Abstract
Background
Deguelin, a naturally occurring rotenoid, is known to be an Akt inhibitor and to have an anti-tumor effect on several cancers.
Aims
This study was performed to elucidate the effect of deguelin on apoptotic pathways related to NF-κB signaling in colon cancer cells and on the anti-tumor effect in colon cancer xenograft mice.
Methods
We studied COLO 205 and HCT116 cells in the presence or absence of deguelin. NF-κB signaling was examined by real-time RT-PCR for interleukin (IL)-8, by Western blotting for IκB phosphorylation/degradation, and by the electrophoretic mobility shift assay. Cell death was determined by the MTT assay, and apoptosis by Annexin V-FITC staining and caspase-3 activity. We also assessed the expression of antiapoptotic and proapoptotic factors by use of RT-PCR. In colon cancer xenograft mice, we evaluated the effect of deguelin on inoculated tumor growth, and apoptotic index was measured by the in vivo TUNEL assay.
Results
Deguelin significantly inhibited IL-8 gene expression, IκB phosphorylation/degradation, and DNA binding activity of NF-κB in colon cancer cells. Deguelin induced cell death and apoptosis in colon cancer cells in a dose and time-dependent manner. Deguelin down-regulated expression of NF-κB-mediated antiapoptotic factors such as cFLIP, Bcl-2, and Bcl-XL. In the colon cancer xenograft model, the volume of the tumor treated with deguelin was significantly lower than that of the control, and the apoptotic index for deguelin-treated mice was much higher.
Conclusion
Deguelin might be a potential therapeutic agent for treatment of colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the second leading cause of cancer-related mortality in USA. Moreover, the incidence of colorectal neoplasms in Asia, including Korea, is increasing, perhaps because of Westernized lifestyle, which in Asians is associated with increased fat intake and reduced physical activity [1]. The prognosis of advanced colorectal cancer is poor and most currently available therapeutic agents can be toxic, and are expensive and lacking in efficacy. Cytotoxic chemotherapy has been used with limited success for treatment of patients with advanced colorectal cancer, often at the expense of severe side-effects. Therefore, identifying novel effective chemotherapeutic agents for treatment of colon cancer with fewer side-effects is essential. Although prevention of cancer is preferred over treatment, recent evidence involving targeted therapy suggests convergence between prevention and treatment of cancer [2, 3].
Deguelin, a rotenoid isolated from several plant species, including Mondulea sericea, has been shown to be effective in chemoprevention in reducing the incidence of tobacco-induced lung tumorigenesis [4], chemically induced skin tumors in mice [5], mammary tumors in rats [5], and preneoplastic lesion formation in mouse mammary glands [6]. The mechanisms by which deguelin mediates its chemopreventive effects and inhibits carcinogenesis have not been fully elucidated. However, several mechanisms have been described, including inhibition of the PI3K/Akt pathway [4, 7], down-regulation of cyclooxygenase-2 [8], suppression of ornithine decarboxylase [9], induction of apoptosis by dysregulation of the cell-cycle checkpoint protein retinoblastoma [10], and an angiopreventive effect by acting on the NF-κB pathway. Activation of the NF-κB pathway is of critical importance in the development of colon cancer and inflammation-driven tumor progression in other mouse models. NF-κB is a transcription factor known to be linked to tumorigenesis, apoptosis, inflammation, and angiogenesis [11, 12].
In this study we evaluated the effect of deguelin on apoptotic pathways and its mechanisms related to NF-κB signaling in colon cancer cell lines in an in-vitro study and its anti-cancer effect in colon cancer xenograft mice in vivo.
Materials and Methods
Colon Cancer Cells and Treatment with Deguelin
Human colon cancer cell lines COLO 205 (KCLB 10222, Korean Cell Line Bank (KCLB), Seoul, Korea) and HCT 116 (KCLB 10247) were used between passages 15 and 30. Both cell types were grown as described elsewhere [13, 14]. Deguelin (Sigma, St Louis, MO, USA) was dissolved in DMSO as a 10 mM stock solution and stored at −20 °C. Cells were treated for 24 h with different concentrations of deguelin (0–300 nM) or with vehicle. Cells were stimulated with TNF-α (10 ng/ml; BioSource, Camarillo, CA, USA) for 4 h after exposure to deguelin or vehicle.
