Abstract
Background
Intestinal absorptive capacity shows a circadian rhythm synchronized with eating patterns. Disrupting these coordinated rhythms, e.g., with shift work, may contribute to metabolic disease. Circadian expression of nutrient transporters has not been studied in metabolic disease. We studied the circadian rhythm of intestinal transporter sodium glucose co-transporter type 1 (SGLT1) in an obese diabetic rat.
Methods
We compared obese Zucker diabetic fatty (ZDF) rats to lean ZDF littermates. Temporal feeding patterns were assessed, then rats were harvested at Zeitgeber (ZT, ZT0 = 7:00 a.m.) 3, 9, or 15 to measure insulin resistance, SGLT1 expression and intestinal glucose absorption capacity. Regulators of SGLT1 (sweet taste receptor T1R2/3; clock genes) were measured to elucidate underlying mechanisms.
Results
Both groups exhibited altered circadian food intake. Obese ZDF rats lost circadian rhythmicity of SGLT1 mRNA expression and functional activity. Lean ZDF rats maintained rhythmicity of SGLT1 mRNA expression but that of functional glucose absorption was blunted. Circadian rhythms of intestinal clock genes were maintained in both groups. Neither group had discernible rhythms of intestinal GLUT2 (glucose transporter) or T1R2 (sweet taste receptor component) mRNA expression. In summary, lean and obese ZDF rats exhibited similar disruptions in circadian feeding. Glucose intolerance was evident in lean rats, but only obese rats further developed diabetes and exhibited disrupted circadian rhythmicity of both SGLT1 mRNA expression and function.
Conclusions
Our findings suggest that disrupted circadian feeding rhythms contribute to glucose intolerance, but additional factors (genetics, changes in nutrient sensing/transport) are needed to lead to full diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Shift work has become increasingly common in today’s society and is associated with increased levels of metabolic disease. For example, extended adherence to a rotating shift pattern significantly increases the risk of developing the metabolic syndrome [1–5]. The central circadian clock, located in the suprachiasmatic nucleus of the brain, is entrained to the light:dark cycle and establishes the overall activity rhythms of the organism. Under normal circumstances, peripheral tissues also exhibit circadian rhythms, which are linked to the central clock, thus coordinating many temporal metabolic rhythms. However, the repetitive nocturnal activity and food consumption coupled with daytime sleeping necessitated by shift work alter the normal temporal alignment of these activities, thereby desynchronizing peripheral clocks from the central clock. This state, known as shift work disorder, is most likely due to the resultant mistiming of various metabolic processes, e.g., release of intestinal secretions, enzymes, and hormones.
This hypothesis is supported by several animal and human studies. Advancing feeding times along the light–dark cycle leads to weight gain in mice, while concomitantly altering the circadian pattern of leptin further exacerbates weight changes [6, 7]. Furthermore, animal studies have revealed that circadian rhythms of body temperature and activity are blunted prior to the onset of glucose intolerance [8] and are progressively attenuated with weight gain in obesity [9]. In humans, intentionally misaligning light and dark cycles with behavioral activities can increase glucose intolerance in as little as 10 days [10], while a disruption of the circadian rhythm of insulin secretion has been shown in patients with type 2 diabetes (T2D) as well as in their first-degree relatives with apparently normal glucose tolerance [11].
Diurnal rhythms of intestinal nutrient transporters such as the sodium-glucose co-transporter, sodium glucose co-transporter type 1 (SGLT1) have been demonstrated in health [12–15]. SGLT1 is responsible for the majority of secondary active transport of dietary glucose. While over-expression of SGLT1 is implicated in T2D [16], it is unknown whether the diurnal rhythm of SGLT1 expression changes in metabolic disorders. We previously demonstrated that the SGLT1 rhythm can be shifted by restricting feeding to the light phase, possibly via the parallel shifts observed in clock genes Reverbβ and Per1 [17]. As a picture of SGLT1 expression in normal physiology continues to emerge, we sought to extend our analyses to pathological conditions.
