Abstract
Introduction
Controlled attenuation parameter (CAP) is a new method for the diagnosis of steatosis. Until now, CAP was available only with the M probe of the Fibroscan. The aim of this study was to evaluate the diagnostic performance of CAP with the XL probe versus CAP with the M probe, using liver biopsy (LB) as gold standard.
Patients and Methods
A total of 236 patients with chronic liver disease undergoing LB had CAP measurement with M and XL probes the same day. All LB were analyzed independently by two experienced pathologists.
Results
Median CAP was 240.5 and 239.5 dB/m with the M and XL probes, respectively. For the detection of steatosis grade with the M and XL probes, AUROCs were 0.82/0.83 for S ≥ 1, 0.89/0.88 for S ≥ 2, and 0.92/0.93 for S3, respectively. Cutoffs were (M and XL probes) 246/242 for S ≥ 1, 269/267 for S ≥ 2, and 285/286 dB/m for S3, respectively. The factor significantly associated with CAP with the M and XL probes was steatosis grade. In multivariate analysis, a low CAP value with XL probe was negatively associated with waist circumference, triglycerides, albumin, and the alcohol consumption, and positively with alkaline phosphatases. In multivariate analysis, a high CAP value with the XL probe was positively associated with waist circumference and triglycerides.
Conclusion
CAP with the XL probe is a new tool for the diagnosis of steatosis. This parameter could be useful for the diagnosis and the follow-up of obese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases worldwide and the second leading etiology among adults awaiting liver transplantation in the USA [1, 2]. The prognosis depends heavily on histological severity. While patients with simple steatosis have excellent prognosis, those with nonalcoholic steatohepatitis tend to progress and have hepatic complications.
Recently, a novel physical parameter based on the properties of ultrasonic signals acquired by the Fibroscan has been developed using the postulate that fat affects ultrasound propagation [3]. This novel parameter, named controlled attenuation parameter (CAP), measures the ultrasound attenuation at the center frequency of the Fibroscan M probe (3.5 MHz). Values range from 100 to 400 dB/m. CAP can be used for steatosis detection and quantification and present several advantages: It is non-invasive and easy to perform, provides immediate results and is inexpensive in comparison with other measurement modalities. CAP can be performed, simultaneously to liver stiffness measurement and in the same liver volume, making it possible to simultaneously evaluate both fibrosis and steatosis and consequently enhancing the spectrum of non-invasive methods for the exploration and follow-up of patients with chronic liver disease.
Many studies have shown that CAP is significantly correlated with the percentage of steatosis and steatosis grade, and that median CAP is higher among patients with significant steatosis in patients with chronic liver disease [3,4,5,6,7,8,9]. The largest study reported correlations between CAP and steatosis grade according to LB in 440 patients [5]. Moreover, 5323 CAP examinations in patients with chronic liver diseases showed that factors significantly associated with elevated CAP were BMI, metabolic syndrome, alcohol >14 drink/week, and liver stiffness >6 kPa. Recently, individual meta-analysis showed that CAP values in dB/m (95% CI) were influenced by several covariates with an estimated shift of 10 (4.5–17) for NAFLD/NASH patients, 10 (3.5–16) for diabetics, and 4.4 (3.8–5.0) per BMI unit [7]. In some cases, no valid measurements were obtained with the M probe, mainly in obese patients. Therefore, a new probe was developed, named XL probe, especially for patients with elevated skin–liver length [10]. However, until recently, CAP measurement was not possible with XL probe.
Up to now, CAP with the XL probe of the Fibroscan® was assessed in studies with a small number of patients showing satisfactory results when taking steatosis evaluation by MRI as a Ref. [11]. However, even if those preliminary results were very promising, there was a need of an independent study of the performance of CAP with the XL in a large cohort of patients taking liver biopsy as a reference.
