Abstract
Background
CT-P13 is the first biosimilar monoclonal antibody to infliximab. However, the antibody was tested only in rheumatoid arthritis and ankylosing spondylitis, which demonstrated equivalence to the originator in efficacy, safety, and pharmacokinetic profile. Extrapolation of its efficacy and safety to other pathologies is tenuous. Interchangeability with its originator is another unclear area.
Aim
We aimed to describe the experience of CT-P13 use in inflammatory bowel disease at a tertiary center.
Methods
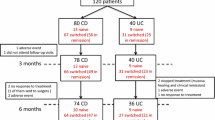
Seventeen subjects diagnosed with Crohn’s disease (CD, n = 8) or ulcerative colitis (UC, n = 9) who were administered CT-P13 from November 2012 to October 2013 at Dongguk University Ilsan Hospital were retrospectively enrolled. Medical records analyzed included patients’ characteristics, previous history of anti-tumor necrosis factor administration, response and remission to this biosimilar antibody, disease flare-up, and adverse drug reaction.
Results
Male–female ratio was 1.8. Mean age was 35.4 years (range 15–57). Mean number of CT-P13 administrations was 4.2 ± 1.9. Induction treatments were done in five UC and three CD patients. Clinical response and remission at 8 weeks were achieved in seven patients (five UC and two CD). One CD patient did not respond to CT-P13. Nine patients in maintenance with the originator were interchanged with CT-P13 (four UC and five CD patients). One UC patient experienced arthralgia and CT-P13 was discontinued. One patient experienced loss of response during the study period.
Conclusions
CT-P13 may have biosimilarity and interchangeability with its originator in inflammatory bowel disease. A large, randomized, double-blind, prospective study is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-tumor necrosis factor alpha (TNF-α) is an important treatment option for several chronic inflammatory autoimmune disease, such as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, Crohn’s disease (CD), and ulcerative colitis (UC). The introduction of biologic therapeutics for treatment of inflammatory bowel disease (IBD) has significantly improved patient outcomes [1]. However, their use is associated with much higher cost compared with traditional treatment options. Therefore, biologic therapies have caused a significant rise in the cost of therapy for IBD during the last few years [2]. For this reason, much interest in biosimilar products has developed.
A biosimilar is a biotherapeutic product similar in quality, safety, and efficacy to an already licensed reference biotherapeutic product [3]. Unlike generics, which are virtually identical copies of traditional drugs, biosimilars are not the same as the original biologic medicine. This is an inevitable outcome because biologics are made of living cells as opposed to the chemical composition of traditional drugs. When dealing with living organisms, even the slightest variation in the cell line or raw materials or even in the laboratory conditions can impact the way these medicines are created [4]. Because of unavoidable differences in the manufacturing processes, a biosimilar and its respective drug product will not be entirely identical [5].
Development of generics for small-molecule drugs has reduced prices by up to 80 % compared with their branded counterparts [6]. A recent report by the Generic Pharmaceutical Association indicates that the use of generics saved the US health care system an estimated $824 billion during the previous decade [7]. The introduction of biosimilars could reduce the drug costs for IBD by an estimated 20–30 % [8]. However, if the biosimilar molecule is not an exact copy, it could potentially lead to patient harm due to loss of response and/or adverse events.
Biosimilars may improve access to expensive biologic agents; however, concerns raised regarding their clinical use include clinical efficacy, safety, and interchangeability with the originator. Whether data from clinical trials of a certain pathology should be extrapolated to other diseases is also a matter of debate [9].
CT-P13 is the world’s first biosimilar monoclonal antibody to infliximab (INX). It is produced in the same type of cell line (Sp2/0-AG14; ATCC, Cat.CRL-1581) and has an identical amino acid sequence to INX. CT-P13 and INX have demonstrated in vitro comparability in primary pharmacodynamics in a range of studies (unpublished data from Celltrion). CT-P13 was recently approved in South Korea and Europe for all the six indications of INX. However, this biosimilar was tested only in rheumatoid arthritis and ankylosing spondylitis, which demonstrated its equivalence to the originator in efficacy, safety, and pharmacokinetic profile [10, 11]. Extrapolation of its efficacy and safety to other pathologies is tenuous. In addition, interchangeability with its originator is unclear. The present study chronicles our experience with CT-P13 in IBD patients at a tertiary center.