In this study, we investigate circadian rhythms of feeding and intestinal SGLT1 mRNA expression and functional activity in Zucker diabetic fatty (ZDF) rats, a model of obesity-induced T2D, hypothesizing that disrupted circadian feeding patterns lead to glucose intolerance and T2D by disrupting the circadian rhythm of SGLT1. We also assess expression of the intestinal sweet taste receptor T1R2/3 and intestinal clock genes, two factors we have found to be involved in SGLT1 regulation [17, 18].
Materials and Methods
Animals
Experimental animal protocols were prospectively approved by the Harvard Medical Area Standing Committee on Animals. Male obese and lean ZDF rats were acquired from Charles River (strains 370 and 380, Wilmington, MA). ZDF rats with the homozygous fa/fa genotype are leptin resistant and hyperphagic, which leads to obesity on a high-carbohydrate diet. Obesity-induced diabetes ensues, with frank T2D developing around 12 weeks of age. Heterozygous fa/+ ZDF rats have some degree of hyperglycemia, but remain lean and do not develop the full T2D phenotype. Eleven-week-old rats (n = 30 per group) were acclimatized for 5 days under a strict 12:12-h light–dark cycle (lights on at 7:00 a.m., Zeitgeber Time ZT0) with constant temperature and humidity and ad libitum access to a high-carbohydrate diet (Purina 5008) [19] and water. Subsequently, animals were weighed daily, with food intake recorded on a subset (n = 24 per group) during light (ZT0-ZT12) versus dark hours (ZT12-ZT0) for 12 days. Concurrently, eight male Sprague–Dawley (SD) rats were similarly housed and food consumption (standard chow ad libitum) and blood glucose monitored and compared to ZDF rats. Circadian rhythms of intestinal glucose transporters and clock genes have been previously well documented in SD rats and therefore were not repeated here [13–15].
Food Consumption
Excess chow was provided to each cage housing two rats at ZT12 (7:00 p.m.), and chow remaining at ZT0 (7:00 a.m.) was removed and weighed, and used to calculate the nocturnal food intake. Difference in chow remaining from ZT0 to ZT12 was similarly recorded, and used to calculate total daily food intake. Consumption per rat in any given cage was calculated by halving the mass consumed by both for each period. The mean consumption per rat for each group was then calculated by averaging the values for all rats in that group.
Tissue Harvest
At 13 weeks, an age that obese ZDF rats exhibit their diabetic phenotype, animals were harvested at ZT3, ZT9, or ZT15 to assess diurnal rhythms (n = 11 at ZT3 and ZT9 to appropriately power the study, given smaller expected differences; and n = 8 at ZT15). These three time points, rather than the traditional four time points across a circadian time, were selected based on previous studies of diurnal rhythms in rodent intestine, where maximum changes in amplitude (peaks and nadirs) lie within this time span. Animals were anesthetized with isoflurane (2–3 %) and a midline laparotomy was performed. Jejunum was retrieved from 2 cm distal to the ligament of Treitz and flushed with ice-cold normal saline (0.9 %). A 5-cm section of jejunum was excised, and divided into equal thirds. Each segment was everted and mounted onto a glass rod for functional glucose uptake assays. The next 10 cm of jejunum was opened longitudinally over ice, blotted to remove debris, and scraped with glass microscope slides to collect the mucosa. Mucosal scrapings were flash-frozen in liquid nitrogen and stored at −80 °C for subsequent RNA extraction.
Functional Glucose Transport Assays
The everted sleeve method was used as previously described [20], taking three sleeves from proximal jejunum. Uptake experiments were performed with Mammalian Ringer’s (128 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 20 mM NaHCO3) containing 50 mM d-glucose (a concentration at which transporter saturation is achieved) and trace quantities of [14C]d-glucose (PerkinElmer, Waltham, MA, USA) and [3H]l-glucose (Moravek Biochemicals, Brea, CA, USA) (1 min, 37 °C). A further single sleeve from each animal was incubated in uptake solution containing 500 μM phloridzin, an SGLT1-specific inhibitor (Sigma, St. Louis, MO, USA). The glucose uptake rate was calculated from the difference between the uptake of [14C]d-glucose and that of the non-transported [3H]l-glucose used as a passive control.
