Abstract
Background
Complex perianal fistulas occurring in the absence of luminal inflammation (isolated perianal disease, IPD) may represent a specific phenotype of Crohn’s disease (CD).
Aim
We assessed the effectiveness of tumor necrosis factor (TNF)-antagonists in patients with IPD compared to those with perianal CD (PCD) with luminal inflammation.
Methods
Patients were identified through our institutional radiology database and were classified as PCD or IPD based on the presence or absence of luminal inflammation by ileocolonoscopy and abdominal enterography. Consecutive adults (> 17 years) with recurrent IPD who were treated with TNF antagonists were matched by age and gender to patients with complex PCD (1:2 ratio). Fistula remission was defined as an absence of fistula drainage. Surgery-free survival was assessed by Cox proportional hazard models.
Results
Twenty-two patients with IPD treated with a TNF antagonist were compared with 44 matched patients with PCD. A similar proportion of patients with IPD and PCD were treated with concomitant immunomodulators (55% vs. 66%) and underwent examinations under anesthesia prior to therapy (36% vs. 46%). Fistula remission at 3, 6, and 12 months was lower for the IPD cohort: 9.5% versus 34%; 19% versus 39%; and 19% versus 43%. Surgical intervention after initiating anti-TNF therapy was more common for patients with IPD (HR 3.99: 95% CI, 1.62–9.83; p = 0.0026).
Conclusions
Fewer patients with IPD achieved fistula remission, and more required surgical intervention after anti-TNF therapy, suggesting that TNF antagonists may not be as effective in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Complex perianal fistulas are associated with substantial morbidity, disability, and health care costs [1,2,3]. They occur in 21–26% of patients with Crohn’s disease (CD), based on population-based studies, and as many as 38% of patients in referral-based centers [4, 5]. Fistulas can also occur in the absence of CD, as a complication of infected anal crypts, referred to as fistula-in-ano or cryptoglandular fistulas [4, 5]. These fistulas are most commonly transient and low-lying in relation to the anal sphincter complex (simple fistulas) [6]. However, some patients with cryptoglandular fistulas develop complex fistula anatomy and recurrent complications, similar to patients with perianal CD (PCD). In a proportion of patients, isolated perianal fistulas can be the initial manifestation of CD, prior to the development of luminal symptoms [5, 7]. Therefore, the distinction between isolated perianal disease (IPD) and PCD can be challenging.
Current treatment algorithms for perianal fistulas depend on the complexity of the fistulas and the underlying diagnosis [6, 8]. While IPD is typically treated with antibiotics and local surgical intervention alone [6, 9, 10], PCD is commonly managed with TNF antagonists, such as infliximab or adalimumab [8]. The efficacy of anti-TNF therapy for PCD is supported by a number of pivotal trials demonstrating a clear benefit of therapies for the induction and maintenance of fistula remission [11,12,13]. In contrast, similar studies have not been conducted for patients with IPD.
When symptoms related to IPD become chronic, and particularly when the fistula anatomy is complex, isolated PCD is often suspected. Although anti-TNF therapy is a reasonable consideration in this clinical context, its utility remains unknown. Therefore, the aim of our study was to assess the efficacy of TNF antagonists in patients with recurrent and complex IPD after failed conventional treatment, and compare these patients with those with PCD treated with TNF antagonists.
Methods
Study Design and Patient Population
A single-center, retrospective comparative cohort study was conducted at the Ottawa Hospital between January 1, 2013, and May 1, 2018, to assess the effectiveness of anti-TNF therapy for the treatment of complex IPD. The Ottawa Health Sciences Network Research Ethics Board approved the study protocol.
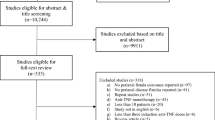
Adult patients (> 17 years of age) with perianal fistulas who underwent a pelvic MRI during the study period were identified by a search of our institutional radiology database. A manual chart review was conducted to classify patients as having IPD or PCD, assess which patients received anti-TNF therapy, and evaluate fistula response to treatment. We included patients with complex fistulas defined as one or more of the following: high inter-sphincteric, high trans-sphincteric, extra-sphincteric, supra-sphincteric fistula tracts or associated collections, as per criteria established by the American Gastroenterological Association [14]. Patients with simple fistulas, perianal disease related to an alternate identifiable cause, an ileal pouch-anal anastomosis, a diverting ostomy, prior exposure to a TNF antagonist, or incomplete records were excluded from the study. Patients were excluded who did not receive at least three induction doses of TNF antagonists (infliximab or adalimumab).
