Abstract
Background
Percutaneous balloon dilation of benign biliary stricture has been the most widely used alternative to endoscopic treatment; however, the rate of recurrence has varied from 15 to 44 %. Recently, several investigators have reported that percutaneous transhepatic placement of retrievable covered stents is feasible for the treatment of benign biliary strictures. However, these studies had only a small number of patients and had short follow-up periods.
Aim
The purpose of this study was to investigate the mid-term outcomes of a retrievable covered stent for treatment of benign biliary strictures.
Methods
We retrospectively assessed 68 patients who underwent percutaneous transhepatic placement and removal of a retrievable covered stent between March 2007 and November 2012, for treatment of benign biliary strictures. Forty-two patients had not previously undergone interventional treatment, whereas 26 had recurrent or refractory strictures despite previous percutaneous procedures.
Results
Placement of the retrievable covered stents was technically successful in all patients. Stent migration occurred in 11 (16.2 %) patients. The mean indwelling period of drainage catheter and stent were 5.8 months (range, 3–22.5 months) and 3 months (range, 2–6.5 months), respectively. Clinical success was achieved in 59 (86.8 %) patients. During the mean follow-up of 36 months (range, 8.5–65 months), 12 (20 %) of 60 patients had recurrence of clinically significant strictures. The primary patency rates at 1, 2, 3, 4, and 5 years were 91, 89, 76, 68, and 68 %, respectively.
Conclusion
Mid-term outcomes suggested that percutaneous treatment of benign biliary strictures using a retrievable covered stent was a clinically effective method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benign biliary strictures have various etiologies, including anastomotic strictures after surgical bile duct repair or liver transplantation, strictures secondary to intraoperative injury, and other inflammatory strictures [1]. Without adequate and prompt management, these strictures can lead to serious consequences, including a deterioration of liver function, jaundice, cholangitis, abscess, and sepsis. However, these strictures continue to be a difficult problem to treat.
Options include surgery, endoscopic therapy, and percutaneous transhepatic approaches. Surgical revision of benign biliary strictures is still considered the best treatment, with a primary success rate of up to 90 % [2]. If strictures recur after initial surgery, 22–36 % of patients undergoing additional surgery will have recurrent strictures after each procedure. It is often difficult to manage biliary strictures that recur after surgery, and the success rates for surgical repair decrease with each successive surgical intervention [3]. Therefore, the optimal initial management for benign biliary strictures at the present time is endoscopic treatment; however, endoscopic treatment is generally considered to be impossible in patients who have previously undergone bilioenterostomy [4, 5]. In addition, endoscopic cannulation of the ampulla of Vater or tight biliary strictures is often difficult or impossible [6, 7]. In such situations, percutaneous transhepatic treatments, including balloon dilation with or without long-term catheter drainage and retrievable covered stent placement, have been suggested as possible alternatives [3–14].
Percutaneous balloon dilation of benign biliary stricture has been the most widely used alternative to endoscopic treatment; however, the rate of recurrence has varied from 15 to 44 % [7–10]. Enya et al. [11] initially reported that endoscopic placement and removal of covered metallic stent can be an option in refractory benign biliary strictures. Recently, several investigators have reported that percutaneous transhepatic placement of retrievable covered stents is feasible for the treatment of benign biliary strictures [12–16]. However, these studies had only a small number of patients and had short follow-up periods. Therefore, the purpose of this study was to investigate the mid-term outcomes of a retrievable covered stent for the treatment of benign biliary strictures.
Materials and Methods
Patient Population
This retrospective study was approved by the Institutional Review Board of our institution and written informed consent was waived. From March 2007 to November 2012, 68 patients with benign biliary strictures were enrolled. Baseline characteristics of 68 patients with benign biliary strictures are presented in Table 1. The initial diagnosis of biliary stricture was based on combinations of clinical symptoms, biochemical data, and the results of imaging using various modalities, including ultrasonography, computed tomography (CT), magnetic resonance cholangiopancreatography, and percutaneous transhepatic cholangiography. Among 68 patients with benign biliary strictures, 55 had benign postoperative strictures, four of whom had nonanastomotic strictures due to bile duct injury at laparoscopic cholecystectomy and 51 of whom had benign postoperative anastomotic strictures including non-transplant strictures (n = 33) and transplant strictures (n = 18) following living donor liver transplantation. On the basis of the configuration of the cholangiographic findings, biliary strictures were classified as simple or complex strictures, with the complex type including bifurcated or trifurcated biliary strictures.