The objective of this study was to evaluate the diagnostic performance of CAP with the XL probe of the Fibroscan versus CAP with the M probe of the Fibroscan, using liver biopsy as gold standard, in a large cohort of patients who underwent both M and XL probe successively. The second objective of this study was to assess factors associated with CAP and with elevated and low CAP values.
Patients and Methods
Study Population
Between April 2010 and October 2012, consecutive patients with chronic liver disease undergoing LB at the University Hospital of Bordeaux (France) were analyzed. We retrospectively included patients age 18 years or above who underwent a successful liver stiffness measurement with Fibroscan (at least 10 valid measurements with both M and XL probes of the Fibroscan, successively), and a LB suitable for interpretation according to the pathologists. These patients were patients included in a prospective study registered with ClinicalTrials.gov, number NCT02060565. The study protocol was conformed to the ethical guidelines of the 1975 Helsinki Declaration, and all patients signed an informed consent.
Clinical Assessment
Anthropometric tests included body weight, body height, waist circumference measurements. BMI was calculated as weight (kg) divided by height (m) squared. Waist circumference was measured at a level midway between the lower rib margin and iliac crest with the tape all around the body in the horizontal position. Metabolic syndrome was defined according to the ethnic-specific criteria by the International Diabetes Federation, which was modified from the National Cholesterol Education Program, Adult Treatment Panel III Guidelines.
Liver Stiffness and CAP Measurements
Liver stiffness and CAP measurements were taken with Fibroscan (Echosens, Paris, France) by experienced operators blinded to the patients’ bioclinical data and using the acquisition procedure described in [12]. All patients were measured successively using both M and XL probes at the same measurement point.
During the clinical examination, the raw ultrasonic radio frequency signals were stored in the Fibroscan examination file to enable computation of CAP off-line. CAP computation was performed blinded to patients’ clinical and histological data.
CAP has been designed to measure liver ultrasonic attenuation (go and return path) at 3.5 MHz on the signals acquired by the Fibroscan. Principle of CAP measurement has been described elsewhere [3]. CAP was computed only when the associated liver stiffness measurement was valid and using the same signals as the one used to measure liver stiffness. Therefore, both stiffness and CAP were obtained simultaneously and in the same volume of liver parenchyma (namely between 25 and 65 mm for the M and between 35 and 75 mm for the XL probe).
The final CAP results were expressed in dB/m and corresponded to the median of 10 valid individual measurements.
Liver Histology
Liver histology served as the gold standard for evaluating the diagnostic accuracy of CAP. Percutaneous LB was performed using the 16G Menghini needle the same day as CAP measurement. LB specimens were fixed in formalin and embedded in paraffin.
For the purpose of the study, all LB were analyzed independently by two experienced pathologists (PB and VP) who were blinded to the clinical and Fibroscan data. A consensus was reached in cases of disagreement between the two readings.
Steatosis was assessed visually in percent and in grade, of either macro-vacuolar or mixed type, defined as follows: 0 = steatosis < 5%, 1 = 5–33%, 2 = steatosis 34–66%, 3 = steatosis > 66%.
For all patients, fibrosis was staged from F0 to F4 according to the METAVIR score for chronic hepatitis and NASH CRN score for ALD and NAFLD patients, and activity was graded from A0 to A3 [13].
Statistical Analysis
In descriptive analyses, continuous variables were expressed as mean ± standard deviation (SD) or median [interquartile range], and categorical variables as absolute figures and percentages. Confidence intervals were reported for a 95th confidence level.
Correlations were performed using the Spearman ρ correlation except for ordinal variables for which the Kendall τ correlation was used.
Diagnostic performance was assessed using receiver operating characteristic (ROC) curve analysis (AUROC). Cutoffs were selected based on the equality of sensitivity and specificity or to minimize the absolute value of the difference between specificity and sensitivity since both may not be exactly equal. Such a criterion was selected since it facilitates the comparison between the two differences diagnostic tests (CAP with the M probe and CAP with the XL probe) [14]. Specificity, sensitivity, positive predictive value, and negative predictive value associated with each cutoff values were computed. Comparison of AUROCs was performed using the Delong test. Comparison of sensitivity and specificity was made using a McNemar test. Comparison of positive predictive value and negative predictive value was made using a generalized score statistic.