RNA Analysis
RNA was extracted from frozen tissue samples with a mirVana mRNA Isolation Kit (Ambion, Austin, TX, USA) and quantified with a microplate reader (Spectramax M5; Molecular Devices, Sunnyvale, CA, USA). Reverse transcription was performed simultaneously on 0.5 μg of RNA from each rat with Superscript III and oligo-dT (Invitrogen, Carlsbad, CA, USA). To facilitate inter- and intra-group comparisons of gene expression, quantitative PCR of all cDNA samples were run on a single 384-well plate. The cDNA product was diluted and added to forward and reverse primers (Sglt1, T1r2, Glut2, Bmal1, Clock, Reverbβ, Per1, Cyclophylin A (housekeeping gene) Invitrogen; Table 1), together with SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed in duplicate or triplicate with diluted cDNA primers and SYBR Green Master Mix using an Applied Biosystems ABI 7900HT Thermal Cycler (2 min, 50 °C; 10 min, 95 °C; 40 cycles of 15 s, 95 °C and 1 min, 60 °C). Dissociation curves were obtained and PCR products were run on agarose gels to ensure generation of a single, authentic amplicon.
Glucose and Insulin Assays
Blood samples were obtained during harvest of rats in the non-fasted state. Glucose was measured with a One Touch Ultra Glucometer (Life Scan Inc., Milpitas, CA, USA) using tail-vein blood drawn immediately before laparotomy. For insulin, 0.5 cc blood was drawn from the vena cava after harvesting the intestine and added to a serum separator tube. After centrifugation at 4,000 × g for 15 min at 4 °C, serum was removed and frozen at −80 °C. Insulin was measured with the Rat/Mouse Insulin ELISA Kit (Millipore, Billerica, MA, USA) (n = 21 obese and 18 lean rats). The Homeostatic Model Assessment equation was used to calculate Insulin Resistance (HOMA-IR) using the formula:
Statistical Analyses
Data were analyzed with SPSS 16 (SPSS, Chicago, IL, USA). Student’s t test was used for normally distributed data. Data are reported as mean ± SE. Non-parametric analyses were undertaken using the Chi-square and Wilcoxon rank-sum tests for non-normally distributed data. These data are shown as proportions and median values with interquartile ranges, respectively. Differences were considered significant at p < 0.05. Presence of diurnal rhythmicity was assessed using the freely available Cosinor Analysis (www.circadian.org) to determine circadian periodicity, assuming a circadian period of 24 h and treating individual harvests as a cross-sectional study. NS not significant.
Results
Food Intake and Weight Gain
Obese ZDF rats consumed almost twice as much chow as lean littermates (37.5 ± 0.8 vs. 21.1 ± 0.3 g/day, p < 0.01). Despite this, the absolute weight gain between 11 and 13 weeks of age was lower than that in leans (25.5 ± 3.0 g vs. 36.2 ± 1.2 g, obese vs. lean respectively, p < 0.01), with obese rats gaining 6.9 % and lean rats gaining 12.8 % of initial body weight. The measurement period spanned the onset of diabetes (typically age 12 weeks for ZDFs), so the slower rate of weight gain in obese rats can be explained by their disease-induced catabolism and severe polyuria.
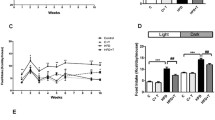
In both groups of rats, the nocturnal pattern of feeding was diminished, with up to 40 % of food intake occurring during light hours. SD rats housed in identical conditions to ZDF rats in this protocol consumed 23 % of their daily intake during the day and 77 % at night. The proportion of food SD rats consumed in daylight hours was significantly lower than that of ZDF rats (n = 8; p < 0.001 vs. lean and obese ZDF rats, Chi-square test).
Blood Glucose, Insulin, and HOMA-IR
Lean ZDF rats were hyperglycemic (mean blood glucose across all time points 206.7 ± 8.7 mg/dl) but obese ZDF rats had significantly higher blood glucose levels (412.4 ± 26.8 mg/dl; p < 0.01) while insulin levels did not differ between groups (39.2 ± 3.7 μU/ml vs. 41.4 ± 11.0 μU/ml, respectively, p = 0.9). Nevertheless, HOMA-IR was increased in obese rats (43.7 ± 9.0 vs. 21.9 ± 2.2 in leans, p < 0.05). Blood glucose levels in SD rats were normal (130.5 ± 9.1 mg/dl, mean fasting blood glucose level).