Patients with benign biliary strictures were selected using the following inclusion criteria: (a) initially documented benign biliary strictures, (b) previously underwent bilioenterostomy (hepaticojejunostomy or choledochojejunostomy), and (c) failed endoscopic cannulation of the intrahepatic or common bile duct. Prior to percutaneous transhepatic biliary drainage (PTBD), endoscopic cannulation of biliary strictures had failed in 11 patients with duct-to-duct anastomotic transplant strictures (n = 8), extrahepatic bile duct strictures due to peripancreatic abscess (n = 2), and traumatic injury (n = 1). Forty-two patients entered the retrievable covered stent placement protocol at initial treatment of a new lesion and 26 had recurrent or refractory benign biliary strictures despite prolonged catheter interposition after balloon dilation procedures. Seventeen of 26 patients had recurrent stricture after initial successful percutaneous treatment (repeat balloon dilation and long-term catheter placement). The mean interval between the end of previous percutaneous treatment (drainage catheter removal) and recurrence was 24 months (range, 3–48 months). Nine of 26 patients were converted to the retrievable covered stent placement protocol after an initial percutaneous treatment (repeat balloon dilation and long-term catheter placement) due to refractory stricture. Patients with documented malignant biliary strictures were excluded from our study.
Technique
Our treatment protocol is summarized in Fig. 1. Experienced interventional radiologists (D.I.G., G.Y.K) performed PTBD and retrievable covered stent insertion and removal. One interventional radiologist had 10 years of experience and treated approximately 800 biliary intervention cases per year; the other had 15 years of experience and treated approximately 300 biliary intervention cases per year. PTBD and retrievable covered stent procedures including insertion and removal were performed under conscious sedation using intravenous pethidine hydrochloride (Demerol, Keukdong Pharmaceuticals, Seoul, Korea) and local anesthesia using intramuscular lidocaine (Jeil Pharmaceuticals, Taegu, Korea). Broad-spectrum antibiotics were administered intravenously 2 h before the procedures and for at least 48 h afterwards. The peripheral intrahepatic bile duct was punctured using a 21-gauge Chiba needle (Cook, Bloomington, IN) under the guidance of fluoroscopy or ultrasound. The needle was then exchanged for a 6-Fr coaxial dilator, and cholangiography was obtained to evaluate the stricture. A 0.035-in. guide wire (Radifocus Guide Wire M; Terumo, Tokyo, Japan) and a 5-F cobra or Kumpe catheter (Cook) were used to traverse the stricture. Before inserting a drainage catheter, dilation of the stricture was performed using a balloon catheter 6–8 mm in diameter (Synergy; Boston Scientific, Galway, Ireland). An internal-external 8.5-F drainage catheter (Cook) was then placed across the stricture.
A retrievable polytetrafluoroethylene (PTFE)-covered stent (Song retrievable stent; TaeWoong Medical, Kimpo, Korea) (13–15) with two drawstrings attached to the upper margin of the stent was used (Fig. 2). An 8.5-F catheter was removed over a 0.035-in. stiff hydrophilic guide wire (Cook), after which a 9 or 10-F stent introduce sheath was inserted. After the dilator was removed from the sheath, the retrievable covered stent was deployed across each stricture site. A stent diameter of 8–12 mm was chosen depending on the diameter of the bile duct and bilioenterostomy site. Two stents were placed in a single session in cases of complex strictures. Balloon dilation was performed using balloon catheter 8–10 mm in diameter (Boston) just before and after stent placement. To prevent distal migration of the stent after deployment, an 8.5–14-F multi-side-holes, pigtail-shaped drainage catheter was placed across the stent, and the catheter tip was placed just beneath the distal margin of the stent. Then, to allow internal drainage, the drainage catheter was capped. Frequent catheter irrigations were performed to remove possible blood clots or sludge on post-stenting days 1 and 2. The decision regarding the indwelling period of the stent was based on the severity of the stricture. If patients had initial benign biliary strictures, the indwelling period was 2–3 months, although if patients had recurrent or refractory strictures from the previous percutaneous treatment, the indwelling period was 3–4 months. Cholangiography was then obtained via the drainage catheter 1 month after stent placement to evaluate stent migration. If stent migration was detected, the stent was repositioned using a balloon catheter. If repositioning of the stent was unsuccessful, another stent was inserted.
