Abstract
Background
Clostridium difficile (CD) infection (CDI) causes marked morbidity and mortality, accounting for large healthcare expenditures annually. Current CDI treatment guidelines focus on clinical markers of patient severity to determine the preferred antibiotic regimen of metronidazole versus vancomycin. The antimicrobial resistance patterns for patients with CD are currently unknown.
Aim
The aim of this study was to define the antimicrobial resistance patterns for CD.
Methods
This study included all patients with stools sent for CD testing to a private laboratory (DRG Laboratory, Alpharetta, Georgia) in a 6-month period from across the USA. Patient data was de-identified, with only age, gender, and zip-code available per laboratory protocol. All samples underwent PCR testing followed by hybridization for CD toxin regions A and B. Only patients with CD-positive PCR were analyzed. Antimicrobial resistance testing using stool genomic DNA evaluated presence of imidazole- and vancomycin-resistant genes using multiplex PCR gene detection.
Results
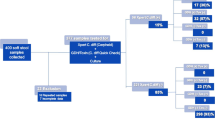
Of 2743, 288 (10.5%) stool samples were positive for CD. Six were excluded per protocol. Of 282, 193 (69.4%) were women, and average age was 49.4 ± 18.7 years. Of 282, 62 were PCR positive for toxins A and B, 160 for toxin A positive alone, and 60 for toxin B positive alone. Antimicrobial resistance testing revealed 134/282 (47.5%) patients resistant to imidazole, 17 (6.1%) resistant to vancomycin, and 9 (3.2%) resistant to imidazole and vancomycin.
Conclusions
CD-positive patients with presence of imidazole-resistant genes from stool DNA extract was a common phenomenon, while vancomycin resistance was uncommon. Similar to treatment of other infections, antimicrobial resistance testing should play a role in CDI clinical decision-making algorithms to enable more expedited and cost-effective delivery of patient care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clostridium difficile (CD) was first reported as a major cause of infectious diarrhea in 1978 [1]. Beginning in 2000, the incidence of CD infection (CDI) has risen rapidly in North America [2, 3]. The rising incidence of CDI has been further compounded by the emergence of a hyper-virulent strain of CD identified as the NAP1/BI/027 strain (North American pulsed-field gel electrophoresis type 1 by pulsed-field gel electrophoresis; group BI by restriction endonuclease analysis; and ribotype 027), often associated with more severe CDI [4, 5]. There were an estimated 453,000 cases of CDI annually by 2011 in the United States of America (USA), with approximately 83,000 cases classified as first recurrence [6]. CDI is responsible for substantial morbidity and is the leading cause of gastroenteritis-associated mortality in the USA with an attributable estimated 29,300 deaths annually [6, 7]. Further, CDI accounts for large healthcare expenditures annually with estimates ranging from $1.1 billion to $3.2 billion per year in the USA alone [8, 9].
Current treatment guidelines for CDI focus on clinical markers of patient severity to determine the preferred antibiotic regimen of metronidazole or vancomycin [2, 10, 11]. The latest American College of Gastroenterology (ACG) guidelines categorize mild CDI as diarrhea alone, while moderate CDI is diarrhea but without additional symptoms/signs classified as severe or complicated CDI [2]. These determinants of severe CDI include serum hypoalbuminemia (<3.0 g/dL) in conjunction with leukocytosis ≥15,000 cells/mm3, or abdominal tenderness without complication [2]. Complicated CDI is defined by the presence of at least one of the following features: intensive care unit admission, hypotension (use of vasopressor agents is not required), temperature ≥38.5 °C, ileus, significant abdominal distension, mental status changes, profound leukocytosis (WBC ≥ 35,000 cells/mm3) or leukopenia (<2000 cells/mm3), elevated serum lactate level (>2.2 mmol/L), or evidence of end organ damage [2]. The recommended treatment for mild-to-moderate CDI is oral metronidazole. Those with severe CDI are recommended to be treated with oral vancomycin, with the addition of intravenous metronidazole and potentially vancomycin enemas if severe and complicated CDI is present [2]. In those with recurrent CDI, the recommended treatment for the first recurrence is the same regimen as recommended for the original therapy, unless it is severe in which case vancomycin is preferred, with a pulsed vancomycin regimen for second recurrence, and a consideration for fecal microbiota transplant for third recurrence [2].