Functional Glucose Uptake
Glucose uptake rates measured in everted sleeves revealed a blunted circadian rhythmicity of glucose uptake in lean control rats (Fig. 1; p = 0.07 for assessment of 24-h periodicity). The highest glucose uptake rate occurred at ZT15, coinciding with the expected peak of feeding activity, with the nadir occurring at the ZT3 time point (7.1 ± 0.4 vs. 5.5 ± 0.6 nmol/mg/min, respectively). Obese rats showed no circadian rhythm of intestinal glucose uptake (Fig. 1; p = 0.26 for assessment of 24-h periodicity). Attribution of the observed glucose transport activity to SGLT1 at all harvest times was confirmed by its inhibition following pre-incubation with phloridzin, which led to a 72–76 % reduction in glucose uptake.
Intestinal Sglt1, Glut2, T1r2, and Clock Gene mRNA Expression
To determine whether the functional changes were due to altered Sglt1 transcription, we measured mRNA levels. Median levels of Sglt1 mRNA were not different between obese and lean rats (1.2-fold difference, p = 0.14). Lean ZDF rats maintained circadian rhythmicity of intestinal Sglt1 mRNA levels (p < 0.05 for assessment of 24-h periodicity; Fig. 2) with the peak as expected at ZT9 just prior to onset of the dark phase (2.0-fold increase vs. ZT3, p < 0.001), but the amplitude of rhythmicity was less than expected (cf. 4.6-fold increase between ZT3 and ZT9 seen in SD rats [14]). In obese rats, diurnal rhythmicity of SGLT1 was lost (p = 0.21 for assessment of 24-h periodicity).
There was no correlation between intestinal Sglt1 mRNA expression levels and amount of food intake in either group of ZDF rats (Fig. 3).
Mean daily food consumption and intestinal Sglt1 mRNA expression. Food intake was recorded over 12 days for each pair of rats housed together, and mean daily food intake calculated per pair of rats. Pearson’s correlation coefficient was used to assess for correlation between food intake and jejunal Sglt1 mRNA expression (n = 12 pairs of rats per group)
There was no discernible circadian rhythm of the glucose transporter Glut2 or the glucose sensor T1r2 in either group of rats (Supplementary Figure 1). The median levels of intestinal Glut2 and T1r2 mRNA expression at all time points were not significantly different between obese and lean groups (1.3-fold (p = 0.12) and 1.4-fold differences (p = 0.09), respectively) (Fig. 4).
Rhythmicity of intestinal clock genes Bmal1 (Brain-muscle Arnt-like 1), Clock, Per1, and Reverbβ was preserved in both groups of rats, with the positive clock regulators Bmal1 and Clock showing higher expression around dark hours, and the negative clock regulators Per1 and Reverbβ showing corresponding decreases at these times as previously reported [17] (Fig. 5).
Discussion
We set out to determine whether the temporal expression pattern of intestinal SGLT1 is dysregulated in obesity and T2D. Baseline SGLT1 expression is known to increase in these conditions [16, 21–23], but the impact on SGLT1 rhythmicity is unknown. To address this gap, we examined mRNA expression of SGLT1 and its function in the ZDF rat, a model of obesity and T2D. We examined several factors that may contribute to such disrupted rhythm, including diurnal food consumption, expression of intestinal clock genes, and intestinal sweet taste receptor expression as potential causative factors. Our findings can be summarized as follows: Obese ZDF rats consumed a significantly greater amount of their daily food intake during light hours. This was associated with an increased HOMA-IR, a disrupted rhythm of intestinal Sglt1 mRNA expression and functional glucose uptake, and diabetes as evidenced by chronic hyperglycemia. Sweet taste receptor mRNA rhythm was absent, and Sglt1 mRNA levels were elevated despite low T1r2 levels. Lean littermates similarly consumed a significantly greater amount of their daily food intake during light hours, showed hyperglycemia and an ablated rhythm of intestinal glucose uptake, but a lesser degree of insulin resistance without manifestation of the full diabetic phenotype. mRNA rhythms of Sglt1 were maintained, though not of the same amplitude as observed in physiologically healthy SD rats (two-fold change in Sglt1 mRNA across the circadian period, compared to the 4.6-fold change seen in SD rats) [12–15, 17]. Intestinal clock gene rhythms were retained in both obese ZDF rats and their lean littermates.