“End on” view of the retrievable polytetrafluoroethylene (PTFE)-covered stent used in the procedures. A 2-mm-diameter nylon loop is hooked inside each bend of the proximal end of the stent and secured with a suture. Another nylon thread is passed through each of these small nylon loops to form a larger loop that filled the circumference of the inside of the stent. Two drawstrings made from a nylon monofilament are attached to the upper inner margin of the stent. Radiopaque markers are attached at both ends and center to aid in fluoroscopic visualization. 1 nylon loop, 2 two drawstrings, 3 radiopaque marker
For each patient, the stent was electively removed. A safety wire was placed into the small bowel or the common bile duct in order to maintain access. A 9- or 10-F sheath with a dilator was then passed over the guide wire into the proximal stent lumen. A 9-F sheath was used to remove an 8–10-mm-diameter stent, and a 10-F sheath was used to remove a 12-mm-diameter stent. After the dilator was removed from the sheath, a retrieval hook wire was introduced into the sheath to remove the stent, and the hook was pulled out of the stent so that the hook caught the drawstring. Traction on any one drawstring caused the end of the stent to pull together and collapse into a funnel configuration. When this occurred, the retrieval hook wire was withdrawn through the sheath to collapse the stent. The stent and retrieval hook wire were then pulled out of the biliary duct through the sheath (Fig. 3). Cholangiography immediately after stent removal was then obtained via the sheath to evaluate the stricture. If there was persistent stricture after stent removal, an additional retrievable covered stent placement was performed across the stricture for an additional 3 months.
A 10–14-F external drainage catheter was placed in the intrahepatic bile duct and was left in place with the tip clamped for 1 month for follow-up cholangiography. The drainage catheter was removed when a cholangiogram revealed fluent passage of contrast medium without recurrence of patient symptoms or changes in biochemical data. However, if there was recurrent stricture, a retrievable covered stent placement was performed across the stricture for an additional 3 months or one to three sessions following balloon dilation for a 1-month interval. The decision regarding the method of the additional treatment was based on clinical symptoms and liver enzyme abnormality. If there were any symptoms related to the recurrent stricture and/or liver enzyme abnormality, additional stent placement was performed. If there were no symptoms and liver enzyme abnormality, only additional balloon dilation was performed.
All patients were then followed in our radiology outpatient clinic at 1, 3, 6 and 12 months after drainage catheter removal to check for recurrence. Liver enzyme assay was also measured at this follow-up period and imaging studies including CT was performed at 6–8 months after drainage catheter removal. Thereafter, patients were followed in our radiology outpatient clinic at yearly intervals for 3 years. Beginning 3 years after drainage catheter removal, patients were interviewed by telephone at yearly intervals. Patients were advised to visit our clinic if they experienced any recurrence of symptoms.
Study Endpoints, Definitions, and Statistical Analysis
Major study endpoints included the assessment of technical success, clinical success, complications, and primary patency. Technical success was defined as successful stent placement across the stricture and stent removal without bile duct injury. Complications were classified as major and minor according to the guidelines of the Society of Interventional Radiology Standards of Practice Committee [17]. Total stent migration was defined as migration of the stent beyond the stricture segment, whereas partial stent migration was defined subjectively as the migration of the stent more than 10 mm from its original location but remaining within the stricture segment. Clinical success was defined as the disappearance of patient symptoms and normalization of chemical data or their decrease to less than 1.5 times the normal levels after drainage catheter removal. Recurrence was defined as development of clinically significant restricture based on clinical manifestation, serum biochemical tests, and imaging necessitating subsequent intervention. Primary patency was defined as the interval between the removal of the stent and the recurrence of stricture.
All statistical analyses were conducted using SPSS software (version 14.0, SPSS). Primary patency was estimated using life-table analysis according to the Kaplan–Meier method and was compared with the results of the log-rank test.
Results
Procedural Outcomes and Complications
The results are summarized in Fig. 4. Stent deployment was technically successful in all patients, and correct positioning was verified after each placement (Fig. 5). None of the stents had migrated immediately after deployment. Six patients required two stent placements because of two separated, simple-type, bilioenterostomy strictures (n = 4) and complex-type strictures (n = 2); a single stent was sufficient to treat the biliary strictures in the remaining 62 patients. However, four patients underwent a second stent placement because of total downward stent migration and 13 patients underwent a second stent (n = 10) or third stent (n = 3) placement because of recurrent or refractory strictures following removal of the initial stents. Therefore, in total, 91 stents were placed in 68 patients. Nine of the 13 patients who underwent second or third stent placement had bilioenterostomy strictures, three had transplant strictures including duct-to-duct anastomotic strictures (n = 2) and bilioenterostomy stricture (n = 1), and one had chronic cholangitic stricture. Among the 13 patients, 12 patients had simple stricture and one had complex stricture.
