Abstract
Background
Perifosine, an alkylphospholipid, is an Akt inhibitor which inhibits the growth of diverse cancer cells. We have reported its inhibitory effects on the growth of gastric cancer cells recently, but its molecular mechanisms are still largely unknown.
Aims
The purpose of this study was to investigate the effect and regulatory mechanism of perifosine in gastric cancer.
Methods
Cell viability was determined by sulforhodamine B assay after transiently transfected with AEG-1 specific siRNAs. qRT-PCR and western blot assay were used to determine the mRNA expression and proteins levels of cell signaling molecules examined. Immunohistochemistry was used to detect the AEG-1 expression in 87 gastric carcinomas, 60 dysplasia, and 47 normal gastric mucosa.
Results
Perifosine decreased AEG-1 gene expression along with inhibition of Akt/GSK3β/C-MYC signaling pathway. Knockdown of AEG-1 using siRNA led to significant down-regulation of cyclin D1 expression at both mRNA level and protein level, and inhibited the growth of gastric cancer cells. AEG-1 expression was elevated in gastric dysplasia and cancer tissues compared to normal gastric mucosa (P < 0.01). AEG-1 over-expression correlated with diffuse type of gastric cancer and advanced tumor stages.
Conclusions
Perifosine inhibits the growth of gastric cancer cells possibly through inhibition of the Akt/GSK3β/C-MYC signaling pathway—mediated down-regulation of AEG-1 that subsequently down-regulated cyclin D1. AEG-1 may play an important role in the carcinogenesis and progression of gastric cancer and could be a therapeutic target of perifosine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer remains the fourth most common cancer in the world in 2008 [1]. It is the third leading cause of cancer death in men and the fifth leading cause in women [1]. In recent years, the morbidity and mortality from gastric cancer have decreased worldwide, however, the relative five-year survival rate remains low due to late diagnosis at advanced stages and lack of effective therapy [2, 3]. Understanding the molecular mechanisms is the key for early diagnosis and treatment of gastric cancer. Therefore, it is urgently needed for identifying the key molecules that play important roles in gastric cancer development and progression.
Perifosine is an oral Akt inhibitor [4]. It has been shown to inhibit the MAPK pathway, induce c-Jun NH2-terminal kinase (JNK), and up-regulate death receptor 5 (DR5) [5–7]. As an alkylphospholipid, it perturbs the cell membrane and induces apoptosis, cell cycle arrest and autophagy [8]. Perifosine can also enhance the effects of histone deacetylase inhibitor, 3-Phosphoinositide-dependent protein kinase-1 (PDK1) inhibitor, epithelial growth factor receptor–tyrosine kinase inhibitors (EGFR-TKI), and some chemotherapeutic reagents [9–12]. However, the mechanisms of perifosine on cancer cells have not been fully elucidated.
Astrocyte elevated gene-1 (AEG-1) was originally identified as an inducible gene of human immunodeficiency virus (HIV)-1 in primary human fetal astrocytes in 2002 [13]. It has been reported to play an important role in many cellular processes, such as proliferation, apoptosis, differentiation, and invasion. AEG-1 has been shown to induce cell survival through activating PI3K/Akt signaling [14] and seems to be a downstream target of C-MYC. However, the relationship between perifosine and AEG-1 has not been studied.
In this study, we studied the effect of perifosine on gastric cancer cells and the relationship between perifosine and AEG-1. We also investigated the expression of AEG-1 in gastric cancers and its precancerous dysplasia.
Materials and Methods
Reagents
Perifosine was purchased from Selleck Chemicals LLC (Houston, TX, USA). It was dissolved in PBS at a concentration of 50 mmol/L, and stored at −20 °C. Stock solution was diluted to the appropriate concentrations with growth medium immediately before use. Lipofectamine 2000 transfection reagent was purchased from Life Technologies Co. Invitrogen (Carlsbad, CA, USA). AEG-1 and cyclin D1 antibodies were purchased from Zhongshan Goldenbridge Biotechnology Co., Ltd. (Beijing, China). p-Akt (Ser473), p-GSK3β (Ser9), Akt, and GSK3β antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA). C-MYC antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). GAPDH antibody was purchased from Bioworld Technology Inc. (Louis Park, MN).
