Abstract
Background and Aims
Epstein-Barr virus (EBV) is present in the malignant epithelial cells of 10% of all gastric adenocarcinomas; however, localization of the virus in normal gastrointestinal mucosa is largely unexplored. In the present study, we measured EBV DNA and localized viral gene products in gastritis specimens (n = 89), normal gastric and colonic mucosa (n = 14), Crohn’s disease (n = 9), and ulcerative colitis (n = 11) tissues.
Methods
A battery of sensitive and specific quantitative polymerase chain reactions targeted six disparate regions of the EBV genome: BamH1 W, EBNA1, LMP1, LMP2, BZLF1, and EBER1. EBV infection was localized by EBV-encoded RNA (EBER) in situ hybridization and by immunohistochemical stains for viral latent proteins LMP1 and LMP2 and for viral lytic proteins BMRF1 and BZLF1. B lymphocytes were identified using CD20 immunostains.
Results
EBV DNA was essentially undetectable in normal gastric mucosa but was present in 46% of gastritis lesions, 44% of normal colonic mucosa, 55% of Crohn’s disease, and 64% of ulcerative colitis samples. Levels of EBV DNA exceeded what would be expected based on the numbers of B lymphocytes in inflamed tissues, suggesting that EBV is preferentially localized to inflammatory gastrointestinal lesions. Histochemical staining revealed EBER expression in lymphoid cells of some PCR-positive lesions. The viral lytic viral proteins, BMRF1 and BZLF1, were expressed in lymphoid cells of two ulcerative colitis tissues, both of which had relatively high viral loads by quantitative PCR.
Conclusion
EBV-infected lymphocytes are frequently present in inflamed gastric and colonic mucosa. Active viral replication in some lesions raises the possibility of virus-related perpetuation of gastrointestinal inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic gastritis, which is typically Helicobacter pylori-related, is the earliest precursor lesion for gastric adenocarcinoma, followed by multistep progression of atrophic gastritis, intestinal metaplasia, dysplasia, carcinoma in situ, and invasive adenocarcinoma [1]. Epstein-Barr virus (EBV), a ubiquitous gamma herpesvirus, has been implicated in the pathogenesis of a variety of diseases including 10% of gastric adenocarcinomas where it is localized to the malignant epithelial cells [2]. The amount of EBV and its localization in normal gastric mucosa and gastritis lesions is largely unexplored even though the presence of monoclonal EBV genomes in gastric cancer suggests that infection occurs before neoplastic transformation [3, 4]. A better understanding of the role of EBV in disease pathogenesis could be useful in devising better preventive measures and treatments.
Anecdotal evidence of gastritis in infectious mononucleosis patients suggests that EBV can infect the gastric mucosa at the time of primary infection [5–8]. Interestingly, the proximal segment of the stomach is more inflamed, and this is the same segment where EBV-related carcinomas commonly arise. Virions are periodically shed in saliva of most healthy adults, suggesting one route of viral entry to the stomach is by swallowing, while another route is by hematogenous spread of the occasional infected B lymphocytes that are present in nearly all adults [2, 9]. In older adults, polyclonal or monoclonal EBV-driven lymphoproliferations are thought to result from age-related decline in immune response [10]. EBV-related mucosal ulcers have been described in immunosuppressed individuals [11].
Still unknown is the extent to which EBV is found in normal gastric mucosa and in the full range of gastritis lesions. Its presence in dysplasia and in chronic atrophic gastritis has been reported [4, 12–14], but there are conflicting data regarding frequency of infection and localization of EBV in various premalignant lesions [3, 4, 12, 14–22].
An interesting case report describes a lymphoepithelioma-like carcinoma arising in the sigmoid colon of a patient with ulcerative colitis in which EBER was localized to the malignant epithelial cells and also to many tumor-infiltrating lymphocytes [23]. Cross-reactive humoral immune responses to EBV antigens are proposed as a mechanism of autoimmunity, as is infection-driven persistence of autoreactive B cells [24–28]. It is feasible that active viral replication could also contribute to pathogenesis of inflammatory processes.