The Infectious Disease Society of America (IDSA) guidelines base initial treatment recommendations for CDI solely on clinical markers to define severity using the presence of leukocytosis (WBC ≥ 15,000 cells/mm3) and elevated creatinine (rise of ≥1.5× baseline) to differentiate the mild-to-moderate from severe cases, and define complicated CDI as the presence of hypotension or shock, ileus, or megacolon, with the same corresponding antibiotic treatment recommendations as the ACG guidelines [10].
European guidelines again recommend similar antimicrobial therapy selection as the ACG and IDSA guidelines. These recommendations are again based on clinical markers of severity, with severe CDI being defined by at least one marker of severe colitis, or complications including systemic effects of CD toxin or shock, which lead to ICU admission, colectomy, or death [11]. These proposed clinical markers of severe colitis include physical examination findings including temperature ≥38.5 °C, respiratory failure, and signs of peritonitis; laboratory data including leukocytosis, elevated creatinine, elevated serum lactate, and hypoalbuminemia; endoscopic evidence of pseudomembranes; and radiographic findings of colonic distension.
While the focus of the outlined CDI treatment guidelines is clinically based, current CD treatment regimens remain suboptimal given high recurrence rates. The antimicrobial resistance patterns for patients with CD remain unknown, and antimicrobial resistance may play a role in the decreased efficacy of CD treatment. The aim of this study was to define the antimicrobial resistance patterns for CD.
Methods
The present study included all patients with stools sent for testing for CD to a private laboratory (DRG Laboratory, Alpharetta, Georgia) in a 6-month period (July–December, 2015). Stool samples were submitted from across the USA, coming from both outpatient and inpatient settings, as part of routine clinical care. Samples were collected using sterile laboratory containers. Per laboratory protocol, samples were rejected for testing if appropriate patient identifying information was not provided; if the package containing the stool specimen was leaking; or if the specimen did not meet the minimum weight requirement (20.69 g for tube with sample collected via colonoscopy; 23.29 g for patient collected tube with sample in saline solution; and 25.96 g for patient collected tube with sample in Cary-Blair solution). Patient data were de-identified per laboratory protocol, with only age, gender, and zip code available for this evaluation. There was no exclusion based upon patient age. Institutional Review Board approval was obtained (University of Miami, Leonard M. Miller School of Medicine, Miami, Florida).
Samples were assigned an individual laboratory identification number and processed immediately for crude genomic DNA extraction using EZNA® stool DNA kit (Omega Bio-Tek, Inc. Norcross, GA). All samples then underwent quantitation of DNA using a spectrophotometer (Jenway 7315; Bibby Scientific US, Burlington, NJ), followed by multiplex polymerase chain reaction (PCR) testing (Eppendorf Mastercycler® pro, Eppendorf, Hamburg, Germany). Samples were then processed for hybridization for CD toxin regions A and B and quantitated using fluorescent nanoparticles (MAGPIX xMAP, Luminex Corporation, Austin, TX) with sensitivity 91% (95% CI 69–98); specificity 100% (95% CI 98–100); PPV 100% (95% CI 80–100); and NPV 99% (95% CI 97–99) [12, 13].
A sample was defined as positive if PCR testing for toxin A or toxin B was positive and above the cutoff value 349 MFI (median fluorescent intensity). Only patients with positive PCR for CD were included in the study analysis. Antimicrobial resistance testing was further performed using stool genomic DNA of a patient to evaluate for the presence of imidazole-resistant genes (a class of antimicrobial agents which includes metronidazole—nimA), and vancomycin resistant genes (vanA and vanB) using multiplex PCR gene detection (sensitivity 95%; specificity 99%). If a patient with one sample positive for CD had a second sample submitted that was positive for CD during the study period, the second sample was excluded from the primary analysis. A per protocol subgroup analysis was performed on the patients with two positive samples for CD to examine any changes in toxin positivity and patient’s antimicrobial resistance pattern.
