Abstract
Patients with long-standing ulcerative colitis (UC) or Crohn’s colitis are at increased risk of developing colorectal cancer (CRC). Given that most cases of CRC are thought to arise from dysplasia, previous guidelines have recommended endoscopic surveillance with random biopsies obtained from all segments of the colon involved by endoscopic or microscopic inflammation. However, recent evidence has suggested that the majority of dysplastic lesions in patients with inflammatory disease (IBD) are visible, and data have been supportive of chromoendoscopy with targeted biopsies of visible lesions versus traditional random biopsies. This review article will discuss the risk of colon cancer in patients with IBD, as well as current recommendations for CRC screening and surveillance in patients with UC or Crohn’s colitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with long-standing ulcerative colitis (UC) or Crohn’s colitis are at increased risk of developing colorectal cancer (CRC). Given that most cases of CRC are thought to arise from dysplasia, previous guidelines have recommended endoscopic surveillance with random biopsies obtained from all segments of the colon involved by endoscopic or microscopic inflammation [1–4]. However, recent evidence has suggested that the majority of dysplastic lesions in patients with inflammatory disease (IBD) are visible [5, 6], and data have been supportive of chromoendoscopy with targeted biopsies of visible lesions versus traditional random biopsies [7]. This review article will discuss the risk of colon cancer in patients with IBD, as well as current recommendations for CRC screening and surveillance in patients with UC or Crohn’s colitis.
Epidemiology of CRC Risk in IBD
Although the link between UC and CRC has long been established [8], the association between Crohn’s colitis and colon cancer was more recently discovered [9]. Regardless, the risk of CRC in UC versus Crohn’s colitis appears to be similar, assuming similar colonic extent of inflammation [10, 11]. In a meta-analysis from 2001, the risk of CRC in patients with UC was reported to be 0.5–1 % per year, with a cumulative CRC risk of 2 % at 10 years, 8 % at 20 years, and 18 % after 30 years of colitis [12]. However, more recent population-based studies have shown a decreasing risk of CRC in IBD [13–15]. Declining rates of CRC in patients with IBD in recent years have been potentially attributed to the effects of 5-aminosalicylates [16], immunosuppressive drugs [17, 18], as well as the initiation and implementation of CRC surveillance programs with early colectomy [19].
Age of Disease Onset, Duration of Disease, and Extent of Disease
CRC is increased, the younger the age of diagnosis with a cumulative risk of 40 % in those diagnosed before 15 and 25 % in those aged 15–39 [20]. More recent studies found similar results of early age at diagnosis increasing the risk of CRC, but also noted that patients diagnosed at an older age were also at increased risk [21, 22].
One of the most important risk factors for CRC in patients with IBD, as compared to age- and sex-matched non-IBD patients, is thought to be related to intestinal inflammation [23]. The extent of colonic inflammation is based on the maximal extent of either endoscopic or histologic criteria identified at any point during the disease [3]. In a recent French cohort study, patients with long-standing extensive colitis defined by at least 10 years of colonic inflammation involving over 50 % of the colon were found to have an increased risk of CRC [24]. In a prior population-based study of patients with UC, Ekbom reported an incidence ratio for CRC of 1.7 for isolated proctitis, 2.8 for left-sided colitis, and 14.8 for pancolitis [20]. Importantly, by the fourth decade of disease duration, the incidence of CRC in patients with extensive colitis and left-sided colitis is approximately equal [25, 26]. More recent population-based cohort studies have also confirmed the association of extent of disease with CRC risk but at lower rates with an incidence ratio for CRC of 1.7 for proctitis, 2.1 for Crohn’s colitis, and 5.6 for pan-colitis [27]. Therefore, patients without significant colonic inflammation and patients with ulcerative colitis limited to the rectum are not at increased risk of CRC [20], whereas patients with Crohn’s colitis are only at excess risk of CRC if more than 30 % of the colonic surface was ever involved [23].