Colonic mucosa tends to harbor more chronic inflammatory cells than does gastric mucosa, suggesting that levels of EBV DNA might be different in these two anatomic sites depending on the proportion of infected B cells that reside there. The extent to which EBV levels correlate with levels of inflammatory infiltrate in various tissues could help solve whether EBV is an innocent bystander or is contributing in a pathogenic fashion to inflammatory lesions. Furthermore, if EBV localizes to benign epithelial cells, it could implicate EBV at an earlier stage of carcinogenesis than was previously recognized.
In this study, we examined the prevalence of EBV infection in normal and inflamed gastric and colonic mucosa using a battery of quantitative polymerase chain reaction (Q-PCR) assays targeting six disparate regions of the EBV genome. The rationale for using multiple Q-PCR assays was to confirm the specificity of positive results and to more precisely quantify viral load by targeting multiple genomic loci [29]. When EBV was detected by Q-PCR, infection was further characterized as latent or lytic using histochemical assays that also pinpoint the cell type that was infected. The number of B lymphocytes in each mucosal sample was estimated using CD20 immunohistochemical stains so that viral loads could be interpreted in the context of cell counts.
Materials and Methods
Patient Tissue and Blood Samples
We studied consecutive formalin-fixed, paraffin-embedded tissues from the clinical archives of two hospitals, the University of North Carolina Hospitals in Chapel Hill, and the Western Regional Hospital in Santa Rosa de Copan, Honduras. The gastritis tissues were from three subsets of gastritis patients—six adults from Honduras, 33 adults from the United States (U.S.), and 50 children (age 2 months to 20 years) from the United States. To explore gastritis lesions in association with cancer, the U.S. adult gastritis lesions were identified within gastrectomy specimens from patients with gastric adenocarcinoma. The colitis lesions were from U.S. inflammatory bowel disease patients—nine with Crohn’s disease and 11 with ulcerative colitis. All studies were done with approval of our Institutional Review Board.
Residual normal gastric mucosa was obtained, with informed consent, from five patients undergoing gastric bypass surgery. Paraffin blocks were prepared following fixation in 10% neutral buffered formalin. Additional controls included paraffin-embedded blocks of normal colonic mucosa from nine patients and meningioma tumors from 11 patients. Meningiomas were chosen as a control because they are not EBV-related and they have minimal lymphocytic infiltrate. Paraffin sections were placed on coated glass slides for histochemical stains, or placed in a microfuge tube for DNA extraction as previously described [29]. Whole blood samples (n = 10) from healthy donors served as controls for baseline levels of circulating EBV DNA. Total DNA was extracted from 200 μl of blood using the QIAmp Blood Kit (Qiagen Inc., Valencia, CA) following the manufacturer’s protocol. Purified DNA was eluted into 50 μl of AE buffer (Qiagen).
Quantitative Real-Time PCR as a Measure of EBV Viral Load
A battery of Q-PCR assays targeting six disparate regions of the EBV genome was used to measure EBV viral load in tissue and blood samples. We previously developed five of these Q-PCR assays targeting BamH1 W, EBNA1, LMP1, LMP2, and BZLF1 regions of the EBV genome [29], while the sixth assay targeting EBER1 DNA was developed by Ling et al. [30]. A Q-PCR assay targeting the human APOB gene was used to control for efficacy of DNA extraction and to normalize for the number of cells in the sample [29].