Multivariate linear regression using a stepwise backward elimination was performed to understand which histological parameters (steatosis grade, fibrosis, and activity stages) are influencing CAP and LSM values. Due to its skewed distribution, LSM was 1/X-transformed to perform the multivariate linear regression analysis.
To assess what is associated with a low CAP value (high CAP value, respectively), the optimal cutoff obtained in the ROC analysis was used for the detection of steatosis grade lesser than S1 and greater than S3. Univariate analysis using odds ratio was performed between a low CAP value (high CAP value, respectively) and different parameters. Parameters significantly associated with a low CAP value (high CAP value, respectively) were then entered into the multivariate analysis.
To assess the link between metabolic syndrome and CAP, patients were separated into three groups according to their CAP value (low: < 250 dB/m, intermediate: 250–300 dB/m, high: > 300 dB/m). The prevalence of metabolic syndrome and distribution of metabolic syndrome parameters were evaluated. The difference between CAP groups was assessed using Chi-square test for binary variables and Kruskal–Wallis test for continuous variables.
All statistical analyses were performed using R software [15]. A p value of less than 0.05 was considered statistically significant.
Results
Characteristics of the Study Population
From April 2010 to October 2012, 236 consecutive patients with chronic liver disease who had both 10 valid measurements using the Fibroscan M and XL probe performed successively and a LB suitable for interpretation according to the pathologists were retrospectively evaluated.
Patient characteristics are summarized in Table 1. 56% of the patients were male, mean age was 52 ± 14 years, and mean BMI was 25.5 ± 5.2 kg/m2. Mean length of LB was 23.5 ± 7.7 mm (range 6–40 mm). 27.1% of the patients had hepatitis C virus (HCV) infection, 20.8% NAFLD, 12.3% hepatitis B virus (HBV) infection, 3.8% ALD, and 36.0% other chronic liver disease (unexplained chronic cytolysis n = 32, alcoholic disease n = 12, chronic cholestasis n = 14, autoimmune hepatitis n = 8). Steatosis grade distribution was as follows: S0 n = 122 (51.7%), S1 n = 53 (22.5%), S2 n = 24 (10.2%), and S3 n = 37 (15.7%).
Comparison of CAP with the M and XL Probes
Median CAP (interquartile range, IQR) was 240.5 (88.8) and 239.5 (90.8) dB/m for the M and XL probes, respectively. The Pearson correlation coefficient was r = 0.83, p < 10−16. The mean difference between CAP with the M and with the XL probe was 0.03 [−77.0; 77.0] dB/m and was not statistically different of 0 (p = 0.99) (Supplementary File Fig. 1).
Diagnostic Performance of CAP with the M and XL Probes
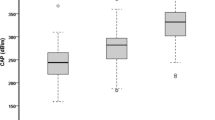
The Kendall correlation coefficient between steatosis grade and CAP was κ = 0.52, p < 10−16 for both the M and XL probe. The Spearman correlation coefficient between steatosis in percent and CAP was ρ = 0.65, p < 10−16 and ρ = 0.64, p < 10−16 for the M and XL probe, respectively. A boxplot of CAP values with both the M and XL probes versus the steatosis grade is given in Fig. 1.
A summary of CAP diagnostic performances for both probes is given in Table 2. ROC curves for the diagnostic of each steatosis grade using both probes are shown in Fig. 2. For the detection of each steatosis grade using both the M and the XL probes, AUROCs were 0.82/0.83 for S ≥ 1, 0.89/0.88 for S ≥ 2, and 0.92/0.93 for S3, respectively. The diagnostic performance in terms of AUROCS was not statistically significant between both probes. The cutoffs were assessed for both M and XL probe as sensitivity equals specificity. Computed cutoffs were for the M and XL probes 246/242 dB/m for S ≥ 1, 269/267 dB/m for S ≥ 2, and 285/286 dB/m for S = 3, respectively. Sensitivity, specificity, and positive and negative predictive values were not significantly significant between the probes. Cutoffs determined for the M probe were applied to the XL probe results showing very similar results with not statistical difference in sensitivity, specificity, and positive and negative predictive values.