An equally important risk factor is the duration of disease [23]. Although a Dutch cohort study reported that 22 % of patients with IBD developed CRC before surveillance was initiated 8 years after diagnosis [28], a recent meta-analysis demonstrated that the risk of CRC becomes apparent after 7 years and increases linearly, with a higher risk of patients with extensive colitis [15]. In a large report of surveillance colonoscopy in patients with extensive UC, the CRC risk also increased linearly, from 2.5 % at 20 years to 10.8 % at 40 years [29]. In that study, the median duration of UC at time of CRC diagnosis was 23.5 years (range 11–48 years).
Family History and Concomitant Primary Sclerosing Cholangitis
Both family history and concomitant primary sclerosing cholangitis (PSC) significantly increase the risk of CRC. The additional risk attributed to family history is the same as that seen in the general population (twofold to threefold) [30, 31]. However, when PSC is also present, there is a substantial increase in the underlying risk of CRC [32]. Broomé et al. [33] reported the risk to be 9 % at 10 years, 31 % at 20 years, and 50 % after 25 years. A subsequent meta-analysis did not substantiate risks this high, but still found a fourfold increased risk of CRC [34]. Unfortunately, this risk is not eliminated post-transplant for PSC with risks up to 14 % at 5 years and 17 % at 10 years post-transplant [35].
Histologic and Anatomic Changes
Changes within the colon are also associated with an increased risk of CRC. Severity of the underlying inflammation, prior dysplasia, development of strictures, pseudopolyps, and a foreshortened (tubular) colon are all risk factors for CRC [23, 36, 37]. In a case control study of patients with UC and colorectal neoplasia, the severity of histologic inflammatory activity was associated with a 3.7 increased odds of developing colorectal neoplasia [38]. In a retrospective study, Lasher et al. [39] reported colonic strictures in 3.2 % of their UC cohort. In those patients, 73 % had dysplasia (n = 11) and 13 % (n = 2) had colon cancer in the strictures. Interestingly, after colectomy, 40 % (n = 6) of the patients were diagnosed with cancer at the stricture site [39]. Predictors of malignant strictures include the development of strictures after 20 years of disease, location proximal to the splenic flexure, and symptomatic obstruction [40]. Another risk factor for CRC is the presence of pseudopolyps (OR 2.29) [41, 42]. While the pseudopolyps themselves do not develop into cancer, they likely serve as a surrogate for areas with prior severe inflammation. Similarly, a foreshortened colon in UC is also a risk factor for colon cancer and likely a marker of prior inflammation [23].
Definitions
The development of CRC in patients with IBD is thought to be preceded by dysplasia, thereby providing a rationale for endoscopic surveillance. The term dysplasia is reserved for epithelial changes that are unequivocally neoplastic, and a classification scheme was originally described by Riddell et al. [43] which included the following: no dysplasia, indefinite for dysplasia (Fig. 1), low-grade dysplasia (Fig. 2), and high-grade dysplasia (Fig. 3). Cases of indefinite dysplasia were further subdivided into mucosa that was thought to be related to inflammatory-associated regenerative changes versus probable presence of dysplasia. In order to distinguish between these two subsets, aggressive medical therapy is typically recommended in order to reduce active inflammation followed by short-term surveillance colonoscopy with repeat biopsies [44].
Indefinite for dysplasia (×200). Some columnar cells show enlarged, elongated nuclei with stratification and retained nuclear polarity. However, many crypts are inflamed (intraepithelial neutrophils and crypt microabscesses), and it is unclear whether these changes can be entirely attributed to epithelial regeneration or whether these changes represent dysplasia. Furthermore, the luminal surface is stripped, and surface maturation of colonocytes cannot be confirmed. Therefore, this case is best classified as indefinite for dysplasia
The distinction between low- and high-grade dysplasia depends on the distribution of nuclei within the mucosa, with low-grade dysplasia typically characterized by crowded, hyperchromatic nuclei localized in the basal half of the cells compared to high-grade dysplasia demonstrating nuclear stratification and loss of cellular polarity [45]. Although interobserver agreement between pathologists is higher at the extremes of low-grade and high-grade dysplasia, there is poor concordance even among experienced gastrointestinal pathologists in cases indefinite for dysplasia and in the gray zone between low- and high-grade dysplasia [44].
