Abstract
Background
Gut inflammation is prevalent in chronic kidney disease (CKD) and likely contributes to systemic inflammation via disruption of the epithelial tight junction with subsequent endotoxin and bacterial translocation.
Aims
To study the expression profile of inflammatory and tight junction proteins in the colon from CKD rats compared to healthy controls, and demonstrate the role of Nrf2 (transcription factor nuclear factor erythroid 2-related factor 2) using a potent Nrf2 activator.
Methods
CKD was induced via 5/6 nephrectomy in Sprague–Dawley rats, and dh404 (2 mg/kg/day) was used to study the effects of systemic Nrf2 activation. The experimental groups included sham, CKD and CKD+ dh404 rats. Blood and colon tissues were analyzed after a 10-week study period.
Results
Colon from CKD rats showed histological evidence of colitis, depletion of epithelial tight junction proteins, significant reduction of Nrf2 and its measured target gene products (NQO1, catalase, and CuZn SOD), activation of NFkB, and upregulation of pro-inflammatory molecules (COX-2, MCP-1, iNOS, and gp91phox). Treatment with dh404 attenuated colonic inflammation, restored Nrf2 activity and levels of NQO1, catalase and CuZn SOD, decreased NFkB and lowered expression of COX-2, MCP-1, iNOS, and gp91phox. This was associated with restoration of colonic epithelial tight junction proteins (occludin and claudin-1).
Conclusions
CKD rats exhibited colitis, disruption of colonic epithelial tight junction, activation of inflammatory mediators, and impairment of Nrf2 pathway. Treatment with an Nrf2 activator restored Nrf2 activity, attenuated colonic inflammation, and restored epithelial tight junction proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impairment of the intestinal barrier (“leaky gut”) is now increasingly recognized as a pathogenic mechanism in a number of chronic non-infectious diseases including heart failure, nonalcoholic fatty liver disease, diabetes mellitus, and fibromyalgia [1–3]. Almost 30 years ago, Vaziri et al. [4] showed the presence of inflammation throughout the gastrointestinal tract (including gastritis, enteritis, and colitis) in a postmortem analysis of 78 hemodialysis patients. Recent studies by our group have demonstrated disruption of the epithelial tight junction throughout the gastrointestinal tract and its association with endotoxemia, local and systemic inflammation, and oxidative stress in animal models of CKD [5–7]. Subsequent in vitro studies revealed the role of urea and its conversion to ammonia and ammonium hydroxide as a main cause of the breakdown of the gut epithelial tight junction [8, 9]. In addition, the intestinal wall inflammation can lead to disruption of the epithelial barrier structure by retraction and endocytosis of the transcellular tight junction proteins, claudins and occludin [10–12]. Disruption of the epithelial barrier structure and function in turn amplifies local and systemic inflammation by allowing translocation of bacterial toxins and other noxious products into the intestinal wall and systemic circulation. Indeed, inflammation in the CKD population is frequently associated with endotoxemia without signs of clinical infection [13, 14], and recent papers highlight bacterial translocation and its association with systemic inflammation in end-stage renal disease (ESRD) patients maintained on hemodialysis [15, 16]. Serum pro-inflammatory cytokine levels (IL-1, TNF-α, IL-6, IL-13) and circulating endotoxin are significantly associated with increased mortality in hemodialysis patients [17–19], and systemic inflammation is strongly associated with comorbidities including cardiovascular disease, anemia, and malnutrition [20–22].