When selecting the probe according to the BMI (M probe for the 190 patients with a BMI < 30 kg/m2 and XL probe for patients with a BMI ≥ 30 kg/m2), results in terms of AUROCs were 0.81 [0.76–0.87] for S1, 0.89 [0.84–0.94] for S2, and 0.92 [0.88–0.97] for S3, respectively.
Factors Associated with CAP
Association of CAP with Histological Parameters
CAP with the M probe was related in univariate analysis to steatosis grade κ = 0.52, p < 10−16; activity grade κ = 0.31, p < 10−9; and fibrosis stage κ = 0.12, p = 0.02. In multivariate analysis, the only factor significantly associated with CAP with the M probe was steatosis grade (p < 10−16).
CAP with the XL probe was related in univariate analysis to steatosis grade κ = 0.52, p < 10−16; activity stage κ = 0.28, p < 10−7; and fibrosis stage κ = 0.12, p = 0.02. In multivariate analysis, the only factor significantly associated with CAP with the XL probe was steatosis grade (p < 10−16).
Bioclinical Parameters Associated with a Low CAP Value (<246 dB/m)
Bioclinical parameters significantly associated with a low CAP value (<246 dB/m) with the XL probe are summarized in Table 3. In multivariate analysis, a low CAP value was negatively associated with waist circumference (p < 10−8): OR = 0.91 [0.87–0.93], triglycerides (p = 0.02): OR = 0.55 [0.33–0.88], albumin (p = 0.03): OR = 0.91 [0.84–0.99], and the alcohol consumption (p = 0.03): OR = 0.97 [0.94–1.00] and positively with ALP (p = 0.01): OR = 1.01 [1.00–1.01].
The same analysis of a low CAP value (<246 dB/m) with the M probe yielded similar results. In multivariate analysis, parameters significantly related to a low CAP value (<246 dB/m) with the M probe were the following: waist circumference (p < 10−10): OR = 0.87 [0.83–0.91], alcohol consumption (p = 0.002): OR = 0.95 [0.92–0.98], albumin (p = 0.02): OR = 0.90 [0.93–0.98], and ALP (p = 0.05): OR = 1.00 [1.00–1.01].
Bioclinical Parameters Associated with a High CAP Value (>285 dB/m)
Bioclinical parameters significantly associated with a high CAP value (>285 dB/m) with the XL probe are summarized in Table 3. In multivariate analysis, a high CAP value was positively associated with waist circumference (p < 10−9) and triglycerides (p = 0.04) and negatively with ALP (p = 0.02).
The same analysis of a high CAP value (>285 dB/m) with the M probe yielded to similar results. Indeed, the exact same bioclinical parameters were found to be correlated with a high CAP in univariate analysis. In multivariate analysis, the parameters significantly related to a high CAP value (>285 dB/m) with the M probe are the following: waist circumference (p < 10−7): OR = 1.11 [1.08–1.15], albumin (p < 10−3): OR = 1.21 [1.09–1.37], fasting glucose (p = 0.01): OR = 1.34 [1.07–1.71].
Association of Metabolic Syndrome and Metabolic Syndrome Parameters with CAP Values
Patients were separated into three groups: patients with a low CAP value <250 dB/m, patients with an intermediate CAP value comprised between 250 and 300 dB/m, and patients with a high CAP value >300 dB/m. The prevalence of metabolic syndrome and distribution of metabolic syndrome parameters are given in Fig. 3. The prevalence of metabolic syndrome increased with the CAP values as well as the prevalence of diabetes and hypertension. Waist circumference increased with increasing CAP values and was significantly different between each CAP group two by two. Patients with low CAP value were correctly classified (S0/S1) in 94% of cases, and patients with a high CAP value were correctly classified (S2/S3) in 80% of cases.