Endoscopic Surveillance
Given the increased risk of CRC, endoscopic surveillance for both colitis-associated neoplasia and CRC is recommended by multiple international gastrointestinal (GI) societies [1, 46–48]. The rationale of endoscopic surveillance is to detect and potentially resect dysplasia/CRC or refer for a colectomy at an earlier and potentially curable stage. Although there have not been any randomized controlled trials demonstrating mortality benefit of endoscopic surveillance, population-based cohort studies and case–control studies have shown improved CRC-related survival [49–53]. In a recent retrospective cohort study, having a recent colonoscopy was found to be associated with a reduced incidence of CRC in patients with IBD, as well as lower mortality rates in those patients diagnosed with CRC [54].
Societies differ in regard to timing of initial screening colonoscopy, timing of surveillance intervals, optimal method of detecting dysplasia, and management of dysplastic lesions. Most societal guidelines recommend performing an initial screening of patients with a colonoscopy with staging biopsies approximately 8–10 years [3, 4, 46] after onset of symptoms in order to evaluate the extent of disease and determine the need for ongoing surveillance. However, some guidelines suggest starting screening 6 years after onset of symptoms, depending on the presence of other risk factors such as severity and extent of colonic inflammation, family history of CRC, presence of pseudopolyps, and age of onset [47]. All societies recommend initiation of surveillance colonoscopy for patients with UC who have at least left-sided colitis [1, 46], and for patients with Crohn’s colitis involving more than one segment or at least one-third of the colon.
The different international societies differ in terms of recommend surveillance intervals after the initial screening colonoscopy. See Table 1 for description of guidelines by major international societies. For patients with risk factors for IBD-associated neoplasia including PSC, extensive colitis, active endoscopic or histologic inflammation, family history of CRC at age <50 years, prior history of dysplasia, or colonic strictures, all guidelines recommend annual colonoscopy surveillance. The British Society of Gastroenterology (BSG), National Institute for Health and Clinical Excellence (NICE), European Crohn’s and Colitis Organisation (ECCO), all suggest a risk-stratified approach to determining the timing of endoscopic surveillance. For example, the ECCO guidelines suggest performing colonoscopy every 2–3 years in intermediate-risk patients, defined by patients with extensive colitis with mild to moderate active inflammation, presence of post-inflammatory polyps, or CRC in a family member >50 years [55]. Additionally, low-risk patients, without risk factors, can have colonoscopy performed every 5 years. Similarly, the NICE guidelines suggest that surveillance can be performed every 5 years in low-risk patients who have left-sided UC, or extensive but quiescent UC or Crohn’s colitis [56]. At present, the United States GI societies (American Gastroenterological Association (AGA), American College of Gastroenterology (ACG), and American Society of Gastrointestinal Endoscopy (ASGE)) do not explicitly recommend extending surveillance intervals to greater than 3 years. However, the ASGE guidelines from 2014 suggest potentially lengthening surveillance intervals for patients with endoscopically and histologically normal mucosa on at least two sequential surveillance colonoscopies [57].
Technique for Colonoscopy and Detection of Dysplasia
Traditionally, dysplasia in UC and Crohn’s colitis was thought to be flat and difficult to detect with colonoscopy [1]. The standard screening modality has been random biopsies every 10 cm for a total of 33 biopsies. This method, however, is limited in that it samples approximately only 1 % of the entire colon [45]. However, recent data have shown that most dysplastic lesions are visible, and consequently targeted biopsies have shown to be potentially superior to random biopsies [58–61]. In order to detect visible dysplasia when using white-light colonoscopy, high-definition colonoscopy is recommended over standard definition colonoscopy (Fig. 4) [7].
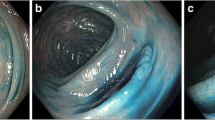
Chromoendoscopy improves visualization of the colonic epithelium via application of dye to the colon mucosa. Typically, methylene blue or indigo carmine is sprayed on the colonic mucosa via a water jet channel or catheter. The colonoscopy is performed, and then the colonic mucosa is sprayed in a segmental fashion with the contrast agent (Fig. 5). The rationale for chromoendoscopy compared to white-light colonoscopy is that the former technique can enhance and highlight areas of mucosal irregularities, as well as help delineate the borders of suspected lesions (Fig. 6).
