconi D, Prager G. Challenges and perspectives for immunotherapy in adenocarcinoma of the pancreas: the cancer immunity cycle. Pancreas. 2018;47:142–157.
Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol. 2016;27:1492–1504.
Das S, Berlin J, Cardin D. Harnessing the immune system in pancreatic cancer. Curr Treat Options Oncol. 2018;19:48.
Marisa L, Svrcek M, Collura A, et al. The balance between cytotoxic T-cell lymphocytes and immune checkpoint expression in the prognosis of colon tumors. J Natl Cancer Inst. 2018;110:68–77.
Walker EJ, Carnevale J, Pedley C, et al. Referral frequency, attrition rate, and outcomes of germline testing in patients with pancreatic adenocarcinoma. Fam Cancer. 2019;18:241–251.
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413.
Lupinacci RM, Goloudina A, Buhard O, et al. Prevalence of microsatellite instability in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2018;154:1061–1065.
Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin Cancer Res. 2017;23:3129–3138.
Chandana S, Babiker HM, Mahadevan D. Therapeutic trends in pancreatic ductal adenocarcinoma (PDAC). Expert Opin Investig Drugs. 2019;28:161–177.
Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–348.
Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209.
Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52.
Aroldi F, Zaniboni A. Immunotherapy for pancreatic cancer: present and future. Immunotherapy. 2017;9:607–616.
Young K, Hughes DJ, Cunningham D, Starling N. Immunotherapy and pancreatic cancer: unique challenges and potential opportunities. Ther Adv Med Oncol. 2018;10:1758835918816281.
Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. 2018;4:418–428.
Peng J, Sun BF, Chen CY, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29:725–738.
Batista IA, Melo SA. Exosomes and the future of immunotherapy in pancreatic cancer. Int J Mol Sci. 2019;20:567.
Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: where are we now? World J Gastroenterol. 2018;24:2137–2151.
Ducreux M, Seufferlein T, Van Laethem JL, et al. Systemic treatment of pancreatic cancer revisited. Semin Oncol. 2019;46:28–38.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218.
Acknowledgments
This work was supported by the grant from the National Natural Science Foundation of China (81401919), the grant from the Science Technology Department of Zhejiang Province (2014C03041-1), and the grant from Zhejiang Provincial Natural Science Foundation of China (LY16H160031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, J., Cai, J. Dilemma and Challenge of Immunotherapy for Pancreatic Cancer. Dig Dis Sci 66, 359–368 (2021). https://doi.org/10.1007/s10620-020-06183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06183-9