Antimicrobial resistance patterns to imidazole and vancomycin were established. Antimicrobial resistance was mapped geographically using an online Google Maps®-based mapping software [14]. Antimicrobial resistance data were then examined using Spearman correlation testing to evaluate any relationship between antimicrobial resistance and age, gender, or the presence of a specific CD toxin positivity pattern (toxin A positive alone, toxin B positive alone, or toxins A and B both positive).
Results
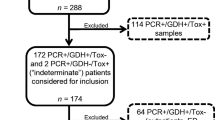
A total of 2743 stool samples were submitted for CD testing during the study period, of which 288 (10.5%) were positive for CD. Of those 288 that were CD positive, six patients had a second specimen submitted during the study period that was positive for CD. The second specimen was excluded per protocol, and this left a total of 282 specimens that were CD positive and were analyzed in this study Fig. 1.
Of the 282, 193 (69.4%) samples were from female patients with an average age of 49.4 years (standard deviation ± 18.7 years, range 1–90 years). Patient age and gender were unavailable for four patients, and these data points were excluded. Of 282 62 (22.0%) were PCR positive for both toxins A and B; 160 (56.7%) were positive for toxin A alone; 60 (21.3%) were positive for toxin B alone. Antimicrobial resistance testing revealed that 134 of 282 (47.5%) specimens were resistant to imidazole (nimA), while only 17 of 279 (6.1%) were resistant to vancomycin (vanA and vanB). Nine specimens (3.2%) were resistant to both imidazole and vancomycin.
There was no statistically significant association between antimicrobial resistance and age (imidazole: Spearman correlation −0.049, 95% CI −0.072 to 0.17, p = 0.41; vancomycin: Spearman correlation −0.06, 95% CI −0.18 to 0.059, p = 0.30) nor was there a significant association among antimicrobial resistance and gender (imidazole: Spearman correlation −0.024, 95% CI −0.14 to 0.095, p = 0.68; vancomycin: Spearman correlation −0.075, 95% CI −0.19 to 0.045, p = 0.21). No significant association existed between specific toxin positivity profile and antimicrobial resistance (imidazole: Spearman correlation −0.040, 95% CI −0.079 to 0.16, p = 0.50; vancomycin: Spearman correlation −0.0012, 95% CI −0.12 to 0.12, p = 0.98).
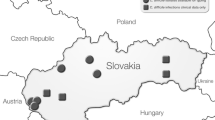
Geographic mapping of specimens with CD was performed, as well as mapping for antimicrobial resistance Fig. 2. Regional antimicrobial resistance rates were calculated and stratified by geographic region based on the first digit of the zip code Table 1. Five specimens had no zip code. A majority of the CD-positive specimens were clustered in the southeastern USA and the mid-Atlantic region with some extension into the northeastern USA, in general accordance with the pattern of all specimens submitted. The imidazole resistance pattern followed the general distribution of CD-positive specimens. The vancomycin resistance pattern, however, was disproportionately highest in the northeastern USA. This did not follow the overall distribution pattern for CD-positive cases.
Map showing distribution of all specimens submitted for CD testing (a); distribution of CD-positive specimens in accordance with the pattern of all specimens submitted (b); imidazole resistance pattern following the general distribution of CD-positive cases (c); and vancomycin resistance pattern not following overall distribution of CD-positive specimens with highest resistance in the northeastern USA when examining regions with 10+ CD-positive specimens (d)
Per protocol subgroup analysis of the six patients with two positive samples revealed that one patient was initially positive for both toxins A and B but was only positive for toxin A in the second sample. Three patients were only positive for toxin A and had the same results in the second sample, and two patients who were only positive for toxin A in the first sample eventually developed both toxins A and B. Only one of the six patients was resistant to imidazole initially. Imidazole resistance in this sample persisted in addition to the development of imidazole resistance in two additional patients. None of the patients had vancomycin resistance initially, while one developed resistance to vancomycin in the second sample. No patients in the subgroup had resistance to both imidazole and vancomycin.