Flow chart of the study patients. n = number of patients. * The retrievable stent was removed 1 day after stent placement due to markedly elevated liver enzymes. † Failure of percutaneous stent removal. The stent was removed surgically. ‡ One patient who underwent living donor liver transplantation needed drainage catheter maintenance because of the progression of the recurrent hepatocellular carcinoma
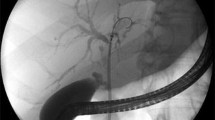
A 64-year-old man with hepaticojejunostomy stricture following pylorus-preserving pancreatoduodenectomy. The patient had refractory stricture despite 18 sessions of percutaneous balloon dilation and long-term catheter placement. a Cholangiography shows severe stricture at hepaticojejunostomy anastomosis. b A 12-mm-diameter and 6-cm-long retrievable covered stent (white arrows) was inserted across the stricture, and a 12-F pigtail catheter was inserted across the stent for follow-up cholangiography and stent removal. To prevent distal stent migration, the pigtail-shaped drainage catheter tip (black arrow) was placed just beneath the distal stent margin. c A cholangiography obtained immediately after stent removal shows patent the anastomosis. d A follow-up cholangiography obtained 4 weeks after stent removal shows the anastomosis to be patent without recurrence. The drainage catheter was removed immediately after the follow-up cholangiography. The patient remained healthy and without a recurrence for 47 months after the drainage catheter removal
In 38 of 68 patients, cholangiography immediately after stent placement revealed ipsilateral or opposite intrahepatic bile ducts occluded by the stents; the stents had been placed in situ in 37 patients because their liver enzymes were within normal range despite branching duct occlusion. However, the stent was removed in one patient 1 day after stent placement due to markedly elevated liver enzymes; the stricture was then managed using repeated balloon dilation and interposition of a 14-F drainage catheter. Procedure-related minor complications occurred in four patients (5.9 %). These four patients experienced self-limiting hemobilia after balloon dilation that was completely resolved 1–2 days later, without transfusion.
Cholangiography obtained 1 month after stent placement showed stent migration in six patients. Four of these six patients experienced total downward stent migration into the jejunum; therefore, an additional stent placement was performed. The other two patients showed partial upward stent migration; the stent was successfully repositioned using a balloon catheter.
Cholangiography obtained at the time of stent removal in 67 patients revealed total (n = 3) and partial (n = 2) downward migration of the stent. In the three patients who showed total stent migration, the stent had migrated and was evacuated from the bowel and no longer radiographically visible within the abdomen at the time planned for stent removal; however, the strictures were improved. Therefore, the drainage catheters were repositioned above the stricture to check for any recurrence. The overall stent migration rate was 16.2 % (11 of 68 patients). During the stent removal procedure in one patient, the drawstring became untied with the stent remaining in the peripheral intrahepatic bile duct, and the stent could not be removed under fluoroscopic guidance. The stent was removed surgically. Except for this one patient, stent removal was successfully achieved in the remaining 66 patients at the initial attempt using the standard stent removal technique described in the Methods sections. Cholangiography immediately after stent removal in 63 patients revealed widened stricture with free passage of contrast media. However, the remaining three patients showed significant refractory stricture. These three patients underwent a second stent placement for 3 months.
Follow-up cholangiography performed 1 month after stent removal revealed patent stricture with free passage of contrast media in 51 patients. Because the 51 patients had a normal range of liver enzymes and were without any symptoms related to the strictures, their drainage catheters were then removed. However, one patient who underwent living donor liver transplantation needed drainage catheter maintenance because of the progression of the recurrent hepatocellular carcinoma, and this patient died 6 months after stent removal. In the remaining 14 patients with recurrent strictures, seven patients needed a second session of stent placement, three of these seven patients needed a third session of stent placement because of recurrent strictures of the treated strictures, and four patients needed 1–3 sessions (mean, 2 sessions) of balloon dilation. Drainage catheters were finally removed in eight of the 14 patients. However, six patients showed refractory stricture after the second (n = 3) or third (n = 3) stent removal. In the six patients with refractory strictures, four patients had bilioenterostomy strictures, one had distal common bile duct stricture caused by traumatic injury, and one had left intrahepatic bile duct stricture caused by chronic cholangitis. Four patients who had bilioenterostomy strictures finally underwent surgical reanastomosis. One patient was converted to drainage catheter interposition and one other patient was converted to endoscopic plastic stent placement until the end of follow-up. In 59 patients with successful drainage catheter removal, there was a mean indwelling period of drainage catheter and retrievable stent of 5.8 months (range, 3–22.5 months) and 3 months (range, 2–6.5 months), respectively. In total, clinical success was achieved in 59 (86.8 %) of 68 patients following single (n = 50) or dual sessions (n = 9) of stent placement. Major complications included total stent migration needing a second stent placement in four patients, markedly elevated liver enzymes after stent placement in one patient, and failure of percutaneous stent removal in one patient. Therefore, the overall complication rate was 14.7 % (10 of 68 patients).