Human Tissue Samples
Specimens used in this study consisted of 87 cases of gastric adenocarcinoma, 30 cases of low-grade dysplasia, 30 cases of high-grade dysplasia, and 47 cases of normal gastric mucosa that were surgically resected at the Nanjing Medical University Affiliated Nanjing Hospital between July 2010 and Feb 2012. Median age of 87 cases of gastric adenocarcinoma was 48.3 years (32–79 years), and 57 % of the patients were male. Tumors were histologically classified according to Lauren classification and tumor stages were classified according to the American Joint Committee on Cancer (2010 version). None of the patients received preoperative treatment such as chemotherapy or radiotherapy.
Cell Lines and Cell Culture
The human gastric epithelial cell lines (GES-1) and gastric cancer cell lines (SGC7901, MGC803, MKN45, AGS) were obtained from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China. The cells were cultured in RPMI-1640 medium supplemented with 10 % fetal bovine serum (Gibco BRL, Grand Island, NY) at 37 °C in a humidified atmosphere consisting of 5 % CO2.
Growth Curve of Tumor Cells
Cells after transfection with siRNA for 24 h were seeded in 96-well plates at 1500/well density and plates were fixed with 10 % (w/v) trichloroacetic acid at 4 °C each day for 5 days. Cell number was determined by the sulforhodamine B (SRB) assay as previously described [15, 16].
Western Blot Analysis
Radio-immunoprecipitation assay (RIPA) lysis buffer was purchased from Cell Signaling Technology, Inc. (Beverly, MA) and was stored at 4 °C. Protease inhibitors were added just before use. Cells were harvested at the end of treatment and subjected for western blotting assay as described previously [16].
Gene Silencing by siRNA
Control (non-target) small interfering RNA (siRNA) was purchased from Invitrogen. AEG-1 siRNAs that target 5′- AAC AGA AGA AGA AGA ACC GGA -3′ were described previously and synthesized by Invitrogen [14]. Cells in the exponential phase of growth were plated in 6-well plates at 5 × 105 cells/well and transfected with AEG-1 siRNA or control siRNA with a final concentration of 100 nmol/L for 48 h using Lipofectamine™ 2000 Reagent (Invitrogen Life Technologies Inc., Carlsbad, CA), according to the manufacturer’s protocol. Then the total RNAs and whole-cell lysates were prepared and subjected for qRT-PCR assay and Western blotting assay, respectively. To determine the cell number, cells after transfection for 24 h were reseeded into 96-well plates and subjected for a 5-day SRB assay.
RNA Isolation and Quantitative RT-PCR
Total RNA from cells was extracted using Trizol reagent (Gibco Carlsbad, CA, USA) according to the manufacturer’s protocol. The reverse transcription was conducted according to standard procedure using M-MLV reverse transcriptase from Promega (Madison, WI, USA). Quantitative PCR (qRT-PCR) reactions were conducted using Power SYBR Green PCR Master Mix from Applied Biosystems, Inc. (Foster, CA, USA), according to manufacturer’s instructions. Forward (F) and reverse (R) primers were used as follows: AEG-1, F: 5′- ACGACCTGGCCTTGCTGAAGAATCT -3′ and R: 5′- CGGTTGTAAGTTGCTCGGTGGTAA -3′; cyclin D1, F: 5′- ACAAACAGATCATCCGCAAACAC -3′ and R: 5′- TGTTGGGGCTCCTCAGGTTC -3′; GAPDH, F: 5′- ATGGGGAAGGTGAAGGTCG -3′, and R: 5′- GGGGTCATTGATGGCAACAATA -3′, and synthesized by Invitrogen [14, 17, 18]. All real-time amplifications were performed with the ABI Prism 7300 sequence detection system in triplicate (Applied Biosystems, Inc.). The fold-change of the target genes were calculated using the 2−ΔΔCT method.