RNA Extraction and Real-Time RT-PCR Analysis
Deguelin-pretreated cells were stimulated with TNF-α for 1–4 h. Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) and 1 μg total RNA was reverse transcribed as described elsewhere [15]. Real-time PCR was performed using an ABI prism 7,000 sequence detection system (Applied Biosystems, Foster City, CA, USA) with specific primers for human IL-8. β-Actin for human IL-8 was used as an endogenous control. All primers were designed by Primer Express v2.0 (Applied Biosystems). The primer sequences used in this study are shown in Table 1. PCR was conducted using SYBR Green PCR Master Mix (Applied Biosystems) in accordance with the manufacturer’s instructions. Thermal cycler conditions were: one cycle of 10 min at 95 °C, followed by 40 amplification cycles for denaturation (15 s at 95 °C) and combined annealing/extension (1 min at 60 °C). Amplification was performed in triplicate and the data were normalized versus β–actin for human IL-8.
Western Blot Analysis
Cells were stimulated with TNF-α for 0-30 min with or without deguelin. Cell lysates containing 20 μg protein were subjected to electrophoresis in 10 % SDS–polyacrylamide gels, transferred to nitrocellulose membranes, and then blotted with primary antibodies at a dilution of 1:1,000. Peroxidase-conjugated secondary antibodies (BioSource) were incubated at a dilution of 1:2,000. Bound antibodies were visualized using a chemiluminescence substrate (ECL; Amersham, Buckinghamshire, UK) and exposed to Kodak X-OMAT film. Primary antibodies included anti-IκBα polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phosphoserine IκB (Cell Signaling, Beverly, MA, USA), and anti-β-actin (Santa Cruz Biotechnology).
Electrophoretic Mobility Shift Assay (EMSA)
Cells were stimulated for 30 min with TNF-α and nuclear extracts were prepared as previously described [13]. For the DNA binding assay, a double-stranded NF-κB oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′; Promega, Madison, WI, USA) was end-labeled with [γ-32P]ATP (3,000 Ci/mmol at 10 mCi/ml; Amersham) using T4 polynucleotide kinase (5–10 μg; Promega). Unlabeled oligonucleotides were removed using a G-25 spin column (Boehringer Mannheim, Germany). Equal amounts (5 μg) of nuclear extracts were then incubated for 20 min with the 32P-labeled DNA probe at room temperature. Complexes were separated on 4 % non-denaturing polyacrylamide gels, dried, and autoradiographed.
Cell Viability
Cells were seeded in 96-well plates at 2 × 104 cells per well and incubated for 24 h with serum starvation. Different concentrations (0–300 nM) of deguelin or vehicle were then added and the cells were incubated for 24 or 48 h. The effect of deguelin on cell viability was determined by use of the MTT (Sigma) assay [13]. Absorbance was determined at 540 nm by use of an ELISA plate reader.
Apoptosis Measurement
Percentage of deguelin-treated cells undergoing apoptosis was determined by use of an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Bioscience, San Jose, CA, USA). Externalization of phophatidylserine, an early apoptotic event, was analyzed by flow cytometry-based Annexin V-binding assays after treatment of COLO 205 and HCT 116 cells with different concentrations of deguelin for 24 h. Approximately 5 × 105 cells were resuspended with 5 μl Annexin V binding buffer (10 mM Hepes/NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2) and then incubated with 5 μl FITC-conjugated Annexin V and 5 μl PI for 15 min at room temperature. Using double labeling for Annexin V-FITC and PI, we separated early apoptotic (Annexin V+/PI−), late apoptotic or necrotic (Annexin V+/PI+), and viable cells (Annexin V−/PI−).
Caspase-3 Activity
Caspase-3 activity was determined by detection of the fluorophore 7-amino-4-trifluoromethylcoumarin (AFC) after cleavage from the labeled substrate DEVD-AFC (ApoTarget Caspase-3/CPP 32 Fluorimetric Protease Assay, BioSource). Briefly, deguelin or vehicle-treated cells (1 × 106) were washed with PBS and resuspended in cell lysis buffer for 10 min. Cells were treated with reaction buffer containing dithiothreitol according to the manufacturer’s instructions. After incubation of lysates with DEVD-AFC for 1 h at 37 °C, fluorescence was determined by use of a fluorimeter equipped with a 400-nm excitation filter and 505-nm emission filter.