Methods
Patients
Seventeen subjects diagnosed with UC or CD and who were administered CT-P13 from November 2012 to October 2013 at Dongguk University Ilsan Hospital were retrospectively enrolled. We analyzed medical records including the patients’ characteristics, previous history of anti-TNF-α administration, response and remission to this biosimilar, disease flare-up, number of administrations, concomitant medication, duration of follow-up, history of surgery, and adverse drug reactions (ADRs).
Indication of CT-P13
For steroid refractory CD, patients displayed active disease despite prednisolone up to 0.75 mg/kg/day over 4 weeks [12]. For steroid dependent CD, patients were unable to reduce corticosteroids below the equivalent of prednisolone 10 mg/day (or budesonide below 3 mg/day) within 3 months of starting corticosteroids, did not have recurrent active disease, or experienced relapse within 3 months of stopping corticosteroids [12]. For steroid refractory UC, patients had active disease despite prednisolone up to 0.75 mg/kg/day over a period of 4 weeks [13]. For steroid-dependent UC, glucocorticoids could be tapered to <10 mg/day within 3 months of starting steroids, there was no recurrent disease, or relapse occurred within 3 months of stopping glucocorticoids [13]. IBD patients who were being treated with INX originator for maintenance could be interchanged with CT-P13 with their consent.
Administration of CT-P13
CT-P13 5 mg/kg was given as part of an intravenous induction regimen at 0, 2, and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks. Each CT-P13 infusion was given over approximately 2 h.
Assessment of Disease Activity
Disease activity was assessed by the Crohn’s Disease Activity Index (CDAI) [14] and the Mayo Scoring System in UC [15]. The induction group was assessed at entry, at 8 weeks and at the last outpatient visit following INX infusion. Patients in whom therapy was changed from the originator to CT-P13 were assessed at first time of originator use, first time of CT-P13 use, and at the last outpatient visit following INX infusion. CD response was defined as a >70-point decrease in CDAI [16, 17]. UC response was defined as >30 % decrease in the activity index plus a decrease in the rectal bleeding and endoscopy subscores [15]. Remission was defined as CDAI below 150 for CD [12], and for UC, it was defined as a Mayo score ≤2, with no individual subscores >1.
Mucosal healing was defined as an endoscopy subscore of 0 or 1 [18]. Loss of response was considered to have occurred after two consecutive infusions of INX of at least 5 mg/kg body weight and assessed treatment failure at 4 weeks [19]. This study was approved by the institutional review board (IRB).
Results
Baseline Characteristics
Male-female ratio was 1.8. Mean age was 35.4 years (range 15–57). The 17 patients comprised nine cases of UC (52.9 %) and eight cases of CD (47.1 %). Mean number of CT-P13 administrations was 4.2 ± 1.9 (range 1–7). Mean disease activity was 6.6 ± 4.4 (range 2–12) in UC and 83.2 ± 68.7 (range 24–230) in CD. Induction treatments were done in five UC and three CD patients. Nine patients in maintenance with originator were interchanged with CT-P13 (four UC and five CD). The characteristics of the study population are presented in Table 1.
Treatment of Infliximab Naïve Patients with CT-P13 (n = 8)
Clinical response and remission at 8 weeks were achieved in seven patients (five UC and two CD). In contrast, one CD patient did not respond to CT-P13. Four UC cases were steroid dependent, and one UC case was steroid refractory. The CD cases were individually steroid refractory, steroid dependent, and fistulating. Only one CD case did not respond to CT-P13; the patient had a history of surgery during study period. He received several adalimumab treatments before CT-P13 and changed to CT-P13 because by loss of response. There were no reports of serious or unexpected ADRs (Table 2).
For patient I-2, colonoscopy and pathologic finding confirmed ulcerative colitis, extensive. Initially, the patient received intravenous hydrocortisone 100 mg q8h for 2 weeks. Although this treatment resulted in temporary resolution of the diarrhea, she subsequently developed bloody diarrhea, occurring as often as 14 times/day, accompanied with tenesmus, crampy lower abdominal pain, and elevated temperatures. She was started on CT-P13 (5 mg/kg) monotherapy with an induction regimen of 0, 2, and 6 weeks followed by maintenance treatment every 8 weeks. She achieved a full remission, confirmed colonoscopically (Fig. 1).