Q-PCR was performed and products were detected using ABI Prism 7900 or 7500 Real-Time PCR instruments and Sequence Detection System software (Applied Biosystems) as previously described [29]. Briefly, each 25-μL reaction contained: 1× TaqMan Universal Master Mix, forward and reverse primers (15 pmol each), and TaqMan probe (10 pmol). The assays targeting LMP1 and BZLF1 used 30 pmol each of the forward and reverse primers. Template volume was 1 μL of DNA from each paraffin-embedded tissue and 5 μL of DNA from whole blood. Thermocycling conditions were: 50°C for 2 min, 95°C for 10 min, and then 95°C for 15 s and 60°C for 1 min for 40 cycles. A standard curve was generated using serial tenfold dilutions of Namalwa DNA (two copies of EBV per cell) varying from 50,000 copies to 0.5 copies of EBV DNA. This curve was acceptable if a difference of 3.3 ± 0.3 cycles was demonstrated between each of the tenfold dilutions and if the correlation coefficient was at least 0.99. To check for amplicon contamination, every run contained at least two “no template” controls in which nuclease-free H2O was substituted for template. All experimental samples were run in duplicate and a mean viral load was calculated based on the ratio of the copies of EBV to cellular APOB in a given sample multiplied by 100,000, which represented the number of copies of EBV DNA per 100,000 cells. For purposes of statistical analysis, samples with no measurable EBV DNA were reported as having a viral load of zero.
EBV-Encoded RNA In Situ Hybridization
EBER in situ hybridization is considered the gold standard assay for detecting latent EBV infection. A manual method of EBER staining relied on a fluorescein-labeled oligonucleotide probe targeting EBER RNA (Biogenex, San Ramon, CA) and the Super-Sensitive Poly-HRP ISH Non-Biotin Detection Kit (Biogenex) with methyl green counterstain. An automated EBER stain method was carried out on the Ventana Benchmark in situ hybridization system according to the manufacturer’s protocol. An oligo d(T) probe targeting poly(A) tails of mRNA served as a control for RNA preservation. No significant discrepancies were observed between the manual and automated EBER staining procedures among 56 samples tested by both methods. A tissue was considered EBER-negative if no EBER signal was seen in any nuclei. EBER-positive cell types were characterized by cytomorphology.
LMP1, LMP2A, BMRF1, and BZLF1 Expression by Immunohistochemistry
Immunohistochemical analysis for the latent viral proteins, LMP1 and LMP2, was performed as described [31] using citrate antigen retrieval and the anti-EBV LMP1 CS1-4 cocktail of mouse monoclonal antibodies (1:100, Dako, Carpinteria, CA) or the LMP2A E411 rat monoclonal antibody (1 mg/ml, Asencion, Munich, Germany). EBER-positive Hodgkin lymphoma slides served as positive controls for both assays. Results were interpreted microscopically by localizing the chromagen to the cytoplasm and membrane of target cells.
Immunohistochemical analysis of the lytic EBV proteins, BMRF1 and BZLF1, was performed using citrate antigen retrieval and BMRF1 G3-E31 antibody (1:200, Research Diagnostics, Inc., Flanders, NJ) or BZLF1 BZ.1 antibody (1:25, Dako). Sections were incubated with primary antibody for 30 min at 37°C using the manufacturer’s blocking and detection protocols in the Super-Sensitive Non Biotin HRP Detection Kit (Biogenex). Bound antibody was detected by diamino-benzidine chromogen (Biogenex) and tissues were counter-stained with hematoxylin (Dako) to permit microscopic nuclear localization of the chromagen. Oral hairy leukoplakia paraffin sections served as the positive control.
Estimation of B Lymphocyte Number Using CD20 Immunohistochemistry
Immunohistochemical analysis of CD20 was performed using prediluted CD20 (L26) primary antibody (Chemicon International, Temecula, CA) and the IHC Select Immunophosphatase Secondary Detection system (Chemicon) with Fast Red chromogen. Conventional light microscopy was used to estimate the proportion of cells that expressed CD20 across nine different 40× fields.