Follow-up and Primary Patency
Clinical follow-up until the end of this study was available for all 60 patients (59 with percutaneous catheter removal and one with surgical removal) and the cutoff date for data analysis was November 30, 2012. During the mean follow-up period of 36 months (range, 8.5–65 months), three patients died and 57 patients were still alive. In the three patients that died, death was caused by rapidly progressed multiple metastases due to pancreatic cancer recurrence at 10.5, 17.5, and 18 months after drainage catheter removal. Thirty-five patients were followed up for more than 3 years and three for less than 1 year. The remaining 22 patients were followed up between 1 and 3 years. The cumulative primary patency rates at 1, 2, 3, 4, and 5 years were 91, 89, 76, 68, and 68 %, respectively (Fig. 6). There were no significant differences in patency rates between initial and refractory stricture groups (p = 0.064), anastomotic and non-anastomotic stricture groups (p = 0.998), and transplant and non-transplant stricture groups (p = 0.855).
Twelve (20 %) of 60 patients had recurrence of clinically significant strictures at a mean of 20.8 months (range, 4–29.5 months) after drainage catheter removal. Seven of the 12 patients had bilioenterostomy strictures, three had due-to-duct anastomotic strictures, one had traumatic stricture and one had chronic cholangitic stricture. Ten of the 12 patients underwent repeated balloon dilations. The strictures were improved in eight patients for 1–3 months (mean, 1.6 months), and then drainage catheters were successfully removed. These eight patients showed no recurrence for 5–41 months (mean, 24.5 months) until the end of follow-up. In two (bilioenterostomy stricture [n = 1], chronic cholangitic stricture [n = 1]) of the ten patients, the strictures did not improve even after repeated balloon dilations; therefore, the 18-F indwelling catheters were maintained in these two patients for ten and 15 months until the end of follow-up. The remaining two (duct-to-duct anastomotic stricture [n = 2]) of the 12 patients who had recurrence underwent endoscopic balloon dilation with subsequent plastic stent placement. The stricture was improved in one patient 5 months after repeated endoscopic treatments, and this patient showed no recurrence for 50.5 months until the end of follow-up. In one patient, the stricture did not improve; therefore, repeated endoscopic treatments were maintained for 10 months until the end of follow-up.
Among the 42 patients who underwent retrievable covered stent placement for initial strictures, 32 patients (76.2 %) showed improvement of the strictures and symptoms until the end of the study period (median of 32.1 months after drainage catheter removal). Among the 26 patients who underwent retrievable covered stent placement for recurrent or refractory strictures after balloon dilation and long-term catheter placement, 16 patients (61.5 %) showed improvement of the strictures and symptoms until the end of the study period (median of 36.4 months after drainage catheter removal).
Discussion
Given the increased expansion force and diameter and the persistent dilation effect of a self-expanding metallic stent, placement of a stent theoretically may be able to increase the chances for curing benign biliary strictures. Placement of uncovered metallic stents has been attempted in patients with refractory benign biliary strictures [18–20]. The efficacy of these stents has been unsatisfactory because of the decreased long-term patency and difficulty in removal caused by hyperplastic tissue ingrowth [19, 20]. To date, a variety of covering materials, such as polyurethane, silicon, and PTFE, have been manufactured and tested to prevent tissue or tumor ingrowth. Previous investigators have reported tears in the polyurethane and silicon covering membrane during or after stent placement, which resulted in tumor ingrowth [21, 22]. Moreover, the polyurethane and silicon covering membrane may be degraded by bile, pancreatic juice or gastric juice [21–23]. However, PTFE covering membrane is more resistant to chemicals, i.e. acids and alkalis and to bacterial growth, both of which reduce the risk of bile incrustation [22–24].