Colonoscopic findings in patient I-2 before and after administration of CT-P13 administration. Colonoscopy revealed, at initial, multiple deep ulcerations spontaneous hemorrhage and mucosal edema throughout the colon and rectum. At 8 weeks, improvement of severe UC was shown. At 16 weeks, clinical remission with mucosal healing was shown
Patient I-4 was diagnosed with UC in 2007. At diagnosis, patient was placed on prednisone, orally 15 mg/day and azathioprine orally 50 mg/day as induction therapy. He had a number of flares requiring intravenous and oral steroids, having failed 5-aminosalicylic acid agents. However, within 8 months, he developed passing in excess of six stools per day, as well as presenting bloody diarrhea and abdominal pain. He had severe anemia, with repeated need for blood transfusions. Colonoscopy confirmed active ulcerative colitis. He was started on CT-P13 (5 mg/kg) as induction therapy. He achieved endoscopic response at 8 weeks, confirmed colonoscopically (Fig. 2).
Switch from Originator to CT-P13 (n = 9)
Biologic orginator included only INX, not adalimumab. Nine patients were switched to CT-P13 from the originator during the remission period. Among them, eight patients showed a similar clinical outcome compared with the originator. One CD patient experienced loss of response during the study period. The other eight patients experienced no ADR. But, one UC patient experienced arthralgia and CT-P13 was discontinued (Table 3).
Discussion
This case series reviewed our clinical experience with CT-P13, a biosimilar of INX in IBD patients at a single tertiary center. We described cases in TNF-α naïve case, but also following the switch from INX to CT-P13. To our best knowledge, this is the first report about the use of CT-P13 in IBD patients.
Randomized clinical trials have proven the efficacy of INX in moderately to severely active luminal CD and in CD with draining fistulas [20, 21]. Moreover, maintenance treatment with INX has shown that this regimen is reasonably safe and that steroid withdrawal can be achieved in the majority of patients [17, 22]. Previous clinical trials demonstrated that 5 mg/kg INX given as an intravenous induction regimen produces a clinical response rate of 81 and 33 % remission rate in moderately to severely active luminal CD [20]. Another trial reported an overall response rate in all forms of IBD of 75 and 48 % remission was in IBD patients [23]. Although presently the sample size was too small to allow statistical comparison, the efficacy of CT-P13 treatment in our study closely resembles the results of earlier controlled trials. Only one CD patient had surgery (7 weeks after induction of remission). Subtotal colectomy was performed because of communication between abscess and terminal ileum. The patient received anti-TNF-α treatment (adalimumab) prior to CT-P13 treatment. Chronic recurring periods of flare-up occurred. Thus, in this patient, it is difficult to determine the effectiveness of CT-P13. Except for this case, clinical response and remission rate for CT-P13 were similar to previous studies.
In the ACCENT 1 trial, at week 30, 39 % of members of group II (repeat infusions of 5 mg/kg INX) patients were in remission [17]. In the ACT-1 trial, 69 and 62 % of patients receiving INX 5 and 10 mg/kg, respectively, at weeks 0, 2 and, 6 displayed a clinical response at week 8, compared with 37 % of those who received placebo (P < 0.002 for both comparisons) [24]. In the ACT-2 trial, 65 and 69 % of patients receiving INX 5 mg/kg and 10 mg/kg, respectively, displayed clinical response at week 8, compared with 26 % of those who received placebo (P < 0.001 for both comparisons) [25]. In our study, clinical response and remission at 8 weeks was 87.5 % in CT-P13 treatment of INX naïve patients. No clinically meaningful difference was shown for CT-P13 and INX.
It is important to prove interchangeability between biosimilar and the reference (innovative) product. Interchangeability means that the biologic product may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product [13]. Presently, nine patients switched to CT-P13 from the originator during the remission period. Among them, eight patients (88.9 %) showed a similar clinical outcome compared with the originator. But, one patient experienced loss of response during study period. Thus, there were not significant interchangeability differences between the CT-P13 groups. From the perspective of the Food and Drug Administration, interchangeability includes the concept of switching and alternating between an innovative biologic product (R) and its follow-on biologics (T). The concept of switching is referred to as not only the switch from “R-T” or “T-R” (narrow sense of switchability) but also “T–T” and “R–R” (broader sense of switchability) [13]. As a result, biosimilarity for “R–T”, “T–R”, “T–T”, and “R–R” needs to be assessed on the basis of some biosimilarity criteria under a valid study design. Our study indicates that only a switch from “R-T” was the limiting point. Further studies need to assess the difference between the switch from T-R versus R-T, and from R-T versus T-R.