Discussion
The 10.5% prevalence of CD in stool samples submitted for testing in our study was consistent with the reported rate in the one prior study from a developed country of 11.4% [15]. CD-positive patients with presence of imidazole-resistant genes from stool DNA extract was a common phenomenon in our study (47.5%), while finding vancomycin-resistant genes was uncommon (6.1%). This high level of antimicrobial resistance to imidazole in patients may explain an increased treatment failure rate in those receiving metronidazole as first-line therapy for CDI. A 2005 prospective observational study of 207 patients with CDI who were treated with metronidazole demonstrated that 22% of patients did not respond to metronidazole by 9 days of therapy, and 28% of patients developed recurrent CDI within 90 days of metronidazole treatment [16]. These results showing a combined 50% treatment failure or recurrence rate with metronidazole are in accordance with our 47.5% metronidazole resistance rate. Further, in a 2014 phase 3 multinational study by Johnson et al. [17] comparing tolevamer, a high molecular weight polymer that binds CD toxin, to metronidazole and vancomycin for patients with CDI revealed clinical success with metronidazole of 66.3% in comparison with 78.5% with vancomycin (p = 0.059). Johnson et al. were limited by the inclusion of all patients with CDI regardless of disease severity with the exception of those with fulminant CDI or acute, life-threatening illness, but perhaps this better reflects the general population and reaffirms our similar rates of vancomycin and metronidazole antimicrobial resistance. It remains unknown whether dose escalation may be able to overcome resistance to vancomycin for CDI at this time.
Our study contradicts the results of two prior smaller laboratory-based studies performed in 1999 showing low or no rates of CD resistance to metronidazole; however, these studies pre-date the rapid rise of CDI since 2003 [18, 19]. More recently, Baines et al. [20] demonstrated a reduced susceptibility of CDI to metronidazole in 24.4% of patients specifically with the 001 strain of CD, whereas there was no reduction in metronidazole susceptibility in patients with the 106 and 027 (NAP1/BI/027) strains of CD. Perhaps our findings indicate an emerging pattern of metronidazole resistance over time, or may be a consequence of prior metronidazole exposure, which is unknown in our study population. Further, while metronidazole resistance patterns geographically followed general CD positivity, the discordance seen geographically of vancomycin resistance may indicate an emerging trend regionally as vancomycin usage continues to rise for treatment of CDI.
In a study by Kamboj et al. of 102 patients with repeated episodes of CDI, 88% of those with a second episode within 8 weeks of the index episode were found to have the same CD strain accounting for both episodes. Even after 8 weeks, the relapse rate with the same strain accounting for both episodes still remained elevated at 65% as opposed to infection with a second CD strain [21]. Our per protocol subgroup analysis of the 6 patients with two CD-positive samples revealed new development of imidazole resistance in two and new development of vancomycin resistance in one. In total, 50% of the patients in this subgroup had new development of antimicrobial resistance in the second specimen. These findings, though limited in number, may further shed light into the pathogenesis of treatment failures and clinical relapses.
Similar to the treatment of other infections, antimicrobial resistance testing should play a pivotal role in CDI clinical decision-making algorithms. For example, in the treatment of catheter-related bloodstream infections, broad spectrum antibiotics are recommended by IDSA guidelines first, followed by tailoring of antibiotic therapy once a specific organism is identified and antimicrobial sensitivity analysis is performed [22]. Further, the results of our mapping of antimicrobial resistance showed regional variability which may impact clinical success rates of CDI treatment. Future studies should evaluate the clinical utility of antimicrobial resistance testing of CD taking into account clinical data, which may eventually lead to a paradigm shift in practice guidelines for CDI treatment if non-response rates rise.