Intestinal wall inflammation and barrier dysfunction in ESRD patients and CKD rats are accompanied by marked alteration of the gut microbiome [23] and dominance of urease-possessing bacterial families [24]. Heavy influx of urea in the gut lumen together with bacterial urease in humans and animals with CKD facilitates generation of ammonia and ammonium hydroxide [25, 26] and the erosion of the epithelial tight junction and local inflammation [9]. Local inflammation, in turn, amplifies disruption of epithelial tight junction by promoting endocytosis of claudins and occludin as mentioned above.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that plays a central role in the regulation of antioxidant and phase 2 detoxifying enzymes and related proteins [27, 28]. In the cytoplasm, Keap1 (Kelch-like ECH-associated protein 1) binds to Nrf2 and facilitates its ubiquitination and thereby prevents its translocation to the nucleus. Increase in intracellular reactive oxygen species enhances nuclear translocation of Nrf2 and expression of its target genes by modifying the reactive cysteine residues on Keap1, thus limiting its ability to bind Nrf2 [29, 30]. Oxidative stress and inflammation in CKD are associated with marked elevation of Keap1 abundance, impaired Nrf2 activity, and significant down-regulation of Nrf2 target gene products in the kidney and vascular tissues [31, 32]. The renal protective effect of Nrf2 is supported by the fact that Nrf2 knockout mice develop lupus-like autoimmune nephritis [33] and show intensified oxidative stress with accelerated renal injury when subjected to experimental diabetes mellitus [34]. Further, Nrf2-deficient mice subjected to ischemic and nephrotoxic insults show impaired upregulation of antioxidant pathways, with more severe acute kidney injury and higher mortality, compared to wild-type controls [35]. Finally, recent studies have demonstrated the salutary effects of a low dose of Nrf2 inducer dh404 on oxidative stress, kidney fibrosis, endothelial dysfunction, and vascular abnormalities in CKD animals [36, 37]. However, to our knowledge, the impact of CKD on the intestinal Nrf2 pathway and the effect of Nrf2 inducers on CKD-associated gut inflammation and barrier disruption have not previously been described.
Given the contribution of gut pathology to systemic inflammation, the central role of Nrf2 in defense against oxidative stress and inflammation, and recent demonstration of renal and vascular Nrf2 deficiency in CKD, we examined the Nrf2 pathway in intestinal tissue and the response to a potent Nrf2 inducer in CKD rats.
Methods
Experimental Groups
Male Sprague–Dawley rats were purchased from Harlan Sprague–Dawley (Indianapolis, IN). They were randomized to undergo sham operation (controls) or 5/6 nephrectomy (CKD) by resection of both poles of the decapsulated left kidney, followed by right total nephrectomy 5 days later. Surgeries were performed under general anesthesia using intraperitoneal injection of ketamine/xylazine. Experimental groups were as follows: sham controls (CTL, n = 5), vehicle-treated CKD (n = 8), and CKD treated with Nrf2 activator dh404 (CKD + dh404, n = 8). Tail blood pressure was measured at 6 weeks. All experiments were approved by the University of California, Irvine Institutional Committee for the Use and Care of Experimental Animals.
The Nrf2 activator analog synthetic triterpenoid dh404 (2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-9,11-dihydro-trifluoroethyl amide or CDDO-dhTFEA) was obtained from Reata Pharmaceuticals (Irving, TX). dh404 interrupts Keap1-mediated Nrf2 ubiquitination by saturating the binding capacity of Keap1, thus facilitating increased Nrf2 translocation to the nucleus [38]. The drug was dissolved in sesame oil and administered by daily oral gavage (2 mg/kg/day) for 10 weeks. The given dh404 dosage was selected by conducting a series of preliminary dose response experiments using doses ranging 0.5–20 mg/kg/day. We found significant weight loss, marked increase in proteinuria, and worsening of kidney pathology in animals treated with doses between 5 and 20 mg/kg, but significant improvements were noted at doses of 1–2 mg/kg.
Tissue Harvest and Blood Chemistries
Rats were killed after 10 weeks by exsanguination using cardiac puncture under general anesthesia. The ascending and descending colon were harvested and processed for Western blot analysis, and histological and immunohistological assays. Blood urea nitrogen (BUN) and creatinine were measured using kits from Bioassay Systems (Hayward, CA).