Serious adverse events concerning INX therapy include postoperative complications, serious infections, malignancies, and death [19, 26]. In a clinical trial, 500 CD patients received a median of three infusions with a median follow-up of 17 months; 8.6 % of the patients experienced a serious adverse event [27]. In our clinical experience with CT-P13 in IBD patients, no serious or unexpected ADRs were evident. Only one UC patient, in whom drug was changed to CT-P13 from its originator, experienced arthralgia. After change to the originator, ADR did not develop. But, interpretation of the finding is limited because the median follow-up of 42.5 weeks is short compared with other clinical trials.
This study has several important limitations. First, the data were retrospectively collected from a single institution. Second, there is no control group in this study. Moreover, enrolled subjects are heterogenous including both CD and UC patients. Third, the major limitation of our study was too small sample size (17 patients). Therefore, our results should be interpreted with caution. Correctly powered studies comparing the drugs are needed to indicate similar efficacy and large post-registration studies are required to address the risk of adverse events. Despite the limitations described above, our results may show meaningful information about the use of biosimilar in IBD patients.
In conclusion, this case series indicates the clinical efficacy, safety, and interchangeability of CT-P13 in the treatment of IBD compared with its originator. CT-P13 may have biosimilarity and interchangeability with its originator in IBD. Data from large, randomized, double-blind, prospective studies would be needed.
References
Rutgeerts P, Van Assche G, Vermeire S. Review article: infliximab therapy for inflammatory bowel disease—seven years on. Aliment Pharmacol Ther. 2006;23:451–463.
Norum J, Koldingsnes W, Aanes T, et al. The economic burden of TNFα inhibitors and other biologic treatments in Norway. ClinicoEconomics Outcomes Res (CEOR). 2011;3:73.
World Health Organization. WHO expert committee on biological standardization. World Health Organ Tech Rep Ser. 2013;978:1–384, back cover.
Kanase SJ, Gavhane YN, Khandekar A, et al. Biosimilar: an overview. Int J Pharm Sci Res. 2013;4:2132–2144.
Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1:457–462.
Malik NN. Controlling the cost of innovative cancer therapeutics. Nat Rev Clin Oncol. 2009;6:550–552.
Generic Pharmaceutical Association. Savings achieved through the use of generic pharmaceuticals 2000–2009. http://www.gphaonline.org/sites/default/files/GPhA%20Savings%20Study%20Book%20Updated%20Web%20FINAL%20Jul23%2010_0.pdf. Accessed 12 May 2011.
Epstein MS, Ehrenpreis ED, Kulkarni PM. Biosimilars: the need, the challenge, the future: the FDA perspective. Am J Gastroenterol. 2014. doi:10.1038/ajg.2014.151.
Weise M, Bielsky M-C, De Smet K, et al. Biosimilars: what clinicians should know. Blood. 2012;120:5111–5117.
Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–1620.
Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–1612.
Dignass A, Van Assche G, Lindsay J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohn’s Colitis. 2010;4:28–62.
Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. Washington, DC: Food and Drug Administration; 2003.
Best WR, Becktel J, Singleton J, et al. Development of a Crohn’s disease activity index. Gastroenterology. 1976;70:439–444.
D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786.
Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti–tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Travis S, Dinesen L. Remission in trials of ulcerative colitis: what does it mean? Pract Gastroenterol. 2006;30:17.
Clark M, Colombel J-F, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology. 2007;133:312–339.
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Engl J Med. 1997;337:1029–1036.
Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405.
Rutgeerts P. A critical assessment of new therapies in inflammatory bowel disease. J Gastroenterol Hepatol. 2002;17:S176–S185.
Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53:849–853.
Rutgeerts P, Feagan B, Olson A, et al. A randomized placebo-controlled trial of infliximab therapy for active ulcerative colitis: act 1 trial. Gastroenterology. 2005;128:A105.
Sandborn W, Reinisch W, Rachmilewitz D, et al. Infliximab induction and maintenance therapy for ulcerative colitis: the act 2 trial. Zeitschrift für Gastroenterologie. 2005;43:V6.
Farrell RJ, Shah SA, Lodhavia PJ, et al. Clinical experience with infliximab therapy in 100 patients with Crohn’s disease. Am J Gastroenterol. 2000;95:3490–3497.
Colombel J-F, Loftus EV Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, YS., Moon, H.H., Lee, S.E. et al. Clinical Experience of the Use of CT-P13, a Biosimilar to Infliximab in Patients with Inflammatory Bowel Disease: A Case Series. Dig Dis Sci 60, 951–956 (2015). https://doi.org/10.1007/s10620-014-3392-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3392-z