Our study is limited by a lack of clinical data for the patients including risk factors for CDI, first episode versus recurrence of CDI as well as clinically symptomatology, prior antibiotic treatment experience or response to therapy, clinical markers of severity, and potential comorbid conditions. Clinical disposition based on CDI testing results and potential response to antibiotic therapy was unavailable based on the lack of clinical data. Further, C. difficile subtyping for specific strains such as the NAP1/BI/027 strain was not performed per protocol but could add further potential data in future studies for clinical or pathological correlations with antimicrobial resistance. Given unknown clinical symptomatology, active CDI is unable to be distinguished from asymptomatic carriage of CD; however, it is likely that stool samples were obtained only from symptomatic patients. Given that the stool sample is preserved in a liquid format prior to testing, we are unable to confirm that all patients with samples submitted for testing have clinical evidence of diarrhea at the time of testing. These limitations, however, are to be expected in a referral laboratory-based study. While this may limit the widespread applicability of our study, our results underscore the need for individual laboratories and centers to determine an antibiogram for their population and to perform individualized testing of patient stool samples of CD for antimicrobial resistance. This will assist in guidance of therapy and hopefully minimize CDI recurrence. Future prospective studies should examine the clinical impact of antimicrobial sensitivity testing on success and relapse rates of antibiotic treatment regimens for CDI.
Conclusion
CDI is an increasingly common, severe, and costly disease. Current guidelines use clinical markers of severity for determination of antibiotic selection. There was, however, a high rate of metronidazole resistance in CD-positive stool samples examined in our study. Based on our findings, a potential shift away from solely clinically based antimicrobial selection for CDI should be considered to enable more expedited and cost-effective delivery of patient care.
References
Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778–782.
Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498.
Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145:758–764.
See I, Mu Y, Cohen J, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis. 2014;58:1394–1400.
Rao K, Micic D, Natarajan M, et al. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clin Infect Dis. 2015;61:233–241.
Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834.
Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55:216–223.
Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2012;34:346–353.
O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–1227.
Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;21:431–455.
Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20:1–26.
Claas EC, Burnham CA, Mazzulli T, Templeton K, Topin F. Performance of the xTAG® gastrointestinal pathogen panel, a multiplex molecular assay for simultaneous detection of bacterial, viral, and parasitic causes of infectious gastroenteritis. J Microbiol Biotechnol. 2013;23:1041–1045.
Patel A, Navidad J, Bhattacharyya S. Site-specific clinical evaluation of the Luminex xTAG gastrointestinal pathogen panel for detection of infectious gastroenteritis in fecal specimens. J Clin Microbiol. 2014;52:3068–3071.
EasyMapMaker. (March 2, 2016). Available from: http://www.easymapmaker.com.
Heimesaat MM, Granzow K, Leidinger H, Liesenfeld O. Prevalence of Clostridium difficile toxins A and B and Clostridium perfringens enterotoxin A in stool samples of patients with antibiotic-associated diarrhea. Infection. 2005;33:340–344.
Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–1590.
Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345–354.
Wong SS, Woo PC, Luk WK, Yuen KY. Susceptibility testing of Clostridium difficile against metronidazole and vancomycin by disk diffusion and Etest. Diagn Microbiol Infect Dis. 1999;34:1–6.
Barbut F, Decré D, Burghoffer B, et al. Antimicrobial susceptibilities and serogroups of clinical strains of Clostridium difficile isolated in France in 1991 and 1997. Antimicrob Agents Chemother. 1999;43:2607–2611.
Baines SD, O’Connor R, Freeman J, et al. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother. 2008;62:1046–1052.
Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitz KA. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin Infect Dis. 2011;53:1003–1006.
Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45.
Funding
The authors report no sources of funding for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nimita Fifadara Ph.D. is the Chief Science Officer of DRG Laboratory. Dr. Fifadara played no role in study design, data analysis, or decision to publish. The authors report no other relevant conflict of interest or disclosures.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional Review Board approval was obtained.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Barkin, J.A., Sussman, D.A., Fifadara, N. et al. Clostridium difficile Infection and Patient-Specific Antimicrobial Resistance Testing Reveals a High Metronidazole Resistance Rate. Dig Dis Sci 62, 1035–1042 (2017). https://doi.org/10.1007/s10620-017-4462-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4462-9