Results
Levels of EBV DNA in “Normal” Tissue
In order to determine baseline levels of EBV in healthy individuals, we examined whole blood from healthy donors (n = 10), normal gastric mucosa (n = 5), and normal colon mucosa (n = 9) for the presence of EBV DNA using a battery of six EBV Q-PCR assays (Table 1). As expected, extremely low level EBV DNA was detected in the peripheral blood of healthy donors (mean viral load = 0.7 EBV DNA copies/100,000 nucleated cells). EBV DNA was rarely detected in normal gastric mucosa in keeping with the paucity of lymphoid tissue in the stomach (Table 2). Only 1/5 (20%) normal gastric mucosa had detectable EBV DNA, and that case had <1 copy of viral DNA per 100,000 cells (about one in a million cells). EBER in situ hybridization was completely negative in normal gastric mucosa, while 4/9 normal colon samples (44%) had detectable EBER-positive lymphocytes in correlation with detectable EBV DNA by Q-PCR in the same four colonic mucosa samples (average viral load = 2 EBV DNA copies/100,000 cells). It was concluded that EBV is present at low levels in normal whole blood, stomach, and colon tissues of immunocompetent individuals.
EBV Levels in Gastritis Lesions
Gastritis lesions (n = 89) from three different clinical settings (cancer-associated, adult, and pediatric) were screened using a battery of six Q-PCR assays targeting disparate regions of the EBV genome. EBV DNA was detected by at least one of the Q-PCR assays in 21/33 (64%) cancer-associated gastritis lesions, in 5/6 (83%) adult gastritis lesions, and in 15/50 (30%) pediatric gastritis lesions. The mean viral load for each type of gastritis was 41, 32, and 47 EBV DNA copies/100,000 cells, respectively, with an average of 40 EBV DNA copies/100,000 cells.
The EBV viral loads for cancer-associated gastritis were fairly consistent across the six Q-PCR assays (see Fig. 1). Interestingly, 3/33 (9%) cancer-associated gastritis samples (cases 3, 6, and 7) had detectable EBV DNA by all six Q-PCR assays and were located adjacent to EBER-positive gastric cancers. It should be clarified that no tumor was seen by microscopy in these gastritis lesions that were sampled from the same stomach where gastric adenocarcinoma was present. The remaining 30 cancer-associated gastritis lesions were adjacent to EBER-negative cancers, and about half of these gastritis lesions had measurable EBV loads by at least one Q-PCR assay. When EBV DNA was detected by only one Q-PCR assay, there was a preference for detecting the BZLF1 region of the viral genome, suggesting that either this assay is more sensitive than the other five assays or that it detects a naturally amplified DNA independent of the complete viral genome.
Trends of Epstein-Barr virus (EBV) viral load in gastritis lesions. a Some cancer-associated gastritis tissues had EBV DNA detected by three or more Q-PCR assays (left panel) while others were detected by only one or two Q-PCR assays (right panel). b Honduran adult gastritis tissues (left panel) consistently had detectable EBV DNA by any of five Q-PCR assays but had unamplifiable LMP1 genes, suggesting that the LMP1 assay is less sensitive than the other five assays, whereas pediatric gastritis tissues (right panel) had preferential detection of the BamH1 W and BZLF1 regions of EBV DNA
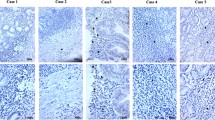
To help resolve the particular cell types that were infected, EBER in situ hybridization was performed on each gastritis case that had detectable EBV DNA by at least one Q-PCR assay (n = 41). Rare EBER-positive cells were observed in 7/21 cancer-associated gastritis and 3/5 adult gastritis tissues. In contrast, no EBER expression was observed in the pediatric gastritis cases. When present, the EBER signal was localized to small to medium lymphoid cells and was never seen in epithelial cells or stromal cells (see Fig. 2). This suggests that the amplifiable EBV DNA was derived, at least in part, from infected lymphocytes.