The concept of retrievable covered stents in the treatment of benign biliary stricture is based on the idea of providing a mechanical barrier that limits tissue ingrowth, helping to maintain stent patency as well as allowing easy removal when no longer needed or desired. We made several observations regarding retrievable PTFE-covered stents that led us to believe that they would be ultimately retrievable. First, because the completely PTFE-covered material serves as an effective barrier to tissue ingrowth and as a relatively friction-free surface, this type of covered stent is particularly suitable for potential removal without becoming incorporated into the bile duct wall. Petersen et al. [12] reported the PTFE covering of the stent appears to prevent early tissue proliferation and incorporation of the stent into the bile duct epithelium. They also discovered the stent did not adhere to the bile duct wall for as long as 4–6 months following its placement. Second, as a nitinol stent is able to recompress to a small volume, this should facilitate its removal through a relatively narrow channel without requiring an increase to the size of the tract. Recently, several investigators have reported that percutaneous transhepatic placement and removal of retrievable PTFE-covered stents were feasible for the treatment of benign biliary strictures [12–16].
In our study, we found that placement of a retrievable covered stent was technically successful in all 68 patients. Only one of our 68 patients required early stent removal, and the stents were successfully removed under fluoroscopic guidance in 64 patients; in three patients, stent removal was not necessary because the stents had migrated completely out of the biliary tree or bilioenterostomy anastomosis and were spontaneously evacuated from the bowel, and in one patient, the stent was removed surgically. Several investigators also reported a 97–100 % technical success rate for stent placement and removal in patients with benign biliary strictures, using the same retrievable covered stents used in our study [14–16].
In our study, clinical success was 86.8 % (59 of 68 patients) following single (n = 50) or dual sessions (n = 9) of stent placement, and the mean duration of retrievable stent placement was 3 months (range, 2–6.5 months). This clinical success rate was similar to previously reported data regarding percutaneous retrievable covered stent treatment (70–91 %) [14–16]. We initially planned to place the stents for at least 2 months because previous reports for treating benign strictures in the esophagus, urethra, and trachea showed acceptable clinical outcomes following 2 months of retrievable covered stent placement [25–27]. In addition, Gwon et al. [14] suggested that the optimal time for stent removal for treatment of benign biliary strictures might be 6 weeks, not only because good stent patency without the development of significant narrowing of the stent was commonly seen within that period, but also because PTFE damage was not a common occurrence during that period. However, persistent or recurrent strictures occurred in a considerable number of our patients in the early phase of our study. Thereafter, when we changed the duration of retrievable stent placement to at least 3 months, we obtained improved clinical outcomes. Because there are no established data on the duration of retrievable stent placement necessary for dilation of benign biliary stricture, a longer duration of retrievable stent placement was preferred especially in patients with recurrent or refractory stricture after percutaneous balloon dilation. Based on our experience, it is recommended that the retrievable covered stent be maintained for longer than 3 months in such patients. Although a longer duration of stent placement may bring about better clinical outcomes, patients must then endure the inconvenience of a drainage catheter. Therefore, further investigation will be necessary to identify the optimal duration of retrievable stent placement.
In our study, the cumulative primary patency rates at 1, 2, 3, 4, and 5 years were 91, 89, 76, 68, and 68 %, respectively. Among the 42 patients who underwent retrievable covered stent placement for initial strictures, 32 patients (76.2 %) showed improvement of the strictures and symptoms until the end of the study period. In addition to the primary use of retrievable covered stents, percutaneous treatment using retrievable covered stent seems to be an effective secondary option for patients with benign biliary strictures that recur and show resistance to percutaneous balloon dilation. In our study, among the 26 patients who underwent retrievable covered stent placement for recurrent or persistent strictures after balloon dilation, 16 patients (61.5 %) showed improvement of the strictures and symptoms until the end of the study period. Although the overall recurrence rate (20 %) of our study was similar to the reported recurrence rate (15–39 %) of percutaneous balloon dilation, the mean indwelling period of drainage catheter (5.8 months; range, 3–22.5 months) of our study was shorter than that of percutaneous balloon dilation (mean, 8.5–19.9 months) [5, 7, 8, 10]. The only series reporting a shorter duration of treatment [9], 1 month or less, was associated with a recurrence rate of 44 %, exceeding that which was reported in other series [5, 7, 8, 10]. Previous investigators reported that the indwelling period of drainage catheter was significantly shorter in a retrievable covered stent group than that in a percutaneous balloon dilation group [15, 16]. Therefore, the duration of treatment can be reduced because of the larger diameter (8–12 mm) of the retrievable covered stent compared to that (8.5–16-F) of indwelling drainage catheters.