Immunihistochemistry
All tissues had been fixed in 10 % neutral-buffered formalin and embedded in paraffin using standard surgical pathology protocols. Immunohistochemical staining for AEG-1 (polyclonal rabbit anti-AEG-1, 1:100 dilution, Abcam Corportation, USA) was done using an EnVsion procedure after autoclave-based antigen retrieval. Sections were incubated for 5 min in 3 % hydrogen peroxide to abolish endogenous peroxidase activity. Immunostaining was performed on a BenchMark XT (Ventana Medical System, Inc., Tucson, AZ) according to the manufacturer’s protocol. Staining was performed with diaminobenzidine and countstaining with hematoxylin. Negative control staining was carried out by substituting PBS for the primary antibody. A tissue section of breast cancer was used as a positive control. All immunohistochemical staining results were evaluated semiquantitatively. The staining intensity for AEG-1 was graded as 0 (negative), 1 (pale yellow), 2 (yellow), and 3 (dark yellow). The frequency (number of tumor cells stained) was scored as 0 (negative), 1 (<10 %), 2 (10–50 %), and 3 (>50 %). The staining index was calculated as staining intensity score multiplied by frequency of positive tumor cells. AEG-1 expression was divided into high expression (≥4) and low expression (<4) according to the staining index score.
Statistical Analysis
All data was represented as the mean ± SD from three separate experiments. Statistical analysis was performed using the two-sided unpaired Student’s t tests. The correlation between the positive expression of AEG-1 and clinicopathological parameters was analyzed using the χ 2 test or the Fisher exact test. Differences were considered statistically significant at P < 0.05. The IC50 value of perifosine was calculated by Bliss software.
Results
Perifosine Inhibited AEG-1/Cyclin D1 Signaling Pathway Along with the Down-Regulation of Akt and C-MYC Signaling
We recently reported that perifosine inhibited the growth of gastric cancer cells, but its mechanisms were still elusive [19, 20]. Since perifosine is a well-known Akt inhibitor, we explored its effects on the Akt/GSK3β/C-MYC signaling pathway. As expected, perifosine decreased p-Akt (Ser 473), p-GSK3β (Ser 9), and C-MYC levels in SGC7901 and MGC803 cells (Fig. 1). Interestingly, the expression of AEG-1 and cyclin D1 was also inhibited by perifosine along with other Akt pathway molecules. These results suggest that AEG-1 is also a target of perifosine in gastric cancer and is probably regulated through inhibition of the Akt/GSK3β/C-MYC signaling pathway.
Perifosine inhibited Akt/GSK3β/C-MYC signaling pathway and AEG-1protein. SGC7901 and MGC803 cells were treated with 0.75 or 10 μmol/L perifosine for 48 h, respectively, and whole-cell protein lysates were purified and subjected for western blot analysis. Western blotting assay was done using specific antibodies against the proteins indicated. GAPDH was used as loading control. C control, P perifosine treatment
To confirm this hypothesis, we examined AEG-1 mRNA expression by qRT-PCR assay after perifosine treatment for 24, 48, and 72 h. As shown in Fig. 2, perifosine treatment significantly decreased AEG-1 expression in SGC7901 cells in both a time-dependent manner (a) and a dose-dependent manner (b). At the same time, we observed a decrease of cyclin D1 transcript by perifosine in a similar pattern as AEG-1 (Fig. 2c, d). Western blotting assay confirmed the down-regulation of AEG-1 and cyclin D1 expression at the protein level at different time points and doses of perifosine treatments (Fig. 2e, f). These results suggest that perifosine down-regulates AEG-1 and cyclin D1 expression in gastric cancer cells through transcriptional regulation.