Western Blot Analyses
Colon segments were homogenized on ice in modified radioimmunoprecipitation assay lysis buffer containing 25 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1 % NP-40, 0.1 % sodium dodecyl sulfate, 1 mM phenylmethylsulfonyl fluoride and Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO). Cellytic™ Nuclear Extraction Kit (NXTRACT-1KT, Sigma-Aldrich) was used for isolation of nuclear proteins. Protein concentration in the tissue homogenates was determined by bovine serum albumin assay kit (Pierce Rockford, IL) and 30–50 μg of total protein per sample was fractionated on 4–12 % Bis–Tris gradient gel (Invitrogen, Carlsbad, CA) at 120 V for 2 h and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with 5 % nonfat dry milk solution in Tris–buffered saline with 0.05 % Tween, then incubated with primary antibodies at the following concentrations: COX-2 @ 3 μg/ml (160126, Cayman Chemical, Ann Arbor, MI), MCP-1 @ 1 μg/ml (5225-100, BioVision, Milpitas, CA), heme oxygenase-1 @ 0.5 μg/ml (H4535, Sigma), catalase @ 1 μg/ml (C0979, Sigma), Keap1 @ 1 μg/ml (AV34728, Sigma), iNOS @ 8 μg/ml (PA1-036, Thermo Scientific, Waltham, MA), gp91phox @ 0.25 μg/ul (611415, BD Transduction Laboratories, San Jose, CA), CuZn SOD @ 33.5 μg/ml (574597, Calbiochem/EMD Millipore, Billerica, MA), and IkB alpha (phospho S32 + S36) @ 1 μg/ml (ab12135, Abcam). GCLC and NQO1 antibodies were purchased from Sigma. Primary antibodies against tight junction proteins included claudin-1, occludin and anti-ZO-1 (Invitrogen) at 1:250 dilutions. Beta-actin antibody (Sigma-Aldrich) at 1:10,000 was used to standardize the data. Nuclear lysates were probed for NFkB-p65 @ 1 μg/ml (SAB4502610, Sigma) and Nrf2 @ 1 μg/ml (SAB4501984, Sigma) with histone H3 @ 1:1,000 dilution (ab1791, Abcam) for standardization. The appropriate horseradish peroxidase-conjugated secondary antibodies (Sigma-Aldrich) were used at a 1:5,000 dilution. The membrane was visualized with SuperSignal West Pico (Pierce) and developed by autoluminography. Band densities were quantified using the free ImageJ software (version 10.2) from the National Institutes of Health (www.imagej.nih.gov/ij/).
Histopathological Analysis
Paraffin sections of rat colon tissue were deparaffinized with xylene, dehydrated in alcohol series, stained with hematoxylin and eosin (H&E), and examined under a photomicroscope (Nikon Eclipse, Japan). For claudin-1 immunohistochemistry, paraffin sections of rat colon were deparaffinized with xylene, and antigens were unmasked using sodium citrate buffer to boil for 10 min and then cooled down to room temperature. Endogenous peroxidase activity was removed using 3 % hydrogen peroxide in water, and blocked with Protein Block Serum-Free solution (Dako North America, Inc. Carpinteria, CA). The sections were incubated overnight at 4 °C with primary antibody (rabbit claudin-1 at 1:50, Invitrogen). Antibody binding was amplified using ImmPRESS™ REAGENT Anti-Rabbit Ig kit (Vector laboratories, Inc., Burlingame, CA), and the was complex visualized using diaminobenzidine (DAB). Nuclei were lightly stained with Mayer’s hematoxylin.
Data Analysis
Data are presented as mean ± SE of the mean. One-way ANOVA was used with Tukey’s post hoc analysis, and P values less than 0.05 were considered significant.
Results
General Data
The experimental timeline is summarized in Fig. 1. General data are summarized in Table 1. As expected, the untreated CKD animals exhibited significant increase in plasma urea and creatinine concentrations, and significant elevation of arterial blood pressure. Long-term dh404 treatment normalized arterial pressure and lowered plasma urea and creatinine in the treated CKD group.
Histological Findings
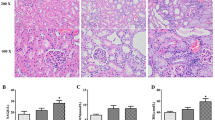
Histological examination of colonic tissues revealed increased wall thickness and accumulation of mononuclear leukocytes in the lamina propria and microvilli in the untreated CKD group (Fig. 2), confirming previous findings in CKD rats [5] and ESRD patients [4]. Colon wall inflammation was significantly reduced in the dh404-treated CKD rats.