In a recent meta-analysis, chromoendoscopy with targeted biopsies of abnormal appearing mucosa was found to be 8.9 times more likely to detect both flat and polypoid dysplasia as well as a 93 % lower likelihood of missing dysplasia as compared to white-light colonoscopy with random biopsies [62]. In a prospective, tandem study using high-definition colonoscopy, dysplasia was identified significantly more frequently in the chromoendoscopy group compared to white-light colonoscopy [63]. However, recently, in a large recent single-center retrospective study, implementation of chromoendoscopy for IBD surveillance did not increase detection of dysplasia compared with white-light endoscopy with targeted and random biopsies [64]. A cost-effectiveness study comparing chromoendoscopy with targeted biopsies to both white-light colonoscopy with random biopsies and no surveillance demonstrated that chromoendoscopy was more effective and less costly than the random biopsy strategy. However, chromoendoscopy was only cost-effective compared to the no surveillance strategy if surveillance intervals were 7 years or greater [65].
When chromoendoscopy with targeted biopsies is performed for surveillance, there does not appear to be a clear role for performing additional random biopsies. However, there may be a role for performing histologic staging biopsies at the time of chromoendoscopy, given that histologic inflammation may play a role in risk stratification [57, 66]. As the yield of detecting neoplasia in the setting of random biopsies has been shown to be very low [61], additional random biopsies in addition to chromoendoscopy are not currently recommended [1, 47, 57].
Currently, chromoendoscopy with targeted biopsies performed by trained operators is the recommended surveillance protocol by BSG and ECCO [48, 55]. As per the SCENIC international consensus statement, chromoendoscopy with targeted biopsy is suggested rather than high-definition, white-light colonoscopy [7]. The more conservative recommendation for chromoendoscopy in this situation was due to the low quality of data and reliance on one small observational study demonstrating that dysplasia was identified in significantly more patients undergoing chromoendoscopy compared to white-light alone [63]. Similarly, the most recent AGA guidelines support chromoendoscopy with targeted biopsies as a reasonable approach to surveillance compared to white-light colonoscopy with random biopsies presuming the operator is experienced and fully trained in the technique [1]. However, the ASGE guidelines suggest that surface chromoendoscopy with resection or targeted biopsies is the preferred surveillance technique [57]. If chromoendoscopy is not available, the ASGE recommends 4-quadrant biopsies every 10 cm for a minimum of 33 biopsies in patients with pancolitis and 4 quadrant biopsies every 10 cm to the maximum extent of endoscopic or histologic inflammation documented previously.
Management of Detection of Dysplasia
While prior recommendations advised using the terms dysplastic-associated lesions or masses (DALM) versus non-DALM [67], current guidelines recommend classifying all dysplasia in IBD as visible or nonvisible [55], with nonvisible lesions referring to those detected on random biopsies. For visible dysplastic lesions that are detected in areas of the colon uninvolved by colitis, management should be consistent with standard polypectomy techniques and continued surveillance based on the patient’s IBD risk without any need for heightened surveillance or colectomy [1, 57, 68]. For all visible lesions in the setting of colitis, lesions should be classified as polypoid or nonpolypoid, based on the Paris classification [7, 69].
Polypoid Visible Lesion
For polypoid visible lesions, endoscopic resection is recommended when feasible. Depending on the size of the polypoid lesion, referral for endoscopic mucosal resection (EMR) may be considered. Separate biopsies of the flat mucosa around the polypectomy site should be performed to ensure the absence of any surrounding dysplasia [1, 55, 57]. Patients with a dysplastic, polypoid lesion that has been completely resected should be followed with close endoscopic surveillance, as long-term follow-up studies have not shown an increased risk of developing CRC in patients with resected polypoid lesions as compared to standard IBD surveillance patients [70–72]. If lesions are not endoscopically resectable or if there is evidence of endoscopically invisible multifocal low-grade dysplasia (LGD) or invisible HGD, total proctocolectomy is indicated [1, 55]. Patients with larger sessile lesions which are removed via EMR or piecemeal resection should have repeat surveillance in 3–6 months, followed by annual surveillance if the initial follow-up procedure shows no residual polyp [7].