IPD required the presence of spontaneously arising perianal fistulas and an absence of intestinal inflammation by ileocolonoscopy (including histologic confirmation of normal intestinal mucosa) and small bowel imaging by CT or MR enterography. To capture the most clinically relevant cohorts, we required patients with IPD and PCD to have persistent symptoms despite at least one course of antibiotics, or an examination under anesthesia (EUA) procedure. The PCD group required an established diagnosis of luminal and perianal fistulizing CD on the basis of standard endoscopic, radiographic, and histologic criteria. For each patient with IPD, we matched two patients with PCD based on age (within 3 years) and gender.
Study Outcomes and Definitions
Our primary outcome was fistula remission, defined as minimal or no fistula drainage, either spontaneously or with gentle pressure. Fistula remission was assessed at roughly 3, 6 and 12 months following anti-TNF initiation. Secondary outcomes included a clinical response (defined as a decrease in fistula drainage by at least 50% [14]), requirement for examinations under anesthesia post-anti-TNF therapy, and persistence of anti-TNF therapy. In situations where the degree of fistula drainage was not explicitly stated, the fistula response was determined based on the treating physicians’ global assessment.
Data Collection
Data collection included: (a) patient demographics—age, gender, family history of IBD, smoking status, and disease duration; (b) fistula characteristics—anatomical location, number of fistula tracts, number of external and internal openings, hyper-enhancement of the fistula tract on T2-weighted images, vaginal involvement, and the presence of fluid collections (defined as greater or equal to 2 cm in the largest special orientation); (c) medication exposure—biologic type, immunosuppressive therapy, and antibiotics; and (d) examinations under anesthesia with or without seton placement. To ensure report consistency, a single radiologist, blinded to the clinical history, and treatment outcomes retrospectively reviewed all pelvic MRI studies.
Statistical Analysis
Descriptive statistics were used to describe patient demographics and disease characteristics. Categorical variables are presented as proportions and compared between groups by chi-squared or Fisher exact tests where appropriate. Continuous variables are summarized using medians with IQR and compared between treatment groups using two-sided Student’s t test or the Wilcoxon rank-sum test where appropriate. Patients who discontinued anti-TNF therapy either for adverse events or for a lack of efficacy were considered non-responders for all subsequent time points. Time to EUA procedures and time to anti-TNF discontinuation were determined using Kaplan–Meier survival analysis, with a log-rank test for statistical comparison of effect. Cox proportional hazard models were also used to assess differences in the requirement for EUA procedures and medication discontinuation.
Results
Disease Characteristics and Treatments
A total of 50 patients with recurrent and complex IPD were identified from our institutional database search, and of these patients 22 (44%) met our study criteria. Twenty-eight patients were excluded: 26 without exposure to anti-TNF therapy, one for a loop ileostomy, and one for inadequate documentation. Of the 22 patients included in the study, a median of two ileocolonoscopies (range 1–3) and one CT or MRI enterography procedure (range 1–2) were performed per patient over a median of 32-month follow-up (range 0–144 months). A median of two (range 0–10) EUA procedures per patient were performed prior to initiating anti-TNF therapy. Fourteen patients also underwent a least one procedure with a partial fistulotomy, three patients underwent ligation of inter-sphincteric fistula tract (LIFT), one patient had an advancement flap, and three patients had a fistula plug prior to starting anti-TNF therapy. Despite these measures, all patients had ongoing symptoms at the time of initiating anti-TNF therapy.
These patients were compared with 44 patients with complex PCD who received anti-TNF therapy. The demographics and disease characteristics for both cohorts are summarized in Table 1. Fistula anatomy, aggregate number of fistula tracts, and the number of collections were similar between both groups. The majority of patients in both cohorts were treated with infliximab: 19 patients with IPD (86%) and 33 patients with PCD (75%). The remaining patients were treated with adalimumab (Table 2). The time from diagnosis until the initiation of anti-TNF therapy was longer for patients with IPD (45 months vs. 16 months; p < 0.002) (Table 1). Finally, the percentage of patients who received concomitant therapy with an immunomodulator and the number of patients who underwent an examination under anesthesia within 6 months prior to initiating anti-TNF therapy were similar between each cohort (Table 2).