These observations lend support to the idea that disrupted rhythmicity of food intake is associated with insulin resistance—other studies have also shown that a disruption in circadian rhythms of eating can lead to metabolic derangements. For example, a recent study by Hatori et al. [24] reports that a high-fat diet ad libitum in mice is considerably more deleterious than the same calorie-matched diet provided on a phase-restricted basis for a 6-week period, with time-restricted feeding conferring protection against obesity and hyperinsulinemia. In our study, diurnal food intake was disrupted in both lean and obese ZDF rats, with a loss of phase-defined periods of feeding associated with insulin resistance. However, our data suggest that disrupted feeding is unlikely to be the sole etiologic factor to developing diabetes and that additional factors contribute to the final swing to the T2D phenotype.
Having shown that despite similar disruption of food intake patterns, HOMA-IR is significantly elevated and SGLT1 functional rhythmicity more markedly disrupted in obese ZDF rats, we set out to investigate other known intestinal factors regulating SGLT1, including intestinal clock genes, and luminal nutrient sensors [17]. Previous work in SD rats has shown that rhythms of two clock genes, Per1 and Reverbβ, parallel the rhythm of Sglt1 mRNA, and that there is an inverse relationship between Per1 and Sglt1 expression [25]. Given these findings suggesting clock gene regulation of Sglt1, we analyzed expression of Per1 and Reverbβ as well as Bmal1 and Clock with which they have an interdependent relationship, in ZDF rats. We found that intestinal rhythms of the clock genes Clock, Bmal1, Per1, and Reverbβ were all maintained in both groups of rats. In db/db mice, Kudo et al. [26] found disruption of peripheral hepatic oscillators including Per2 and Bmal1 that could be restored with night-time restricted feeding. This is in contrast to our findings of preserved clock gene rhythms in ZDF rats, however, it may be accounted for by the different disease models, and the tissue-type studies (hepatic vs. intestinal oscillators in our studies).
Diurnal rhythmicity of the basolateral glucose transporter GLUT2 in the gut has been documented in rats [13, 27, 28]. Recruitment of GLUT2 to the intestinal brush border membrane at high glucose concentrations has been proposed in health [29] and in obesity [30]. The physiological significance of this observation, however, continues to be debated [31, 32]. Given the potential interdependence of SGLT1 and GLUT2, we also assessed GLUT2 intestinal rhythms in the ZDF strain. We noted an absence of circadian rhythmicity of GLUT2 in both obese and lean groups in this study, in contrast to findings in the above studies, which used physiologically healthy strains of rat [13, 27]. The loss of rhythmicity of GLUT2 in our study is consistent with the pattern we see for SGLT1 and suggests an overall dysregulation of carbohydrate absorption.
SGLT1 was measured in two ways in this study: mRNA levels by PCR and functional uptake using everted sleeves. The consistent results obtained with these two techniques give us confidence in our conclusions regarding the SGLT1 expression patterns [14, 17, 33]. Despite trialing several different SGLT1 and T1R2 antibodies for protein level assays, we have been unsuccessful in finding ones for which we think the resultant bands are acceptable (clear band at appropriate molecular weight). While we continue to search for acceptable antibodies, functional SGLT1 glucose uptake is taken here as a “surrogate” for SGLT1 protein expression, though we acknowledge that protein data on intestinal SGLT1 will provide a more complete picture on disrupted rhythms and at what transcriptional/translational level any disruptions occur.
Lean ZDF rats were selected as control animals for this study, which introduces increased variability in our data points as the lean rats with a heterozygous leptin receptor genotype are more phenotypically heterogeneous than homozygous wild-type rats. A possibly greater tendency toward impaired glucose tolerance and obesity in heterozygous rats could also explain the disrupted feeding patterns and blunting of circadian rhythm in SGLT1 function, consistent with blunting of other circadian rhythms that is seen with weight gain and progressive hyperglycemia [9, 10].