RT-PCR Analysis for Pro or Anti-Apoptotic Factors
Total cellular RNA was isolated from deguelin-treated cells by use of Trizol (Invitrogen) and 1 μg was reverse-transcribed as described elsewhere [13]. cDNA was then amplified by PCR in 50 μl buffer containing 10 mM Tris (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 200 μM each of dATP, dCTP, dGTP, and dTTP in the presence of 25 pM each of the 5′ and 3′ primers. The primer sequences used in this study are listed in Table 2. PCR amplification was performed in a thermal cycler (GenAmp PCR system 9600; Perkin Elmer Cetus, Norwalk, CT, USA). PCR products were separated on 2 % NuSieve agarose gel (FMC Bioproducts, Rockland, ME, USA) and stained with ethidium bromide. Subsequently, PCR products were semiquantified by comparison with the PCR products of β-actin by use of an imaging densitometer (GS-670, Bio-Rad, Hercules, CA, USA).
Mice
Six-week-old male BALB/c athymic nude mice were purchased from Central Lab Animal (Seoul, Korea). They were allowed to acclimatize for 1 week before initiation of the experiment. They were maintained on a 12 h light/12 h dark cycle under pathogen-free conditions and fed with a standard diet and water ad libitum. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital.
Tumor Xenograft Model
Deguelin (1 or 3 mg/kg) or vehicle was administered by intraperitoneal injection once a day for three days before inoculation of the cells into BALB/c nu/nu mice (n = 6 per treatment group) as shown in Fig. 1a. We determined the dose as previously described [4]. Then, 5 × 106 COLO205 cells in 0.5 ml Matrigel (BD Bioscience) were inoculated subcutaneously into the right flank. Then, daily, mice were weighed, and the size of each tumor was measured with a digital caliper (Fisher Scientific, Pittsburgh, PA, USA). The tumor volume was calculated as 0.5 × width2 × length (mm3). Animals were sacrificed 30 days after inoculation of cells. The experiment was performed twice, independently.
Histological Evaluation, Immunohistochemistry, and In Vivo TUNEL Assay
For the histological examination, tumor tissues were fixed in 10 % buffered formalin, embedded in paraffin, and stained with hematoxylin–eosin. Immunohistochemistry (IHC) was performed as described elsewhere [16, 17]. The tumor proliferation index was determined by anti-Ki-67 antibody staining (Novo Castra, Newcastle, UK). The TUNEL assay was conducted with the ApopTag peroxidase in situ apoptosis detection kit (Chemicon, Temecula, CA, USA) in accordance with the manufacturer’s instructions. We used an anti-VEGF (Abcam, Cambridge, UK) antibody for assessment of the antiangiogenic effect of deguelin. We also used an anti-phospho-NF-κB p65 antibody (Cell Signaling) to evaluate the effect of deguelin on NF-κB signaling in vivo. We then used anti-rabbit biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, USA). We visualized the reaction with diaminobenzidine tetrahydrochloride containing 0.03 % hydrogen peroxide and counterstained with hematoxylin. For quantification of immunoexpression, photomicrographs of 640 × 480 pixels were obtained in noncoincident consecutive fields from each slide, at a magnification of 400×, with a digital camera adjusted to these values. The images obtained were analyzed by use of an imaging processing and analysis system (Softium Informatica, Sao Paulo, Brazil). At least 1,000 cells were counted, and the percentage of labeled cells (PLC) was determined by use of the equation PLC = (number of labeled cells/total counted cells) × 100(%).
Statistical Analysis
We expressed all data as mean ± SE. We used the Kruskal–Wallis test or multiple comparison by use of Tukey’s Studentized range test for comparison of differences between groups. We considered P < 0.05 as statistically significant.
Results
Deguelin Inhibits TNF-α Induced IL-8 mRNA Expression in Colon Cancer Cells
Real-time RT-PCR analysis showed that deguelin inhibited IL-8 gene expression at 1 h and 4 h post-stimulation in COLO 205 cells (Fig. 2). The transcription factor NF-κB is a downstream signal pathway of TNF-α. It is of crucial importance in signal-induced IL-8 gene expression in intestinal epithelial cells [18].