Nonpolypoid Visible Lesions
Nonpolypoid detectable lesions should be evaluated for safety and efficacy of endoscopic resection depending on whether there are features concerning for submucosal invasion or underlying malignancy, such as a depressed or ulcerated lesion [62]. Other features that may be associated with failed resection include ill-defined margins, inability to lift the lesion with submucosal injection, or flat neoplastic changes adjacent to the lesion [1, 62]. Referral to providers experienced in removal of nonpolypoid lesions should be considered, given that techniques such as EMR and endoscopic submucosal dissection (ESD) may be necessary, as well as the fact that attempts should be made to completely resect the lesion during the initial polypectomy [73]. In addition to biopsies being obtained adjacent to the resection site, a tattoo should be placed to aid in future surveillance.
There is limited data regarding the risk of CRC after resection of nonpolypoid dysplastic lesions. For completely resected nonpolypoid, dysplastic lesions, early surveillance colonoscopy at 3–6 months is recommended. Some guidelines have suggested colectomy for nonpolypoid dysplastic lesions, given the belief that the majority of nonpolypoid dysplastic lesions are unresectable [1, 47]. However, if resection is complete, then surveillance colonoscopy is suggested by the SCENIC guidelines as compared to colectomy [7]. For unresectable nonpolypoid lesions, biopsies should be obtained to confirm the presence of dysplasia, and proctocolectomy is recommended.
Endoscopically Invisible Dysplasia
If there is evidence of endoscopically invisible dysplasia at the time of random biopsies, confirming the presence of dysplasia with a pathologist experienced in IBD is recommended, as there may be significant interobserver variability in the diagnosis of IBD-associated dysplasia [74]. In this setting of detecting invisible dysplasia, there is some concern for the presence of a synchronous CRC. A prior systematic review including surveillance studies of patients found to have invisible LGD showed that 22 % of patients who underwent colectomy for LGD had CRC [75]. The risk of synchronous CRC in the setting of invisible high-grade dysplasia (HGD) has been reported to be even higher, with rates of CRC ranging from 45 to 67 % [10, 29, 76, 77]. However, it is possible that many of invisible dysplasia detected in prior studies would have been visible with current availability of high-definition white-light endoscopy and chromoendoscopy. Thus, recent guidelines recommend referral for chromoendoscopy with high-definition endoscopy by an experienced provider as the next step in evaluation after detection of invisible HGD or LGD [7]. Random biopsies should also be considered at the time of chromoendoscopy to assess and confirm invisible dysplasia [57]. If detectable lesions are present on chromoendoscopy, resection and further surveillance can be considered as described above. If no dysplasia is detected on random biopsies, then discussions regarding the risks and benefits of further surveillance versus proctocolectomy should be initiated with the patient.
In the setting of persistent endoscopically invisible dysplasia, the grade of dysplasia may play a role in whether patients should be referred for colectomy versus remain in a surveillance program. In a study of 46 patients with flat LGD, a finding of focal LGD was a predictor for future progression to HGD or CRC [78]. In a recent study including patients with both visible and flat dysplasia detected on random biopsy, the overall frequency of progression of LGD to advanced neoplasia was low, although flat dysplasia in the distal colon was associated with a greater risk of progression [79].
Pouch Surveillance
For patients who have had ileal pouch anal anastomosis (IPAA) surgery, the development of dysplasia in either the ileal pouch mucosa or anorectal mucosa has been reported, although appears to be rare [80]. Potential risk factors for dysplasia post-IPAA surgery include a history of PSC, a history of dysplasia or CRC, refractory pouchitis, and those with atrophic pouch mucosa with severe inflammation [81]. In a recent study of 1200 patients with IBD and prior IPAA, 1.8 % developed pouch neoplasia, with 1.3 % developing adenocarcinoma [82]. In that study, a prior history of CRC was associated with a 25-fold increase in risk of developing pouch neoplasia. Given these risk factors, patients with a history of prior CRC, PSC, or refractory pouchitis should be considered for annual surveillance, with biopsies being obtained in the pouch as well as distally within the anal transition zone [57]. The ideal interval/need for continued surveillance for cancer developing in the pouch for patients without risk factors following an IPAA is unknown.