An interesting finding of this study is that of elevated levels of Sglt1 mRNA in the presence of lower levels of the sweet taste receptor T1r2 component in obese rats. Elements of the sweet taste signaling pathway, including T1R2, T1R3, α-gustducin and Transient Receptor Potential Membrane 5, have all been demonstrated in the proximal small bowel of humans, and Young et al. [34] found each to be inversely correlated with blood glucose levels in T2D subjects. They also showed that jejunal perfusion of glucose in mice reduces T1R2 expression by over 80 %. Our current findings of low levels of intestinal T1r2 mRNA in obese diabetic rats are consistent with this reported T1R2–glucose relationship, and suggest that it is the response of SGLT1 to T1R2/3 signaling that may be disrupted in T2D [34].
The leptin receptor gene mutation in obese and heterozygous lean ZDF rats, leading to hyperphagia, may explain the disrupted circadian feeding patterns. However, that the diabetic phenotype is only seen in obese rats implies that diabetes is the cumulative result of several factors. In these experiments, we limited our analysis to elements of intestinal tissue, but serum analysis for hormones involved in regulation of circadian rhythms, as well as those involved in the intestinal sweetness sensing pathway, will be the next step in trying to explicate etiology. Incretins (GLP-1, GIP) and 5-hydroxytryptamine acting on vagal receptors may be sending inappropriate feedback signals to intestinal glucose transporters and altered levels and/or disrupted rhythms of these hormones should be elucidated.
Determining whether changes in intestinal glucose handling directly contribute to impaired glucose tolerance and the diabetes phenotype or are a subsequent consequence will be an important step toward understanding the putative role of the intestine as an endocrine organ. In our study, both the fa/fa genotype (obese) and the fa/+ genotype (lean) of ZDF rat had some degree of disrupted circadian rhythms of intestinal genes and feeding. Understanding the contribution of phenotype and genotype to rhythmic changes is therefore critical. Improved glucose tolerance in both these rat strains upon limiting food intake to the dark phase (phase-restricted feeding) would provide evidence for the influence of feeding patterns on metabolic parameters, and may help determine the contribution of genotype to the disease phenotype in these rats. This is a future direction for our work.
In summary, we report disruption of circadian rhythmicity in intestinal glucose absorption as animals develop insulin resistance and then full-blown diabetes. These data suggest that in shift workers, disrupted eating patterns may predispose an individual to insulin resistance, which can progress to full T2D depending on other environmental factors and genotype. Elucidating the cause-effect relationships between obesity, T2D, disrupted SGLT1 rhythms and disrupted feeding rhythms may provide crucial new targets in the treatment of metabolic disease.
References
Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141.
Biggi N, Consonni D, Galluzzo V, Sogliani M, Costa G. Metabolic syndrome in permanent night workers. Chronobiol Int. 2008;25:443–454.
Culpepper L. The social and economic burden of shift-work disorder. J Fam Pract. 2010;59:S3–S11.
De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–854.
Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B. Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int. 2009;26:544–559.
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2102.
Arble DM, Vitaterna MH, Turek FW. Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS ONE. 2011;6:e25079.
Mathews CE, Wickwire K, Flatt WP, Berdanier CD. Attenuation of circadian rhythms of food intake and respiration in aging diabetes-prone BHE/Cdb rats. Am J Physiol. Regul Integr Comp Physiol. 2000;279:R230–R238.
Murakami DM, Horwitz BA, Fuller CA. Circadian rhythms of temperature and activity in obese and lean Zucker rats. Am J Physiol. 1995;269:R1038–R1043.
Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458.
Boden G, Chen X, Polansky M. Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 1999;48:2182–2188.
Corpe CP, Burant CF. Hexose transporter expression in rat small intestine: effect of diet on diurnal variations. Am J Physiol. 1996;271:G211–G216.
Houghton SG, Iqbal CW, Duenes JA, Fatima J, Kasparek MS, Sarr MG. Coordinated, diurnal hexose transporter expression in rat small bowel: implications for small bowel resection. Surgery. 2008;143:79–93.