Experimental procedure for the xenograft model in mice (n = 6 for each group) and the effect of deguelin in the xenograft mouse model. a 5 × 106 COLO 205 cells were inoculated subcutaneously into the flank of BALB/c athymic nude mice. b Effect on gross morphology. Tumor volume was measured with a caliper on the day the mice were sacrificed. c Effect on tumor volume. Treatment with deguelin 3 mg/kg/day inhibited tumor growth by 51.5 % compared to vehicle. Values are means ± SE. P values were for comparison with the control group. d Representative microscopic images of tumor sections from animals treated with vehicle or deguelin at 1 or 3 mg/kg/day. In H&E sections (×100), an area showing eosinophilic cytoplasm and loss of nuclei within preserved cellular contours was increased with deguelin treatment in a dose-dependent manner. TUNEL assay sections (×200) showed increased apoptosis in deguelin-treated mice compared with vehicle-treated mice. Ki-67 sections (×200) showed fewer proliferating cells in tumors from deguelin-treated mice. VEGF sections (×200) showed a reduction in VEGF-expressing cells in tumors from deguelin-treated mice, compared with those from the vehicle-treated mice. Phospho-NF-κB p65 sections (×200) showed inhibition of NF-κB activation in tumors from deguelin-treated mice compared with those from vehicle-treated mice. e Quantification of the immunohistochemical staining. For deguelin-treated mice, statistical analysis showed higher expression in the TUNEL assay and lower expression of Ki-67, VEGF, and phospho-NF-κB p65 compared with vehicle-treated mice. PLC, percentage of labeled cells
Deguelin Blocks TNF-α-Induced IκBα Phosphorylation/Degradation and DNA Biding Activity of NF-κB in Colon Cancer Cells
We investigated levels of phosphorylation of a variety of downstream signaling proteins involved in NF-κB activation by use of Western blot analysis. Figure 3a shows that TNF-α induced IκBα phosphorylation and IκBα degradation in COLO 205 cells, effects which were reversed by deguelin. Phosphorylation and degradation of IκBα in TNF-α stimulated cells suggested that NF-κB could be translocated into the nucleus. We next evaluated the binding activity of NF-κB in TNF-α stimulated cells with deguelin or vehicle, using EMSA. As shown Fig. 3b, nuclear extracts from TNF-α-treated cells had strong DNA binding activity, which was markedly inhibited by deguelin pretreatment. These findings indicate that deguelin blocks NF-κB signaling by inhibiting IκBα phosphorylation/degradation and its binding to target DNA.
Effect of deguelin on TNF-α-induced IL-8 mRNA expression in colon cancer cells. COLO 205 cells were pretreated with deguelin (0–300 nM) for 24 h, and then stimulated with TNF-α (10 ng/ml) for 1–4 h. Total RNA (1 μg) was extracted, reverse-transcribed, and amplified with an ABI Prism 7,000 sequence-detection system using primers for human IL-8. mRNA levels are expressed as multiples of control values and are means from five independent experiments (mean ± SE, n = 5). Veh, vehicle. P values were for comparison with the TNF-α alone
Deguelin Reduced Cell Viability in MTT Assay
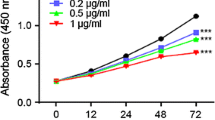
The MTT assay data showed that deguelin reduced cell viability. As shown in Fig. 4, deguelin induced cell death in a dose and time-dependent manner in both cell lines. Cell viability was reduced in deguelin-treated cells (22 % in COLO 205 cells and 61 % in HCT 116 cells).
Effect of deguelin on TNF-α-induced IκB phosphorylation/degradation and DNA binding activity of NF-κB in colon cancer cells. a COLO 205 cells were stimulated with TNF-α (10 ng/ml) for 0–30 min in the presence or absence of deguelin. Total protein was extracted, and 20 μg protein was subjected to SDS-PAGE, followed by phospho-IκB, IκB, and β-actin immunoblotting by use of the ECL technique. Results are representative of five independent experiments. b COLO 205 cells were stimulated with TNF-α (10 ng/ml) for 30 min in the presence or absence of deguelin. NF-κB activation was evaluated by measurement of its binding to an NF-κB oligonucleotide probe, by use of an electrophoretic mobility shift assay. Results are representative of five independent experiments. Veh, vehicle; De 50, deguelin 50 nM; De 300, deguelin 300 nM
Deguelin Potentiated Apoptosis Via Caspase-3 Activation
Apoptosis measurement and caspase-3 activity assay data showed that deguelin increased the proportion of apoptotic cells in a dose-dependent manner (Fig. 5a). Deguelin (300 nM) induced apoptosis in COLO 205 cells from 8 to 26 %. Caspase-3 activity, which is of crucial importance in apoptosis, was also increased by deguelin treatment in a dose-dependent manner (Fig. 5b) and we observed similar results in HCT 116 cells (data not shown).