Chemoprevention
Currently, no therapeutic agents have been firmly established as having a chemopreventive effect in either delaying or inhibiting carcinogenesis in IBD. A pooled analysis of nine observational studies showed a protective association between 5-aminosalicylate (5-ASA) use and risk of CRC [16]. However, a more recent systematic review of nonreferral studies did not support a protective effect of 5-ASA on risk of CRC [83]. In a prospective observational study, patients with long-standing extensive colitis who received thiopurines were less likely to develop HGD or CRC [24]. However, a recent meta-analysis of 15 studies did not show a protective effect of thiopurines on the risk of colorectal neoplasia [84]. Data on the chemopreventive effect of tumor necrosis alpha antagonists are also conflicting [23]. Ursodeoxycholic acid, through reduction in the colonic concentration of potentially carcinogenic bile acids as well as through its antioxidant activity, has been shown to potentially reduce the risk of CRC in patients with UC who also have a diagnosis of PSC [1, 85]. Finally, although aspirin and other nonsteroidal anti-inflammatory medications have a chemopreventive effect in prevention of sporadic CRC [86], there are limited data in whether a similar effect is present in patients with IBD.
Conclusions and Future Research
Patients with UC who have at least left-sided colitis, as well as Crohn’s colitis involving more than 1 segment (1/3) of the colon, should undergo surveillance colonoscopy given an increased risk of CRC. Although the risk of CRC appears to be decreasing based on recent population-based studies, risk factors such as extensive colitis with moderate to severe inflammation, family history of CRC in a first-degree relative less than 50 years old, history of a stricture or prior dysplasia, multiple post-inflammatory polyps, and a history of PSC should warrant more frequent surveillance. Recent data have suggest that the majority of dysplastic lesions are visible, and thus there is a growing role for chromoendoscopy with targeted biopsies versus high-definition white-light colonoscopy with random biopsies, provided that appropriate expertise in chromoendoscopy is available. For visible dysplastic lesions, complete endoscopic resection followed by close surveillance is an option.
Future studies clarifying the risk of CRC in patients with nonpolypoid dysplasia are needed, as the definition of whether these lesions can be completely endoscopic will continue to evolve. Additionally, the role and optimal method of chromoendoscopy need to be further clarified in order to help determine the optimal surveillance strategy in the future.
References
Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–774.
Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745.
Itzkowitz SH, Present DH, Crohn’s, Colitis Foundation of America Colon Cancer in IBDSG. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–321.
Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558–565.
Marion JF, Waye JD, Present DH, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342–2349.
Gunther U, Kusch D, Heller F, et al. Surveillance colonoscopy in patients with inflammatory bowel disease: comparison of random biopsy vs. targeted biopsy protocols. Int J Colorectal Dis. 2011;26:667–672.
Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–651 e28.
Rosenqvist H, Ohrling H, Lagercrantz R, Edling N. Ulcerative colitis and carcinoma coli. Lancet. 1959;1:906–908.
Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–2729.
Friedman S, Rubin PH, Bodian C, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn’s colitis: results of a surveillance program spanning 25 years. Clin Gastroenterol Hepatol. 2008;6:993–998. (quiz 53-4).
Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592.
Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535.
Jess T, Simonsen J, Jorgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–381 e1. quiz e13-4.
Manninen P, Karvonen AL, Huhtala H, et al. The risk of colorectal cancer in patients with inflammatory bowel diseases in Finland: a follow-up of 20 years. J Crohn’s Colitis. 2013;7:e551–e557.
Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789–799.
Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–1353.