Perifosine decreased the expression of AEG-1 and cyclin D1 in gastric cancer cells. SGC7901 cells were treated with 0.75 μmol/L perifosine for different times as indicated (a, c) or with different concentrations of perifosine as indicated for 48 h (b, d). Total RNA was extracted and subjected to qRT-PCR assay to detect the mRNA expression of AEG-1 and cylcin D1. Data are expressed as mean ± SD of three replicate determinations; * P < 0.05. e, f SGC7901 cells were treated with 0.75 μmol/L perifosine for different times as indicated or treated with different concentrations of perifosine as indicated for 24 h. Whole-cell protein lysates were purified and subjected to western blot analysis. C control, P perifosine treatment
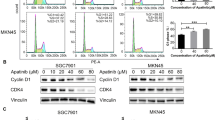
Cyclin D1, but Not C-MYC, Is Regulated by AEG-1 in Gastric Cancer Cells
To explore the relationship among AEG-1, cyclin D1 and C-MYC, we knocked down AEG-1 expression by using AEG-1 specific targeting siRNA. As shown in Fig. 3, AEG-1 siRNA significantly down-regulated AEG-1 expression in SGC7901 and MGC803 cells (a, b). Interestingly, we observed significant down-regulation of cyclin D1 expression at both transcription level (c, d) and protein level (g) upon knockdown of AEG-1. However, the mRNA (e, f) and protein (g) levels of C-MYC were not decreased after AEG-1 knockdown, rather the mRNA of C-MYC increased in MGC803 cells (f) after AEG-1 knockdown (P < 0.05). These results indicate that cyclin D1 may be a downstream target of AEG-1. However, C-MYC is not a downstream target of AEG-1, rather an upstream regulator of AEG-1 in gastric cancer cells examined.
Knockdown of AEG-1 inhibited expression of cyclin D1 but not C-MYC in gastric cancer cells. SGC7901 and MGC-803 cells were transfected with 100 nmol/L AEG-1 siRNA or control siRNA for 24 h; cells were seeded into 6-well plates. qRT-PCR assay was performed to detect mRNA expression of AEG-1 (a, b), cyclin D1 (c, d), and C-MYC (e, f). Data are expressed as mean ± SD of three replicate determinations; * P < 0.05. g Whole-cell protein lysates were purified and western blot analysis was performed to detect AEG-1, cyclin D1, and C-MYC protein expression. GAPDH was used as loading controls. Ctrl control siRNA, AEG-1 AEG-1 siRNA
Down-Regulation of AEG-1 Expression Inhibits the Growth of Gastric Cancer Cells
To explore AEG-1’s function in gastric cancer, we transiently transfected control siRNAs or AEG-1 specific siRNAs into SGC7901 and MGC803 cells, and the cell number was determined by a 5-day SRB assay. Our results showed that knockdown of AEG-1 expression inhibited the growth of tumor cells (Fig. 4).
Knockdown of AEG-1 inhibited the growth of gastric cancer cells. SGC7901 and MGC-803 cells were transfected with 100 nmol/L AEG-1 siRNA or control siRNA for 24 h; cells were seeded into 96-well plates. Sulforhodamine B assay was used to determine cell numbers from day 1 to day 5. Data are expressed as mean ± SD of three replicate determinations
AEG-1 Expression Was Elevated in Gastric Dysplasia and Cancers
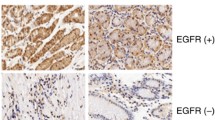
We examined AEG-1 expression levels in the gastric cancer cell lines, SGC7901, MGC803, AGS, and MKN45, as well as in human gastric epithelial cell line, GES-1. AEG-1 mRNA expression was higher in all the gastric cancer cells examined than in GES-1 cells (P < 0.05) (Fig. 5a), and AEG-1 protein expression was over-expressed in 3 of 4 gastric cancer cell lines (SGC7901, MGC803 and AGS cells), as compared with that in GES-1 cells (b).