However, there are several limitations to the use of a retrievable covered stent. First, there is a risk that the stent will cause branching duct occlusion. In our study, cholangiographic occlusions of the ipsilateral or opposite intrahepatic bile duct by the stents were observed in 38 of 68 patients. Except for one patient who had the stent removed 1 day after stent placement due to markedly elevated liver enzymes, the stents had been placed in situ in 37 patients because their liver enzymes were within normal range despite branching duct occlusion. Gwon et al. [28] suggested that cholangiographic occlusion of bile ducts by the retrievable covered stent did not represent functional occlusion. Petersen et al. [12] also observed flow of contrast material between the bile duct wall and the covering of the PTFE-covered stent for as long as 4–6 months following stent placement. Therefore, we suggest that blockage of intrahepatic bile ducts caused by a retrievable covered stent may not induce major complications although close follow-up is necessary in such cases.
Second, there is a risk of stent migration. We initially expected that a pigtail-shaped drainage catheter tip positioned just beneath the distal margin of the stent could prevent stent migration even though the stent has no anti-migration device such as anchoring fins. In our study, stent migration was observed in 11 patients (16.2 %). Previous investigators have also reported 11–21 % of stent migration rates following percutaneous transhepatic retrievable covered stent placement [12, 14, 15]. To prevent stent migration, Wang et al. [29] used a covered stent with anchoring fins (Viabil, Conmed, Utica, NY); however, they found mucosal ulceration of the bile duct in four of six patients following stent removal. They suggested that this may have been related to the anchoring fins of the stents. Therefore, further investigations for reducing stent migration will be necessary.
The present study has several limitations. First, our study was a retrospective design and there was lack of randomization, which may have decreased the statistical strength of the study. However, the retrospective patients were consecutively enrolled and full follow-up data could be obtained. Nevertheless, it remains possible that the sequential nature of the study may have introduced some bias. Future prospective and randomized investigations are required. Second, the mean follow-up was only 36 months. In approximately half of the patients, the duration of the follow-up was even less. Third, our study patients did not undergo homogenous treatments because, prior to retrievable stent treatment, 26 patients with recurrent or refractory benign biliary strictures despite prolonged catheter interposition after balloon dilation procedures were included in the study. However, it is not possible to use a homogenous treatment regime with these patients because biliary strictures are of a different cause, location, and type.
In conclusion, mid-term outcomes suggested that percutaneous treatment of benign biliary strictures using a retrievable covered stent was a clinically effective method. Although primary patency rates and recurrence rates were similar to those of percutaneous balloon dilation, the duration of treatment may be shorter than that for percutaneous balloon dilation.
Abbreviations
- CT:
-
Computed tomography
- PTBD:
-
Percutaneous transhepatic biliary drainage
- PTFE:
-
Polytetrafluoroethylene
References
Judah JR, Draganov PV. Endoscopic therapy of benign biliary strictures. World J Gastroenterol. 2007;13:3531–3539.
Bolton JS, Braasch JW, Rossi RL. Management of benign biliary strictures. Surg Clin North Am. 1980;60:313–332.
Mueller P, Van Sonnenberg E, Ferrucci J, et al. Biliary strictures dilatation: multicenter review of clinical management in 73 patients. Radiology. 1986;160:17–22.
Kim JH, Ko GY, Sung KB, et al. Bile leak following living donor liver transplantation: clinical efficacy of percutaneous transhepatic treatment. Liver Transpl. 2008;14:1142–1149.
Weber A, Rosca B, Neu B, et al. Long-term follow-up of percutaneous transhepatic biliary drainage (PTBD) in patients with benign bilioenterostomy stricture. Endoscopy. 2009;41:323–328.
Kasahara M, Egawa H, Takada Y, et al. Biliary reconstruction in right lobe living donor liver transplantation: comparison of different techniques in 321 patients. Ann Surg. 2006;243:559–566.
Ko GY, Sung KB, Yoon HK, et al. Percutaneous transhepatic treatment of hepaticojejunal anastomotic biliary strictures after living donor liver transplantation. Liver Transpl. 2008;14:1323–1332.
Glas L, Courbiere M, Ficarelli S, et al. Long-term outcome of percutaneous transhepatic therapy for benign bilioenteric anastomotic strictures. J Vasc Interv Radiol. 2008;19:1336–1343.