Clinical Outcomes
The majority of patients in each cohort achieved a clinical response after initiating anti-TNF therapy (Supplementary Table 1). However, only a small proportion of patients with IPD achieved fistula remission: two patients (9.5%) at 3 months, four patients (19%) at 6 months, and four patients (19%) at 12 months (Fig. 1). When compared to patients with PCD, fewer patients with IPD achieved fistula remission at 3, 6 and 12 months, although the difference was statistically significant only at three months (p = 0.035). Of the four patients with IPD who achieved fistula remission, two contained inter-sphincteric fistulas, one contained a trans-sphincteric fistula, and 1 contained a supra-sphincteric fistula. Ten patients with IPD (45%) and 12 patients with PCD (27%) required an examination under anesthesia after initiating anti-TNF therapy. Patients with IPD were significantly more likely than patients with PCD to require an EUA after starting anti-TNF therapy (HR 3.99: 95% CI, 1.62–9.83; p = 0.0026) (Fig. 2). Overall, ten patients with IPD (45%) discontinued anti-TNF therapy: six patients due to a lack of efficacy, two patients for unclear reasons, one patient due to pregnancy, and one patient due to an adverse reaction. A greater proportion of patients with IPD discontinued therapy than patients with PCD, although this did not reach statistical significance (HR: 2.09; 95% CI 0.92–4.77; p = 0.080) (Fig. 3).
Discussion
The management of recurrent and complex perianal fistulas in the absence of luminal inflammation can be challenging, partly due to the uncertainty in the underlying diagnosis (cryptoglandular vs. Crohn’s fistulas). In the current study, we investigated the efficacy of anti-TNF therapy in patients with IPD compared to patients with PCD as a reference population. We found that only a minority of patients with IPD achieved fistula remission and required significantly more EUA procedures after initiating anti-TNF therapy than patients with PCD. These results suggest that anti-TNF therapy may not be as effective in patients with IPD compared to patients with classic PCD.
To our knowledge, this is the first clinical series to assess the efficacy of anti-TNF therapy in patients with IPD. Not only was the rate of fistula healing in patients with IPD lower than our control cohort with PCD, but it was also lower than what has been reported in the literature for patients with PCD. In a meta-analysis of randomized controlled trials evaluating patients with PCD, the aggregate rate of fistula remission at weeks four to 26 following anti-TNF therapy was 33% [15]. Despite the lower rates of remission, the majority of patients with IPD in our study experienced a response to therapy, and 12 patients (55%) remained on therapy over a median follow-up time of 738 days (range 187–1950 days), suggesting that anti-TNF therapy may have some clinical utility.
It is possible that the lower rate of fistula healing in patients with IPD simply indicates that anti-TNF therapy is not an overly effective treatment option in this population. It is unlikely that the difference in fistula remission between our IPD and PCD cohorts is related to treatment factors given that both groups were well balanced with respect to EUA procedures prior to initiating anti-TNF therapy and concomitant use of immunomodulators. However, the time from diagnosis until initiation of anti-TNF therapy was longer in patients with IPD, which may have resulted in a greater degree of fistula tract epithelialization, making it more difficult for this group to achieve fistula healing. Furthermore, it remains possible that the patients with IPD had greater disease severity. Although all patients had complex fistula anatomy, it is important to recognize that the severity of complex fistulas varies widely, and current definitions of “complex” fistulas fail to quantify the degree of complexity. We attempted to address this issue by comparing a number of radiologic characteristics that have been associated with fistula healing in response to anti-TNF therapy [16, 17]. We found that both groups were well balanced with respect to the number of fistula tracts, tract hyper-enhancement, and the presence of fluid collections. Finally, it remains possible that differences in alternate unaccounted variables were responsible for our findings.