Effect of deguelin on colon cancer cell viability. a Treatment of COLO 205 cells with 0–300 nM deguelin for 24 or 48 h reduced cell viability in a dose-dependent and time-dependent manner (mean ± SE, n = 5). IC50 value = 238.05 nM in 24 h, 94.76 nM in 48 h. b Similar results were observed for HCT 116 cells (mean ± SE, n = 5). IC50 value = 1,785.07 nM in 24 h, 113.64 nM in 48 h. P values were for comparison with the vehicle-treated group
Effect of deguelin on apoptosis in colon cancer cells. a Annexin V-FITC assay showing the effect of deguelin (0–300 nM) on COLO 205 cells. Deguelin treatment increased the number of early apoptotic cells (Annexin V+/PI−) (mean ± SE, n = 5). b Fluorimetric assay of caspase-3 activity in COLO 205 cells treated with deguelin 0–300 nM. Deguelin treatment enhanced caspase-3 activity in a dose-dependent manner (mean ± SE, n = 5). P values were for comparison with the vehicle-treated group
Deguelin Down-Regulated the Expression of Anti-Apoptotic Genes
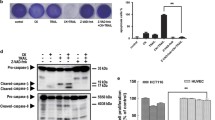
RT-PCR analysis data for anti-apoptotic factors indicated that deguelin down-regulated the expression of bcl-2, bcl-xL, and cFLIP which are NF-κB-regulated anti-apoptotic genes. The extent of down-regulation was more prominent in TNF-α treated cells. However, deguelin did not affect the gene expression of bak, bax, and cIAP (Fig. 6). A semiquantification study by use of a densitometer revealed statistically significant differences in cFLIP, bcl-2, and bcl- xL.
RT-PCR for anti-apoptotic and pro-apoptotic factors in colon cells. a COLO 205 cells were treated with deguelin (50 or 300 nM) or vehicle for 24 h with and without TNF-α stimulation. β-Actin was used as internal control. Deguelin inhibited the gene expression of cFLIP (S), Bcl-2, and Bcl-XL. These results are representative of data from five separate experiments. b Semi-quantification of RT-PCR products by use of an imaging densitometer. Veh, vehicle; De 50, deguelin 50 nM; De 300, deguelin 300 nM
Deguelin Inhibited Tumor Growth in the Xenograft Mouse Model by Induction of Tumor Cell Apoptosis, Inhibition of Tumor Angiogenesis, and Suppression of NF-κB
Deguelin treatment inhibited tumor growth in the xenograft mouse model in a dose-dependent manner (Fig. 1b, c). The mean size of the tumor was 1,614 mm3 in vehicle-treated mice and 782 mm3 in deguelin-treated mice (3 mg/kg/day). No statistically significant difference in body weight was observed between groups (data not shown).
Microscopic examination revealed that tumors from the vehicle-treated group contained dense viable tumor cells. In contrast, large areas of ischemic necrosis with eosinophilic cytoplasm and loss of nuclei within preserved cellular contours were observed in tumors from the deguelin-treated group, suggesting induction of apoptosis. Data from the TUNEL assay were consistent with these findings; tumor cell apoptosis increased with deguelin treatment. In addition, Ki-67 immunohistochemical staining revealed that tumors from deguelin-treated mice had fewer proliferating tumor cells than those from vehicle-treated mice, and the number of VEGF-expressing cells was markedly reduced (Fig. 1d). The results from phospho-NF-κB p65 staining showed that NF-κB signaling was inhibited by deguelin treatment in xenograft tumor similar to the in-vitro experiment. Taken together, these results show that deguelin suppressed tumor growth by inhibiting proliferation, increasing apoptosis, reducing angiogenesis, and suppressing NF-κB activation. Quantification revealed statistical differences in the immunohistochemical staining (Fig.1e).