AEG-1 expression was elevated in both human gastric cancer tissues and cancer cell lines. a Total-mRNA were purified from GES-1, SGC7901, MGC803, AGS, and MKN45 cells after 24 h culturing, and then subjected to qRT-PCR assay. Relative AEG-1 expression was determined by 2−ΔΔCT method. Data are expressed as mean ± SD of three replicate determinations; * P < 0.05. b Whole-cell protein lysates were purified and western blot analysis was used to detect AEG-1 protein expression. c–f Immunohistochemical staining of AEG-1 in normal gastric mucosa, dysplasia and gastric adenocarcinoma. c Weak staining in a normal gastric mucosa. d Strong staining in a low-grade dysplasia. e Strong staining in a high-grade dysplasia. f Strong staining in a gastric adenocarcinoma. Representatives of IHC staining. Magnification, ×200
To confirm the above results, we further examined AEG-1 expression in gastric cancers and precancerous lesions by immunohistochemistry. The representative immunohistochemistry staining images are shown in Fig. 5c–f. Table 1 summarizes the AEG-1 expression in gastric cancer and precancerous lesions. AEG-1 expression was higher in gastric cancer tissues and precancerous lesions (low grade and high grade dysplasia) than that in normal mucosa (P = 0.000). However, no significant differences was seen among cancers, low-grade dysplasia and high-grade dysplasia (P > 0.05). Table 2 summarizes the correlation between AEG-1 expression and clinical pathological parameters in gastric carcinoma. AEG-1 high expression significantly correlated with Lauren classification (P = 0.027), T classification (P = 0.001), N classification (P = 0.002), and pTNM staging (P = 0.043). Overexpression of AEG-1 was more frequent in diffuse types of cancer than in intestinal and mixed types of cancers, and in more advanced tumors.
Discussion
The Akt inhibitor, perifosine, is an oral alkylphospholipid, which exhibits antitumor activity both in preclinical and clinical studies. It showed an inhibition of tumor growth and induced apoptosis in a variety of types of cancers, such as lung cancer, head and neck cancer, prostate cancer, multiple myeloma, and leukemia [21]. It has been tested in clinical trials both as a single agent or in combination with other anticancer reagents [21]. We recently reported that it inhibits the growth of gastric cancer cells [19, 20]. To date, its mechanisms on gastric cancer are still unclear. In this study, we observed that perifosine inhibited the growth of gastric cancer cells. Administration of perifosine in gastric cancers inhibited expression levels of p-AKT, p-GSK3β, cyclin D1, C-MYC and AEG-1. Furthermore, we demonstrated that cyclin D1 (but not C-MYC) is a downstream target of AEG-1 that is over-expressed in gastric cancer cell lines, gastric dysplasia and primary cancer tissues.
Unlike other kinase inhibitors which compete for the ATP binding sites, perifosine inhibits Akt through targeting its pleckstrin homology (PH) domain and interferes with its recruitment to the plasma membrane and subsequent phosphoylation and activation [22]. In addition to Akt, perifosine also regulates MAPK, JNK and DR5 [5–7]. Furthermore, perifosine interferes with the structure, metabolism, and permeability of the cell membranes [21]. Currently, the mechanism of perifosine has not been fully elucidated. In the present study, for the first time, we found that perifosine down-regulated AEG-1 expression. We also observed that it inhibited the Akt/GSK3β/C-MYC pathway. Even though C-MYC has been suggested to be located both upstream and downstream of AEG-1 [14, 23], our findings that knockdown of AEG-1 does not lead to a decrease of C-MYC levels suggest that C-MYC is not a direct downstream target of AEG-1 in the gastric cancer cell lines we examined. On the other hand, it may locate at upstream of AEG-1 and be negatively regulated by AEG-1, because knockdown of AEG-1 led to an increase of C-MYC transcription. Interestingly, we found that cyclin D1 is significantly down-regulated after AEG-1 knockdown, indicating that cyclin D1 is a downstream target of AEG-1. Cyclin D1 is a major regulator of cell cycle progression and our findings suggest that the inhibition of tumor cell growth observed in perifosine treatment may be through down-regulation of the AEG-1-cyclin D1 pathway. We also showed that perifosine decreased the phosphorylation of GSK3β at serine 9, an indicator of inhibition of GSK3β. These findings indicate that a GSK3β-involved, ubiquitination-mediated protein degradation mechanism may contribute to perifosine’s effects on AEG-1 [24]. More studies are needed to fully elucidate the regulatory mechanisms of perifosine on AEG-1.
Overexpression of AEG-1 has been reported in liver cancer, breast cancer, prostate cancer, lung cancer, and melanoma. In this study, we found that AEG-1 expression was significantly higher in low-grade dysplasia, high-grade dysplasia and gastric cancers than that in normal gastric tissues, but no differences among low-grade dysplasia, high-grade dysplasia and gastric cancers. These results suggest that over-expression of AEG-1 is an early event and may be involved in early carcinogenesis of stomach. Our results are similar to the report of Xu et al. [25]. In line with these findings, we also found that manipulating AEG-1 expression by siRNA inhibited the growth of gastric cancer cells, supporting that it may be an oncogene in gastric cancer.