Balakrishnan A, Stearns AT, Rounds J, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). Surgery. 2008;143:813–818.
Tavakkolizadeh A, Berger UV, Shen KR, et al. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G209–G215.
Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248.
Balakrishnan A, Stearns AT, Ashley SW, Tavakkolizadeh A, Rhoads DB. Restricted feeding phase shifts clock gene and sodium glucose cotransporter 1 (SGLT1) expression in rats. J Nutr. 2010;140:908–914.
Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg. 2010;251:865–871.
Fu WJ, Haynes TE, Kohli R, et al. Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135:714–721.
Karasov WH, Pond RS 3rd, Solberg DH, Diamond JM. Regulation of proline and glucose transport in mouse intestine by dietary substrate levels. Proc Natl Acad Sci USA. 1983;80:7674–7677.
Ferraris RP, Vinnakota RR. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr. 1995;62:540–546.
Bihler I, Freund N. Sugar transport in the small intestine of obese hyperglycemic, fed and fasted mice. Diabetologia. 1975;11:387–393.
Morton AP, Hanson PJ. Monosaccharide transport by the small intestine of lean and genetically obese (ob/ob) mice. Q J Exp Physiol. 1984;69:117–126.
Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:1–13.
Balakrishnan A, Stearns AT, Ashley SW, Rhoads DB, Tavakkolizadeh A. PER1 modulates SGLT1 transcription in vitro independent of E-box status. Dig Dis Sci. 2012;57:1525–1536.
Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436.
Tavakkolizadeh A, Ramsanahie A, Levitsky LL, et al. Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J Surg Res. 2005;129:73–78.
Ramsanahie AP, Berger UV, Zinner MJ, Whang EE, Rhoads DB, Ashley SW. Effect of glucagon-like peptide-2 (GLP-2) on diurnal SGLT1 expression. Dig Dis Sci. 2004;49:1731–1737.
Kellett GL, Brot-Laroche E. Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–3062.
Ait-Omar A, Monteiro-Sepulveda M, Poitou C, et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes. 2011;60:2598–2607.
Gorboulev V, Schurmann A, Vallon V, et al. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196.
Kellett GL. Comment on: Gorboulev et al. Na+-d-glucose cotransporter SGLT1 Is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012;61:187–196. Diabetes 2012; 61: e4; author reply e5.
Stearns AT, Balakrishnan A, Rhoads DB, Ashley SW, Tavakkolizadeh A. Diurnal expression of the rat intestinal sodium-glucose cotransporter 1 (SGLT1) is independent of local luminal factors. Surgery. 2009;145:294–302.
Young RL, Sutherland K, Pezos N, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337–346.
Acknowledgments
We thank Jan Rounds for invaluable managerial support, and Dr. Carel Le Roux at Imperial College, London, UK for his role as co-educational supervisor to HB. The manuscript has been presented as an oral presentation at the Society of Academic and Research Surgery, Nottingham UK (Jan 2012) and the Academic Surgical Congress, Las Vegas (Feb 2012). It was published in abstract form only in a supplementary issue of Journal of Surgical Research as related to this meeting. This study was funded by National Institute of Health Grant 1 R01 DK084064 (AT) and Harvard Clinical and Translational Science Center 5 KL2 RR025757 (AT).
Conflict of interest
HY Bhutta, TE Deelman, SW Ashley, and DB Rhoads have no conflicts of interest. A Tavakkoli has an equity interest in Avaxia Biologics, a company that is developing oral antibodies for treatment of intestinal disorders, with potential applications for treatment of diabetes and obesity. AT’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2013_2669_MOESM1_ESM.pptx
Diurnal expression of T1R2 (A) and GLUT2 (B) in obese (left) and lean (right) ZDF rats. Values are expressed as medians ± interquartile range, n = 8–11 per group per time point (PPTX 69 kb)
Rights and permissions
About this article
Cite this article
Bhutta, H.Y., Deelman, T.E., Ashley, S.W. et al. Disrupted Circadian Rhythmicity of the Intestinal Glucose Transporter SGLT1 in Zucker Diabetic Fatty Rats. Dig Dis Sci 58, 1537–1545 (2013). https://doi.org/10.1007/s10620-013-2669-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2669-y