Discussion
In this study, we demonstrated for the first time that deguelin exerts anti-cancer effects by inhibition of NF-κB signaling and induction of apoptosis in colon cancer cells in vitro and in vivo. A previous study showed that deguelin suppressed colonic preneoplastic lesions in a mouse model, which suggested potential chemopreventive effects of deguelin [19]. However, there has been no study suggesting a chemotherapeutic effect of deguelin or its mechanisms in vivo. We showed that deguelin inhibited colon cancer growth in a xenograft model in mice. Furthermore, deguelin treatment suppressed phospho- NF-κB p65 expression in xenograft tumor similar to the in-vitro experiment.
NF-κB, a key transcription factor in inflammatory responses, activates the transcription of numerous genes capable of suppressing apoptosis, suggesting crucial involvement in inflammation-related carcinogenesis [12]. In particular, a recent study showed that IκB kinase is related to inflammation and tumorigenesis in murine models of colon cancer [11]. NF-κB regulates the expression of numerous proinflammatory and immune-related genes including IL-8. A series of previous studies revealed an inhibitory effect of deguelin on NF-κB signaling in leukemic cells, lung adenocarcinoma, embryonic kidney carcinoma, and histiocytic lymphoma [20]. However, there has been no report of the effects of deguelin on NF-κB signaling in colon cancer cells. Our study showed that deguelin significantly reduced expression of IL-8 in colon cancer cells. To confirm the inhibitory effect of deguelin on NF-κB activation, we used two different methods for determination of NF-κB activity: IκBα phosphorylation/degradation by Western blot analysis, and DNA binding activity of NF-κB by EMSA. Both of these assays showed that deguelin inhibited NF-κB activation in colon cancer cells in a dose-dependent manner.
Next, we hypothesized that deguelin, an Akt inhibitor, could induce apoptosis in colon cancer cells. We expected that deguelin had anticancer effect by inducing apoptosis in colon cancer cells in addition to suppressing NF-κB activity. We used three different methods to determine the effects of deguelin on apoptosis: MTT assay, Annexin V-FITC assay, and fluorimetric assay of caspase-3 activity. All assays revealed that deguelin induced apoptosis in colon cancer cells in a dose-dependent manner. Furthermore, deguelin down-regulated the expression of NF-κB-mediated antiapoptotic factors, including cFLIP, Bcl-2, and Bcl-XL. NF-κB induces the expression of genes that code for anti-apoptotic proteins, for example cFLIP, Bcl-xL, and cIAP, whose products prevent cell death [12, 21]. FLIP inhibits apoptosis by binding to Fas-associated death domain protein (FADD) and procaspase-8 [22, 23]. In addition, NF-κB induces the expression of several Bcl-2 family members, most notably Bcl-xL, which prevents apoptosis by inhibiting permeability transition and depolarization of mitochondria and blocking of cytochrome release [12, 23]. Evasion of apoptosis is one of the hallmarks of cancer, and induction of apoptosis during tumor promotion is a critical step in chemoprevention [11]. In addition to inducing apoptosis in colon cancer cells, deguelin prevented colonic tumor growth in a xenograft model, suggesting it has a potential to be used as a chemotherapeutic agent for colon cancer.
In a xenograft model, deguelin treatment suppressed tumor growth in a dose-dependent manner. Immunohistochemistry showed that deguelin treatment induced apoptosis and inhibited VEGF expression. VEGF is also known to be regulated by NF-κB [24], and, in this study, deguelin inhibited VEGF expression in vivo. As for NF-κB inhibition, the same inhibition by deguelin treatment was observed in vivo, parallel to the in-vitro study. Taken together, these results from a xenograft model suggest that deguelin’s anticancer effects may arise not only from suppression of cell proliferation but also from apoptosis induction, inhibition of tumor angiogenesis, and suppression of NF-κB signaling. Moreover, a pharmacokinetic study using a rat model showed that the concentration of deguelin in the colon is high when administered intravenously [25].