Metabolic syndrome (a) and distribution of the parameters of the metabolic syndrome (b diabetes, c hypertension, d waist circumference, e HDL cholesterol, f triglycerides) according to the CAP values (<250, 250–300, >300 dB/m). The indicated p value represents the difference between groups using Chi-square test for binary variables and Kruskal–Wallis test for continuous variables
Diagnostic Performance of LSM and Influence of Steatosis on LSM
AUROC for the diagnostic of F ≥ 2 was 0.78 [0.72–0.84] and 0.72 [0.65–0.78] for the M and XL probes, respectively. AUROC for the diagnostic of F = 4 was 0.87 [0.77–0.96] and 0.84 [0.74–0.94] for the M and XL probes, respectively.
LSM with the M probe was related in univariate analysis to fibrosis stage κ = 0.40, p < 10−16; activity stage κ = 0.24; p < 10−5, and steatosis grade κ = 0.11, p = 0.03. In multivariate analysis, the only factor significantly associated with LSM with the M probe was the fibrosis stage (p < 10−16).
LSM with the XL probe was related in univariate analysis to fibrosis stage κ = 0.34, p < 10−11, activity stage κ = 0.18, p < 10−3. LSM with the XL probe was not significantly correlated with steatosis grade κ = 0.01, p = 0.77. In multivariate analysis, the only factor significantly associated with LSM with the XL probe was the fibrosis stage (p < 10−12).
Discussion
In the present study, we assessed CAP with the XL probe of the Fibroscan and compared it to CAP with the M probe. To our knowledge, this is the largest study evaluating the diagnostic performances of CAP with XL probe taking steatosis at LB as a reference.
In this large cohort, we have shown that CAP measured with the XL probe of the Fibroscan had good to excellent performance for the diagnosis of each steatosis stage. In addition, we showed that the same cutoff values can be applied for both M and XL probes. This is a very important result since it will allow an ease of interpretation when using either the M or the XL probe. Both probes can now be used very easily by the clinician, just choosing the proper probe for each patient’s morphology [16, 17].
In the present study, we have shown that the performances of CAP with the XL probe are similar to the performance of CAP with the M probe of the Fibroscan. Moreover, the AUROCs and cutoffs for CAP for steatosis ≥S1 and ≥S2 were very similar between our study and the recent published meta-analysis [7]. These results and the fact that CAP is now available with the XL probe of the Fibroscan is a major progress for the management of obese patients. In 2014, more than 13% of the world’s population was obese and around 25% in affluent countries [18]. Nearly one-third of American adults now have NAFLD [19,20,21]. Moreover, the prevalence of NAFLD in non-obese patients is increasing and was recently estimated around 7% [22].
A high CAP value was mainly associated in multivariate analysis with waist circumference and triglycerides, two components of the metabolic syndrome. To further assess the link between metabolic syndrome and CAP, patients were separated into three groups according to their CAP value (low: <250 dB/m, intermediate: 250–300 dB/m, high: >300 dB/m). We choose to use 250 dB/m instead of 246 and 300 dB/m instead of 285 dB/) for more friendly use of CAP in clinical practice. The prevalence of the metabolic syndrome and the distribution of the metabolic syndrome criteria were associated with CAP value. This association had already been shown in a previous study on 5323 examinations [5] and is demonstrated again in the present study. This strong association could let us presume that CAP may have the potential to be a more objective parameter than the metabolic syndrome.
At last, we propose an algorithm for clinical use of CAP. With this algorithm, we can exclude steatosis S2/S3 with a negative predictive value of 94% and suspect steatosis S2/S3 with a positive predictive value of 80%. Our cutoff of 300 dB/m is close to another cutoff (310 dB/m) we proposed with the M probe in NAFLD patients [6]. Therefore, in clinical practice, we can conclude that in patients with CAP >300–310 dB/m, steatosis S2/S3 is very likely and management of the patient should be adapted to this result.