Cantwell CP, Pena CS, Gervais DA, et al. Thirty years’ experience with balloon dilatation of benign postoperative strictures; long-term outcomes. Radiology. 2008;249:1050–1057.
Bonnel DH, Fingerhut AL. Percutaneous transhepatic balloon dilatation of benign bilioenteric strictures: long-term results in 110 patients. Am J Surg. 2011;203:675–683.
Enya M, Yasuda I, Mukai T, et al. Endoscopic treatment for benign biliary strictures: can placement of a covered metallic stent be an option in refractory cases? Dig Endosc. 2003;16:12–20.
Petersen BD, Timmermans HA, Uchida BT, Rabkin JM, Keller FS. Treatment of refractory benign biliary stenoses in liver transplant patients by placement and retrieval of a temporary stent-graft: work in progress. J Vasc Interv Radiol. 2000;11:919–929.
Kuo MD, Lopresti DC, Gover DD, Hall LD, Ferrara SL. Intentional retrieval of viabil stent-grafts from the biliary system. J Vasc Interv Radiol. 2006;17:389–397.
Gwon DI, Shim HJ, Kwak BK. Retrievable biliary stent-graft in the treatment of benign biliary strictures. J Vasc Interv Radiol. 2008;19:1328–1335.
Kim J, Ko GY, Sung KB, et al. Percutaneously placed covered retrievable stents for the treatment of biliary anastomotic strictures following living donor liver transplantation. Liver Transpl. 2010;16:1410–1420.
Kim JH, Gwon DI, Ko GY, et al. Temporary placement of retrievable fully covered metallic stents versus percutaneous balloon dilation in the treatment of benign biliary strictures. J Vasc Interv Radiol. 2011;22:893–899.
Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202.
Rossi P, Bezzi M, Salvatori FM, et al. Recurrent benign biliary strictures: management with self-expanding metallic stents. Radiology. 1990;175:661–665.
Deviere J, Cremer M, Baize M, et al. Management of common bile duct stricture caused by chronic pancreatitis with metal mesh self-expandable stents. Gut. 1994;35:122–126.
van Berkel AM, Cahen DL, van Westerloo DJ, et al. Self-expanding metal stents in benign biliary strictures due to chronic pancreatitis. Endoscopy. 2004;36:381–384.
Jung GS, Song HY, Seo TS, et al. Malignant gastric outlet obstructions: treatment by means of coaxial placement of uncovered and covered expandable nitinol stents. J Vasc Interv Radiol. 2002;13:275–283.
Schoder M, Rossi P, Uflacker R, et al. Malignant biliary obstruction: treatment with ePTFE-FEP- covered endoprostheses initial technical and clinical experiences in a multicenter trial. Radiology. 2002;225:35–42.
Bezzi M, Zolovkins A, Cantisani V, et al. New ePTFE/FEP-covered stent in the palliative treatment of malignant biliary obstruction. J Vasc Interv Radiol. 2002;13:581–589.
Han YM, Kwak HS, Jin GY, Lee SO, Chung GH. Treatment of malignant biliary obstruction with a PTFE-covered self-expandable nitinol stent. Korean J Radiol. 2007;8:410–417.
Song HY, Jung HY, Park SI, et al. Covered retrievable nitinol stents in patients with benign esophageal strictures: initial experience. Radiology. 2000;217:551–557.
Shin JH, Song HY, Park H, et al. Removal of retrievable self-expandable urethral stents: experience in 58 stents. Eur Radiol. 2006;16:2037–2043.
Kim JH, Shin JH, Song HY, Shim TS, Yoon CJ, Ko GY. Benign tracheobronchial strictures: long-term results and factors affecting airway patency after temporary stent placement. AJR Am J Roentgenol. 2007;188:1033–1038.
Gwon DI, Ko GY, Sung KB, Kim JH, Yoon HK. Percutaneous transhepatic treatment of postoperative bile leaks: prospective evaluation of retrievable covered stent. J Vasc Interv Radiol. 2011;22:75–83.
Wang AY, Ellen K, Berg CL, Schmitt TM, Kahaleh M. Fully covered self-expandable metallic stents in the management of complex biliary leaks: preliminary data—a case series. Endoscopy. 2009;41:781–786.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gwon, D.I., Ko, GY., Ko, H.K. et al. Percutaneous Transhepatic Treatment Using Retrievable Covered Stents in Patients with Benign Biliary Strictures: Mid-term Outcomes in 68 Patients. Dig Dis Sci 58, 3270–3279 (2013). https://doi.org/10.1007/s10620-013-2784-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2784-9