Hematoxylin/eosin (H&E) staining of colon tissue. Representative micrograph is shown from each experimental group, with 20X objective. There was wall thickening and increased leukocyte infiltration (indicated by arrow) in the CKD group. These features of inflammation were reduced in the CKD + dh404 group
Inflammatory and Oxidative Stress Markers
Data are shown in Fig. 3. Nrf2 content in colon nuclear lysates from untreated CKD rats was significantly decreased and was restored by dh404 treatment (Fig. 3a). The suppressor protein Keap1 was over-expressed in CKD colon and was decreased with dh404 therapy (P < 0.05, data not shown). Detoxifying enzymes regulated by the Nrf2 pathway such as catalytic glutamate–cysteine ligase (GCLC, key enzyme in glutathione synthesis), and NQO1 were increased with dh404 treatment (Fig. 3a). Likewise, the key antioxidant enzymes catalase and CuZn SOD were decreased in untreated CKD rats and were partially restored with Nrf2 activator therapy. However, the difference did not reach statistical significance (data not shown). Tissue levels of phosphorylated IkB alpha were significantly elevated in CKD rats, corresponding with dissociation of inhibitory phospho-IkB from NFkB and increased nuclear translocation of NFkB-p65 (Fig. 3b). Phospho-IkB alpha levels were lowered in dh404-treated CKD rats (P = 0.02). NFkB-p65 content was significantly increased in colonic tissue of the untreated CKD rats and was reduced with dh404 therapy (P = 0.03). This highlights the emerging interplay between the Nrf2 and NFkB pathways [31, 39].
Representative Western blots depicting protein abundance of key signaling and inflammatory mediators in the colon from sham control (CTL), chronic kidney disease (CKD) and CKD + dh404 groups. a Nuclear Nrf2 levels were significantly increased in CKD + dh404 rats. Detoxifying enzymes downstream of Nrf2 signaling such as NAD(P)H quinone oxidoreductase (NQO1) and catalytic subunit of glutamate–cysteine ligase (GCLC) were increased in the colonic tissue from dh404-treated CKD rats. b Phosphorylated IkB alpha was significantly increased in CKD, indicating increased clearance of this inhibitor of NFkB (and thus increased translocation of NFkB to the nucleus). Levels of phosphorylated IkB alpha were decreased with Nrf2 treatment, to levels equivalent to controls. NFkB was over-expressed in CKD rats and was lowered with Nrf2 activator dh404 treatment. c iNOS, COX-2, and MCP-1 were significantly decreased by dh404 treatment. Data are mean ± SEM, *P < 0.05 versus CKD group, # P < 0.05 versus controls, n = 3–6 animals per group
Inducible nitric oxide synthase (iNOS), MCP-1 (monocyte chemotactic protein 1), and COX-2 were elevated in untreated CKD rats and were significantly decreased with Nrf2 activator treatment (Fig. 3c). Two bands were detected for COX-2, suggesting splice variants versus different glycosylated forms of the enzyme.
Tight Junction Proteins
Data are shown in Fig. 4. In confirmation of earlier studies [5, 6], the key components of the tight junction apparatus, i.e., zona occludens-1 (ZO-1), occludin and claudin-1 were significantly depleted in the colons of untreated CKD group. We previously reported that mRNA levels of these proteins were unchanged or elevated in CKD, suggesting posttranscriptional modification [5]. Tight junction protein levels were increased with dh404 treatment. Indeed, for occludin and claudin-1, levels were restored to values found in the control group.