Due to the lack of definitive imaging characteristics or biomarkers to reliably differentiate between cryptoglandular and Crohn’s-related perianal fistulas, we were unable to determine conclusively if our patients with IPD had CD or simply severe cryptoglandular fistulas. Some groups have begun to investigate this issue, but additional longitudinal cohort studies with sufficient follow-up are required to determine if these entities can be reliability differentiated [18,19,20,21,22]. Notwithstanding these limitations, a number of lines of evidence suggest that anti-TNF therapy could be useful for patients with IPD. Firstly, population-based studies have demonstrated that perianal fistulas can precede the diagnosis of luminal disease in up to 9% of patients with CD [7]. Secondly, small bowel inflammation is detectable by capsule endoscopy in up to 30% of patients with IPD after normal luminal investigations [18]. Finally, 58% of patients with IPD in our cohort had at least one positive antimicrobial marker for IBD (data not shown). Collectively, these findings suggest that in a proportion of patients, IPD may represent a specific phenotype of CD. Furthermore, cryptoglandular fistulas may also benefit from anti-TNF therapy given that biopsies from these fistula tracts contain high levels of pro-inflammatory cytokines, including TNF alpha [23, 24].
The majority of patients with IPD have mild, self-limited disease courses, and some do not undergo pelvic imaging [6]. Therefore, it is likely that 44% of patients in our cohort who underwent anti-TNF treatment is an overestimate of the percentage of patients with chronic, refractory symptoms. It is also important to acknowledge that the majority of patients with IPD do not require anti-TNF therapy, and even when symptoms become chronic, surgical intervention is an option. Typically, a conservative approach using loose-fitting setons is the surgical treatment of choice for patients with complex fistulas in order to promote fistula drainage and to prevent abscess formation without compromising fecal continence. Fibrin glue or fistula plugs also have a favorable side-effect profile, but the long-term efficacy of these strategies has been questioned [25, 26]. Improved rates of healing can be achieved by advancement flaps or ligation of internal fistula tract (LIFT), but each comes at a cost of an increased risk of fecal incontinence [27,28,29]. Further comparative effectiveness studies are required to establish the optimal surgical and/or medical treatment approach.
There are a number of notable limitations in our study. Most importantly, we did not have a placebo arm for patients with IPD to directly assess the efficacy of anti-TNF therapy. Therefore, it remains possible that the clinical responses observed in our cohort occurred irrespective of anti-TNF therapy. Our small sample size and restricted patient population also make it difficult to draw firm conclusions and limit the broader applicability of our findings. Anti-TNF trough levels were not readily available at our center throughout a portion of the study period. Although we cannot exclude the possibility that serum trough levels were not optimized, the majority of patients in both cohorts underwent treatment optimization in the setting of primary or secondary non-response. Therefore, it is unlikely that sub-therapeutic drug exposure alone explains our findings. Finally, our primary outcome relied on patient-reported symptoms rather than an MRI assessment of fistula healing. Although MRI healing is a more objective outcome and likely is able to more accurately predict long-term remission, complete fistula is rare in patients with perianal fistulas and not routinely performed at our center to monitor clinical remission.
In conclusion, the management of patients with complex IPD who have failed conservative measures can be challenging. The current study demonstrates that while the majority of patients appear to have a clinical response to anti-TNF therapy, only a minority achieves fistula remission. Further studies with a larger sample size, and ideally with a dedicated placebo-control arm, are required to establish the true efficacy of anti-TNF therapy in this population and to identify which patients are most likely to benefit from therapy. We believe that until this evidence is established surgical options should be considered first and anti-TNF therapy should be reserved for selected patients.
References
Kasparek MS, Glatzle J, Temeltcheva T, et al. Long-term quality of life in patients with Crohn’s disease and perianal fistulas: influence of fecal diversion. Dis Colon Rectum. 2007;50:2067–2074.
Ng SC, Plamondon S, Gupta A, et al. Prospective assessment of the effect on quality of life of anti-tumour necrosis factor therapy for perineal Crohn’s fistulas. Aliment Pharmacol Ther. 2009;30:757–766.
Chaparro M, Zanotti C, Burgueno P, et al. Health care costs of complex perianal fistula in Crohn’s disease. Dig Dis Sci. 2013;58:3400–3406. https://doi.org/10.1007/s10620-013-2830-7.
Schwartz DA, Loftus EVJ, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880.
Hellers G, Bergstrand O, Ewerth S, et al. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21:525–527.