In conclusion, this study demonstrates that deguelin down-regulates NF-κB activation, induces apoptosis in colon cancer cells, and inhibits tumor growth in a colon cancer xenograft model. Deguelin might be a potential therapeutic agent for treatment of colorectal cancer.
References
Byeon JS, Yang SK, Kim TI, et al. Colorectal neoplasm in asymptomatic Asians: a prospective multinational multicenter colonoscopy survey. Gastrointest Endosc. 2007;65:1015–1022.
Abbruzzese JL, Lippman SM. The convergence of cancer prevention and therapy in early-phase clinical drug development. Cancer Cell. 2004;6:321–326.
Aggarwal BB, Takada Y, Oommen OV. From chemoprevention to chemotherapy: common targets and common goals. Expert Opin Investig Drugs. 2004;13:1327–1338.
Lee HY, Oh SH, Woo JK, et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J Natl Cancer Inst. 2005;97:1695–1699.
Udeani GO, Gerhauser C, Thomas CF, et al. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid. Cancer Res. 1997;57:3424–3428.
Gerhauser C, Lee SK, Kosmeder JW, et al. Regulation of ornithine decarboxylase induction by deguelin, a natural product cancer chemopreventive agent. Cancer Res. 1997;57:3429–3435.
Chun KH, Kosmeder JW II, Sun S, et al. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst. 2003;95:291–302.
Lee HY, Suh YA, Kosmeder JW, et al. Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells. Clin Cancer Res. 2004;10:1074–1079.
Gerhauser C, Mar W, Lee SK, et al. Rotenoids mediate potent cancer chemopreventive activity through transcriptional regulation of ornithine decarboxylase. Nat Med. 1995;1:260–266.
Murillo G, Salti GI, Kosmeder JW 2nd, et al. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur J Cancer. 2002;38:2446–2454.
Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296.
Kucharczak J, Simmons MJ, Fan Y, et al. To be, or not to be: NF-kappaB is the answer–role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982.
Cho SJ, Kim JS, Kim JM, et al. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis-associated colon cancer in mice. Int J Cancer. 2008;123:951–957.
Kim JM, Kang HW, Cha MY, et al. Novel guggulsterone derivative GG-52 inhibits NF-kappaB signaling in intestinal epithelial cells and attenuates acute murine colitis. Lab Invest. 2010;90:1004–1015.
Lee JY, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Simvastatin inhibits NF-kappaB signaling in intestinal epithelial cells and ameliorates acute murine colitis. Int Immunopharmacol. 2007;7:241–248.
Nam SY, Kim JS, Kim JM, et al. DA-6034, a derivative of flavonoid, prevents and ameliorates dextran sulfate sodium-induced colitis and inhibits colon carcinogenesis. Exp Biol Med. 2008;233:180–191.
Cheon JH, Kim JS, Kim JM, et al. Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm Bowel Dis. 2006;12:1152–1161.
Jobin C, Hellerbrand C, Licato LL, et al. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut. 1998;42:779–787.
Murillo G, Kosmeder JW 2nd, Pezzuto JM, et al. Deguelin suppresses the formation of carcinogen-induced aberrant crypt foci in the colon of CF-1 mice. Int J Cancer. 2003;104:7–11.
Nair AS, Shishodia S, Ahn KS, et al. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J Immunol. 2006;177:5612–5622.
Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26.
Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190.
Fang LW, Tai TS, Yu WN, et al. Phosphatidylinositide 3-kinase priming couples c-FLIP to T cell activation. J Biol Chem. 2004;279:13–18.
Huang S, Pettaway CA, Uehara H, et al. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197.
Udeani GO, Zhao GM, Shin YG, et al. Pharmacokinetics of deguelin, a cancer chemopreventive agent in rats. Cancer Chemother Pharmacol. 2001;47:263–268.
Acknowledgments
This study was supported in part by grant no. 03-2009-0060 from the SNUH Research Fund. The authors thank Chi-Yeon Lim, Ph.D., for her assistance with statistics.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, H.W., Kim, J.M., Cha, M.Y. et al. Deguelin, an Akt Inhibitor, Down-Regulates NF-κB Signaling and Induces Apoptosis in Colon Cancer Cells and Inhibits Tumor Growth in Mice. Dig Dis Sci 57, 2873–2882 (2012). https://doi.org/10.1007/s10620-012-2237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2237-x