a Representative Western blots depicting tight junction protein levels in the colon from sham (CTL), CKD and CKD + dh404 rats. Zona occludens 1 (ZO-1), occludin, and claudin-1 were decreased in CKD. The Nrf2 activator dh404 significantly improved tight junction protein expression compared to non-treated rats. *P < 0.05 versus CKD group, # P < 0.05 versus sham group, n = 3 animals per group. b Tissue immunostaining for claudin-1 showing depletion in CKD rat colon (×40 objective). Levels were restored with Nrf2 activator treatment and were equivalent to sham controls
Discussion
In this study, CKD was associated with colon inflammation as evidenced by heavy leukocyte infiltration on histology, with upregulation of inflammatory and oxidative stress mediators including COX-2, MCP-1, and iNOS. Nuclear levels of Nrf2 were decreased in CKD and corresponded with increased nuclear levels of the pro-inflammatory transcription factor NFkB. Treatment with the Nrf2 activator dh404 resulted in the reduction of inflammatory mediators and improvement of histological abnormalities in CKD rat colon. Dh404 therapy also resulted in lesser severity of CKD, with lower plasma urea and creatinine values; dh404 has previously been reported to reduce kidney fibrosis and inflammation in CKD rats [37]. Beneficial systemic effects included amelioration of arterial hypertension. Consistent with prior work [5], tight junction proteins ZO-1, occludin, and claudin-1 were depleted in the CKD colon. Expression levels of all three tight junction constituents were significantly increased with dh404 therapy, in tandem with decreased colonic inflammation.
Similar to previous reports in the remnant CKD kidney and aorta [31, 36], the colon from CKD rats showed inappropriately increased Keap1 levels in the setting of increased inflammation. This suggests that imbalance of the Nrf2 and NFkB pathways is likely a systemic occurrence in CKD. Treatment with dh404 reduced NFkB activity and inflammatory mediators and ameliorated depletion of the tight junction proteins. Earlier studies conducted by our group demonstrated the role of urea-derived ammonia and ammonium hydroxide generated by microbial urease in the gut lumen as a major cause of the disruption of intestinal epithelial tight junction [9], and its partial restoration by oral adsorbent [7]. The other potential mechanism known to cause tight junction breakdown is intestinal inflammation which leads to endocytosis of claudins and occludin as seen in inflammatory bowel diseases [12, 40]. The results of the present study have identified the role of impaired Nrf2 system as another cause of the breakdown of the intestinal epithelial tight junction in CKD. It appears that the rise in urea concentration in body fluids leads to its heavy influx into the intestinal tract and subsequent hydrolysis by microbial urease, triggering breakdown of the tight junction apparatus. The latter in turn triggers local inflammation by enabling the influx of endotoxin and other luminal noxious contents into the submucosal tissue. This inflammation amplifies disruption of the epithelial barrier structure and function, forming a vicious circuit.
Intestinal tight junction breakdown with subsequent bacterial or endotoxin translocation is gaining recognition as a major source of systemic inflammation. Prior histological studies in CKD rats demonstrated increased penetration of bacteria across the intestinal wall and their detection in mesenteric lymph nodes [41]. More recently, using genomic DNA amplification techniques, Wang and colleagues demonstrated increased bacterial translocation from the gut into mesenteric lymph nodes, liver and spleen in CKD rats, which was associated with increased serum interleukin-6 and C-reactive protein [42]. McIntyre et al. reported increasing levels of circulating bacterial endotoxin/lipopolysaccharide with progressive stages of CKD, with levels being highest in dialysis patients [43]. Given the accumulating evidence that gut bacterial translocation is associated with systemic inflammation in ESRD patients [15, 16], and that the latter is an independent predictor of increased mortality in hemodialysis patients [17–19], studies that advance the understanding of gut inflammation are needed as interventions in this area may beneficially impact clinical outcomes. The phase 3 clinical trial of bardoxolone methyl (potent systemic Nrf2 activator) in diabetic CKD patients was terminated early due to unforeseen cardiovascular events in the treatment arm [44], highlighting the need to explore tissue-specific targets for restoration of Nrf2 activity.
In summary, CKD-induced colonic inflammation and disruption of the epithelial tight junction is associated with impaired Nrf2 system. Oral administration of the Nrf2 activator dh404 at a dose of 2 mg/kg/day in CKD rats decreased colon inflammation and restored epithelial tight junction protein levels, ameliorated arterial hypertension, and improved plasma markers of kidney function.
References
Sandek A, Rauchhaus M, Anker SD, von Haehling S. The emerging role of the gut in chronic heart failure. Curr Opin Clin Nutr Metab Care. 2008;11:632–639.
Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691–701.
Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904.
Vaziri ND, Dure-Smith B, Miller R, Mirahmadi MK. Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am J Gastroenterol. 1985;80:608–611.
Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant. 2012;27:2686–2693.
Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol. 2013;38:99–103.
Vaziri ND, Yuan J, Khazaeli M, Masuda Y, Ichii H, Liu S. Oral activated charcoal adsorbent (AST-120) ameliorates chronic kidney disease-induced intestinal epithelial barrier disruption. Am J Nephrol. 2013;37:518–525.
Vaziri ND, Goshtasbi N, Yuan J, et al. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol. 2012;36:438–443.
Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37:1–6.
Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857.
Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778.
Shen L, Turner JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–G582.
Gonçalves S, Pecoits-Filho R, Perreto S, et al. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21:2788–2794.
Szeto CC, Kwan BC, Chow KM, et al. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–436.
Shi K, Wang F, Jiang H, et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci. 2014;59:2109–2117.
Vaziri ND. Gut microbial translocation in the pathogenesis of systemic inflammation in patients with end-stage renal disease. Dig Dis Sci. 2014;59:2020–2022.
Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–244.
Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1507–1519.
Raj DS, Shah VO, Rambod M, Kovesdy CP, Kalantar-Zadeh K. Association of soluble endotoxin receptor CD14 and mortality among patients undergoing hemodialysis. Am J Kidney Dis. 2009;54:1062–1071.
Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538.
Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80:299–307.
Vaziri ND. Oxidative stress in uremia: nature, mechanisms, and potential consequences. Semin Nephrol. 2004;24:469–473.
Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315.
Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–237.
Bourke E, Milne MD, Stokes GS. Caecal pH and ammonia in experimental uraemia. Gut. 1966;7:558–561.
Swales JD, Tange JD, Evans DJ. Intestinal ammonia in uraemia: the effect of a urease inhibitor, acetohydroxamic acid. Clin Sci. 1972;42:105–112.
Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029.
Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036.
Kobayashi A, Kang MI, Watai Y, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229.
Uruno A, Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25:153–160.
Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662–F671.
Kim HJ, Sato T, Rodríguez-Iturbe B, Vaziri ND. Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2-related factor 2 activity in the progression of focal glomerulosclerosis. J Pharmacol Exp Ther. 2011;337:583–590.
Yoh K, Itoh K, Enomoto A, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353.
Yoh K, Hirayama A, Ishizaki K, et al. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells. 2008;13:1159–1170.
Liu M, Grigoryev DN, Crow MT, et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285.
Aminzadeh MA, Reisman SA, Vaziri ND, et al. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores endothelial function impaired by reduced Nrf2 activity in chronic kidney disease. Redox Biol. 2013;1:527–531.
Aminzadeh MA, Reisman SA, Vaziri ND, Khazaeli M, Yuan J, Meyer CJ. The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica. 2014;44:570–578.
Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One. 2009;4:e8391.
Li W, Khor TO, Xu C, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489.
Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252.
de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, Nochi RJ. Bacterial translocation in experimental uremia. Urol Res. 2004;32:266–270.
Wang F, Zhang P, Jiang H, Cheng S. Gut bacterial translocation contributes to microinflammation in experimental uremia. Dig Dis Sci. 2012;57:2856–2862.
McIntyre CW, Harrison LE, Eldehni MT, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:133–141.
Chin MP, Reisman SA, Bakris GL, et al. Mechanisms Contributing to Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus and Stage 4 Chronic Kidney Disease Treated with Bardoxolone Methyl. Am J Nephrol. 2014;39:499–508.
Acknowledgments
This study was funded by an unrestricted research grant from Reata Pharmaceuticals. WLL was supported by a Sanofi renal fellowship award.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Ling Lau and Shu-Man Liu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lau, W.L., Liu, SM., Pahlevan, S. et al. Role of Nrf2 Dysfunction in Uremia-Associated Intestinal Inflammation and Epithelial Barrier Disruption. Dig Dis Sci 60, 1215–1222 (2015). https://doi.org/10.1007/s10620-014-3428-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3428-4