Our study has some limitations. Firstly, patients had chronic liver disease from various etiologies. A study assessing the performance of CAP with the XL probe in NAFLD patients is needed. Secondly, our study was performed in one center and a tertiary center. Our results need to be evaluated in other centers, especially in different countries. At last, most of our patients were non-obese. This was mandatory for our study since we aimed to compare the performance of CAP with the M and with the XL probe and for that purpose we should have patients that could be successfully assessed using both probes and therefore mainly non-obese patients. However, since the XL probe of the Fibroscan is dedicated to overweight and obese patients, it is necessary to assess the performance of CAP with the XL probe of the Fibroscan in that patient’s type.
In conclusion, with the measurement of CAP using the XL probe of Fibroscan, and since CAP is related to the metabolic syndrome, CAP could become an objective parameter of the metabolic syndrome and its evolution could be very useful in the follow-up of obese patients. However, its prognosis role needs further large studies.
Abbreviations
- CAP:
-
Controlled attenuation parameter
- LB:
-
Liver biopsy
- AUROC:
-
Area under the receiver operating curve (ROC)
- NAFLD:
-
Nonalcoholic fatty liver disease
- BMI:
-
Body mass index
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- IQR:
-
Interquartile range
- LSM:
-
Liver stiffness measurement
References
Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131.
Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555.
Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835.
Chon YE, Jung KS, Kim SU, et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102–109.
de Ledinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–1031.
de Ledinghen V, Wong GL, Vergniol J, et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:848–855.
Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi:10.1016/j.jhep.2016.12.022.
Mi YQ, Shi QY, Xu L, et al. Controlled attenuation parameter for noninvasive assessment of hepatic steatosis using Fibroscan(R): validation in chronic hepatitis B. Dig Dis Sci. 2015;60:243–251.
Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20.
Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–1871.
Sasso M, Audiere S, Kemgang A, et al. Liver steatosis assessed by controlled attenuation parameter (CAP) measured with the XL probe of the Fibroscan: a pilot study assessing diagnostic accuracy. Ultrasound Med Biol. 2016;42:92–103.
de Ledinghen V, Vergniol J. Transient elastography (Fibroscan). Gastroenterol Clin Biol. 2008;32:58–67.
Bedossa P, Poitou C, Veyrie N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759.
Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41.
Team. RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2015. https://www.R-project.org/.
Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470–1476.
Myers RP, Pollett A, Kirsch R, et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910.
WHO. Obesity and overweight—fact sheet n°311. http://www.who.int/mediacentre/factsheets/fs311/en/: WHO; 2015.
Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–858.
Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, american college of gastroenterology, and the American gastroenterological association. Hepatology. 2012;55:2005–2023.
Xu C, Yu C, Ma H, Xu L, Miao M, Li Y. Prevalence and risk factors for the development of nonalcoholic fatty liver disease in a nonobese Chinese population: the Zhejiang Zhenhai study. Am J Gastroenterol. 2013;108:1299–1304.
Author’s contributions
VL performed research, analyzed data, designed research study, and wrote paper. JV performed research and analyzed data. JH, WM, and PB performed research. VP performed research and designed research study. All authors approved the final version of the manuscript.
Funding
Funding for this study was obtained from Echosens (Paris).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Victor de Lédinghen: SuperSonic Imagine. Other authors: no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2017_4638_MOESM1_ESM.tiff
Supplementary file Fig. 1. Controlled attenuation parameter (CAP) measured with the M probe versus the CAP measured with the XL probe
Rights and permissions
About this article
Cite this article
de Lédinghen, V., Hiriart, JB., Vergniol, J. et al. Controlled Attenuation Parameter (CAP) with the XL Probe of the Fibroscan®: A Comparative Study with the M Probe and Liver Biopsy. Dig Dis Sci 62, 2569–2577 (2017). https://doi.org/10.1007/s10620-017-4638-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4638-3