In summary, perifosine inhibited the growth of gastric cancer cells, likely through down-regualtion of AEG-1-cyclin D1 expression. AEG-1 was elevated both in the gastric cancer tissues and in some gastric cancer cell lines, and down-regulation of AEG-1 expression inhibited the growth of gastric cancer cells. Our results suggest that AEG-1 may be important in gastric cancer development and progression and could be a therapeutic target by small molecules like perifosine.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615–628.
Orditura M, De Vita F, Muto P, et al. Adjuvant chemoradiotherapy in patients with stage III or IV radically resected gastric cancer: a pilot study. Arch Surg. 2010;145:233–238.
Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062.
Elrod HA, Lin YD, Yue P, et al. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6:2029–2038.
Papa V, Tazzari PL, Chiarini F. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia. 2008;22:147–160.
Tazzari PL, Tabellini G, Ricci F, et al. Synergistic proapoptotic activity of recombinant TRAIL plus the Akt inhibitor perifosine in acute myelogenous leukemia cells. Cancer Res. 2008;68:9394–9403.
Fu L, Kim YA, Wang X, et al. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–8976.
Rahmani M, Reese E, Dai Y, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432.
Nyakern M, Cappellini A, Mantovani I, Martelli AM. Synergistic induction of apoptosis in human leukemia T cells by the Akt inhibitor perifosine and etoposide through activation of intrinsic and Fas-mediated extrinsic cell death pathways. Mol Cancer Ther. 2006;5:1559–1570.
Dasmahapatra GP, Didolkar P, Alley MC, Ghosh S, Sausville EA, Roy KK. In vitro combination treatment with perifosine and UCN-01 demonstrates synergism against prostate (PC-3) and lung (A549) epithelial adenocarcinoma cell lines. Clin Cancer Res. 2004;10:5242–5252.
Festuccia C, Gravina GL, Muzi P, et al. Akt down-modulation induces apoptosis of human prostate cancer cells and synergizes with EGFR tyrosine kinase inhibitors. Prostate. 2008;68:965–974.
Su ZZ, Kang DC, Chen Y, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602.
Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci USA. 2006;103:17390–17395.
Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418.
Luo X, Fan S, Huang W, et al. Downregulation of IRS-1 promotes metastasis of head and neck squamous cell carcinoma. Oncol Rep. 2012;28:659–667.
Hirotsu M, Setoguchi T, Sasaki H, et al. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer. 2010;9:5.
Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3 K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA. 2009;106:268–273.
Liu D, Sun Q, Liang S et al. MicroRNA-27a inhibitors alone or in combination with perifosine suppress the growth of gastric cancer cells. Mol Med Rep. 2013;7:642–648.
Liang S, Guo R, Zhang Z, et al. Upregulation of the eIF4E signaling pathway contributes to the progression of gastric cancer, and targeting eIF4E by perifosine inhibits cell growth. Oncol Rep. 2013;29:2422–2430.
Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110.
Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK, et al. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103.
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3 K-Akt signaling. Oncogene. 2008;27:1114–1121.
Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144.
Jian-bo X, Hui W, Yu-long H, et al. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Med Oncol. 2011;28:455–462.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30873099, 81102458, 81172004) (X. Wang), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (X. Wang), the Nanjing Pharmaceutical Technology Development Project (YKK09050) (W. Huang), “Medical ZhongDianRenCai Project” of Jiangsu Province (RC2011059) (L. Yang), and ‘‘Six RenCai Gaofeng’’ Funding for the Young Academic Leader of Jiangsu Province (2012) (L. Yang). We thank Dr. Heath Elrod for editing of the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wenbin Huang and Li Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, W., Yang, L., Liang, S. et al. AEG-1 Is a Target of Perifosine and Is Over-Expressed in Gastric Dysplasia and Cancers. Dig Dis Sci 58, 2873–2880 (2013). https://doi.org/10.1007/s10620-013-2735-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2735-5