Vogel JD, Johnson EK, Morris AM, et al. Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2016;59:1117–1133.
Eglinton TW, Barclay ML, Gearry RB, et al. The spectrum of perianal Crohn’s disease in a population-based cohort. Dis Colon Rectum. 2012;55:773–777.
Gecse KB, Bemelman W, Kamm MA, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392.
Garcia-Aguilar J, Belmonte C, Wong WD, et al. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–729.
Mahmoud NN, Halwani Y, de Montbrun S, et al. Current management of perianal Crohn’s disease. Curr Probl Surg. 2017;54:262–298.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885.
Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405.
Sandborn WJ, Fazio VW, Feagan BG, et al. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–1530.
Ford AC, Sandborn WJ, Khan KJ, et al. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–59. https://doi.org/10.1038/ajg.2011.73.
Shenoy-Bhangle A, Nimkin K, Goldner D, et al. MRI predictors of treatment response for perianal fistulizing Crohn disease in children and young adults. Pediatr Radiol. 2014;44:23–29.
Brochard C, Landemaine A, L’Heritier AM, et al. Anal fistulas in severe perineal Crohn’s disease: Mri assessment in the determination of long-term healing rates. Inflamm Bowel Dis. 2018;24:1612–1618.
Adler SN, Yoav M, Eitan S, et al. Does capsule endoscopy have an added value in patients with perianal disease and a negative work up for Crohn’s disease? World J Gastrointest Endosc. 2012;4:185–188.
Stevens TW, D’Haens GR, Duijvestein M, et al. Diagnostic accuracy of faecal calprotectin in patients with active perianal fistulas. United European Gastroenterol J. 2019;7:496–506.
Oliveira IS, Kilcoyne A, Price MC, et al. MRI features of perianal fistulas: is there a difference between Crohn’s and non-Crohn’s patients? Abdom Radiol (NY). 2017;42:1162–1168.
Zbar AP, Horesh N, Bucholtz V, et al. Are there specific endosonographic features in Crohn’s patients with perianal fistulae? Journal of Crohn’s and Colitis. 2013;7:490–496.
Blom J, Nystrom PO, Gunnarsson U, et al. Endoanal ultrasonography may distinguish Crohn“s anal fistulae from cryptoglandular fistulae in patients with Crohn”s disease: a cross-sectional study. Tech Coloproctol. 2011;15:327–330.
van Onkelen RS, Gosselink MP, van Meurs M, et al. Pro-inflammatory cytokines in cryptoglandular anal fistulas. Tech Coloproctol. 2016;20:619–625.
Kiehne K, Fincke A, Brunke G, et al. Antimicrobial peptides in chronic anal fistula epithelium. Scand J Gastroenterol. 2007;42:1063–1069.
Owen G, Keshava A, Stewart P, et al. Plugs unplugged. Anal fistula plug: the Concord experience. ANZ J Surg. 2010;80:341–343.
Schwandner O, Stadler F, Dietl O, et al. Initial experience on efficacy in closure of cryptoglandular and Crohn’s transsphincteric fistulas by the use of the anal fistula plug. Int J Colorectal Dis. 2008;23:319–324.
Gingold DS, Murrell ZA, Fleshner PR. A prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn’s disease. Ann Surg. 2014;260:1057–1061.
Soltani A, Kaiser AM. Endorectal advancement flap for cryptoglandular or Crohn’s fistula-in-ano. Dis Colon Rectum. 2010;53:486–495.
Mizrahi N, Wexner SD, Zmora O, et al. Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum. 2002;45:1616–1621.
Author information
Authors and Affiliations
Contributions
SP acquired and analyzed the data. YD and GR acquired the data. BM contributed to MRI analysis and critical review of the manuscript. LO and SM critically reviewed the manuscript. ES analyzed the data. JDM contributed to the study concept design, data analysis, and drafting of the manuscript. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
JD and SM: Honoraria from Takeda, Jansen, Abbvie, and Ferring.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McCurdy, J.D., Parlow, S., Dawkins, Y. et al. Tumor Necrosis Factor Inhibitors May Have Limited Efficacy for Complex Perianal Fistulas Without Luminal Crohn’s Disease. Dig Dis Sci 65, 1784–1789 (2020). https://doi.org/10.1007/s10620-019-05905-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05905-y