Baars JE, Looman CW, Steyerberg EW, et al. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol. 2011;106:319–328.
van Schaik FD, van Oijen MG, Smeets HM, van der Heijden GJ, Siersema PD, Oldenburg B. Thiopurines prevent advanced colorectal neoplasia in patients with inflammatory bowel disease. Gut. 2012;61:235–240.
Loftus EV Jr. Epidemiology and risk factors for colorectal dysplasia and cancer in ulcerative colitis. Gastroenterol Clin North Am. 2006;35:517–531.
Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233.
Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clinical Gastroenterol Hepatol. 2012;10:639–645.
Baars JE, Kuipers EJ, van Haastert M, Nicolai JJ, Poen AC, van der Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J Gastroenterol. 2012;47:1308–1322.
Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–1452.
Beaugerie L, Svrcek M, Seksik P, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166–175 e8.
Greenstein A, Sachar D, Smith H, et al. Cancer in universal and left-sided ulcerative colitis: factors determining risk. Gastroenterology. 1979;77:290–294.
Gyde S, Prior P, Allan R, et al. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29:206–217.
Soderlund S, Brandt L, Lapidus A, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561–1567. (quiz 818-9).
Lutgens MW, Vleggaar FP, Schipper ME, et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246–1251.
Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038.
Askling J, Dickman PW, Karlen P, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356–1362.
Nuako KW, Ahlquist DA, Mahoney DW, Schaid DJ, Siems DM, Lindor NM. Familial predisposition for colorectal cancer in chronic ulcerative colitis: a case-control study. Gastroenterology. 1998;115:1079–1083.
Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497–508.
Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404–1408.
Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54.
Vera A, Gunson BK, Ussatoff V, et al. Colorectal cancer in patients with inflammatory bowel disease after liver transplantation for primary sclerosing cholangitis. Transplantation. 2003;75:1983–1988.
Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. (quiz 340-1).
Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459.
Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601–1608 e1–4.
Lashner BA, Turner BC, Bostwick DG, Frank PH, Hanauer SB. Dysplasia and cancer complicating strictures in ulcerative colitis. Dig Dis Sci. 1990;35:349–352.
Gumaste V, Sachar D, Greenstein A. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992;33:938–941.
Lutgens M, Vermeire S, Van Oijen M, et al. A rule for determining risk of colorectal cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:148–154 e1.
Rutter M, Saunders B, Wilkinson K, et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813–1816.
Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968.
Rubin DT, Turner JR. Surveillance of dysplasia in inflammatory bowel disease: the gastroenterologist–pathologist partnership. Clin Gastroenterol Hepatol. 2006;4:1309–1313.
Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648.
Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. (quiz 24).
Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohn’s Colitis. 2013;7:1–33.
Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666–689.
Nugent FW, Haggitt RC, Gilpin P. Cancer surveillance in ulcerative colitis. Gastroenterology. 1991;100:1241–1248.
Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–153.
Karlen P, Kornfeld D, Brostrom O, Lofberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711–714.
Lutgens MW, Oldenburg B, Siersema PD, et al. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. Br J Cancer. 2009;101:1671–1675.
Löfberg R, Broström O, Karlén P, Tribukait B, Ost A. Colonoscopic surveillance in long-standing total ulcerative colitis—a 15-year follow-up study. Gastroenterology. 1990;99:1021–1031.
Ananthakrishnan AN, Cagan A, Cai T, et al. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2015;13:322–329 e1.
Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohn’s Colitis. 2013;7:982–1018.
Centre for Clinical Practice at NICE (UK). Colonoscopic Surveillance for Prevention of Colorectal Cancer in People with Ulcerative Colitis, Crohn's Disease or Adenomas. London: National Institute for Health and Clinical Excellence (UK); 2011. (NICE Clinical Guidelines, No. 118). Available from http://www.ncbi.nlm.nih.gov/books/NBK82209/. Accessed 1 Apr 2015.
Committee ASoP, Shergill AK, Lightdale JR, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81:1101–1121 e13.
Blonski W, Kundu R, Lewis J, Aberra F, Osterman M, Lichtenstein GR. Is dysplasia visible during surveillance colonoscopy in patients with ulcerative colitis? Scand J Gastroenterol. 2008;43:698–703.
Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998–1004.
Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334–339.
van den Broek FJ, Stokkers PC, Reitsma JB, et al. Random biopsies taken during colonoscopic surveillance of patients with longstanding ulcerative colitis: low yield and absence of clinical consequences. Am J Gastroenterol. 2014;109:715–722.
Soetikno R, Subramanian V, Kaltenbach T, et al. The detection of nonpolypoid (flat and depressed) colorectal neoplasms in patients with inflammatory bowel disease. Gastroenterology. 2013;144:1349–1352. 52 e1-6.
Picco MF, Pasha S, Leighton JA, et al. Procedure time and the determination of polypoid abnormalities with experience: implementation of a chromoendoscopy program for surveillance colonoscopy for ulcerative colitis. Inflamm Bowel Dis. 2013;19:1913–1920.
Mooiweer E, van der Meulen-de Jong AE, Ponsioen CY, et al. Chromoendoscopy for surveillance in inflammatory bowel disease does not increase neoplasia detection compared with conventional colonoscopy with random biopsies: results from a large retrospective study. Am J Gastroenterol. 2015;110:1014–1021.
Konijeti GG, Shrime MG, Ananthakrishnan AN, Chan AT. Cost-effectiveness analysis of chromoendoscopy for colorectal cancer surveillance in patients with ulcerative colitis. Gastrointest Endosc. 2014;79:455–465.
Shergill AK, Farraye FA. Toward a consensus on endoscopic surveillance of patients with colonic inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2014;24:469–481.
Blackstone MO, Riddell RH, Rogers B, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366–374.
Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857.
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–S43.
Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis. Gastroenterology. 1999;117:1288–1294. (discussion 488-91).
Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004;2:534–541.
Vieth M, Behrens H, Stolte M. Sporadic adenoma in ulcerative colitis: endoscopic resection is an adequate treatment. Gut. 2006;55:1151–1155.
Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909–1918.
Odze RD, Goldblum J, Noffsinger A, Alsaigh N, Rybicki LA, Fogt F. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod Pathol. 2002;15:379–386.
Thomas T, Abrams KA, Robinson RJ, Mayberry JF. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007;25:657–668.
Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934–944.
Hata K, Watanabe T, Kazama S, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br J Cancer. 2003;89:1232–1236.
Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311–1319.
Navaneethan U, Jegadeesan R, Gutierrez NG, et al. Progression of low-grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J Crohn’s Colitis. 2013;7:e684–e691.
Borjesson L, Willen R, Haboubi N, Duff SE, Hulten L. The risk of dysplasia and cancer in the ileal pouch mucosa after restorative proctocolectomy for ulcerative proctocolitis is low: a long-term term follow-up study. Colorectal Dis. 2004;6:494–498.
Liu ZX, Kiran RP, Bennett AE, Ni RZ, Shen B. Diagnosis and management of dysplasia and cancer of the ileal pouch in patients with underlying inflammatory bowel disease. Cancer. 2011;117:3081–3092.
Derikx LA, Kievit W, Drenth JP, et al. Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology. 2014;146:119–128 e1.
Nguyen GC, Gulamhusein A, Bernstein CN. 5-aminosalicylic acid is not protective against colorectal cancer in inflammatory bowel disease: a meta-analysis of non-referral populations. Am J Gastroenterol. 2012;107:1298–1304. (quiz 7, 305).
Jess T, Lopez A, Andersson M, Beaugerie L, Peyrin-Biroulet L. Thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:1793–1800 e1.
Pardi DS, Loftus EV Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893.
Arber N, Levin B. Chemoprevention of colorectal neoplasia: the potential for personalized medicine. Gastroenterology. 2008;134:1224–1237.
Author contribution
Feuerstein and Sengupta studied and designed the concept; Feuerstein, Sengupta, and Yee contributed to the literature review; Sengupta, Feuerstein, and Yee wrote and critically revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Sengupta, N., Yee, E. & Feuerstein, J.D. Colorectal Cancer Screening in Inflammatory Bowel Disease. Dig Dis Sci 61, 980–989 (2016). https://doi.org/10.1007/s10620-015-3979-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3979-z