Epstein-Barr virus (EBV) is localized to lymphoid cells in inflamed gastric tissues. Epstein-Barr virus encoded RNA (EBER) is expressed in rare lymphoid cells as shown by nuclear localization of purple signal to a single small round nucleus in the inflammatory cell infiltrate associated with each of two gastric adenocarcinomas. The malignant epithelial cells and stromal cells were EBER-negative in both cases (×200)
Detection of EBV in Crohn’s Disease and Ulcerative Colitis Tissues
Crohn’s colitis (n = 9) and ulcerative colitis (n = 11) tissues were tested for EBV using the six Q-PCR assays and EBER in situ hybridization to quantify and localize EBV infection. EBV DNA was detected by at least one Q-PCR assay in 5/9 (55%) Crohn’s disease and 7/11 (64%) ulcerative colitis tissues (Table 3). The overall mean viral load was higher in ulcerative colitis (176 EBV DNA copies/100,000 cells) compared to Crohn’s disease (27 EBV DNA copies/100,000 cells) or gastritis (40 EBV DNA copies/100,000 cells), but these differences were not statistically significant.
EBER-positive lymphoid cells were observed in 1/5 Crohn’s disease samples and in 5/7 ulcerative colitis samples. Furthermore, EBER localization was limited to rare scattered lymphoid cells in the Crohn’s disease, while three of the ulcerative colitis samples had strikingly positive EBER signal in as many as 20% of lymphocytes. EBER was not seen in epithelial cells or in other stromal cell types besides small to large lymphoid cells.
Histochemical Localization of Latent and Lytic EBV Proteins
A possible explanation for the observed increased viral load in gastritis and colitis compared to normal gastric and colonic mucosa could be infiltrating chronic inflammatory cells, thus escalating the frequency at which random EBV-infected B cells happen to be present. An alternate hypothesis is that EBV infected lymphoid cells are preferentially found in these lesions and they might even contribute to pathogenesis of these diseases. Higher viral loads could be a consequence of viral replication by which cells enter the lytic phase of infection and replicate the viral genome. To address these possibilities, immunohistochemistry for LMP1 and LMP2A was performed to detect latent viral proteins, and immunohistochemistry for BMRF1 and BZLF1 was performed to detect lytic viral proteins. Neither latent nor lytic EBV proteins were expressed in normal gastric or colon mucosa, gastritis, or Crohn’s disease samples. However, BMRF1 and BZLF1 proteins were observed in scattered lymphoid cells in two of five ulcerative colitis cases, and both cases also expressed EBER in many lymphocytes (see Fig. 3). No latent or lytic gene expression was seen in non-lymphoid cells. These results suggest that high viral DNA levels in some ulcerative colitis lesions could result in part from EBV replication and in part from relatively high numbers of latently infected lymphocytes. In contrast, the lower levels of EBV found in gastritis and Crohn’s disease lesions appear to derive from latently infected lymphoid cells.
Sampling error may account for the failure to localize latent or lytic viral infection in some lesions in which EBV DNA was amplifiable. Extracellular EBV DNA cannot be excluded as a source of PCR amplifiable viral DNA.
B lymphocytes in Normal or Chronically Inflamed Gastrointestinal Epithelium
To study whether EBV viral load elevation is merely the result of higher numbers of infiltrating B lymphocytes, we estimated the number of B cells in normal versus inflamed tissue using an immunohistochemical stain for CD20. First, a series of meningiomas (n = 11) were studied because they represent a control tissue that has very little inflammatory cell infiltrate and is not EBV-related. It is thought that this tumor of the membranes lining the brain or spinal cord harbors few inflammatory cells (beyond those transiting inside blood vessels) as a consequence of the tight capillary structure of the “blood–brain barrier.” The six Q-CPR assays and EBER in situ hybridization were applied, and EBV DNA was detected by at least one Q-PCR assay in 2/11 meningiomas (18%). The viral load (0.3 EBV DNA copies/100,000 cells) was similar to that of whole blood from healthy individuals (0.7 EBV DNA copies/100,000 cells). No EBER-positive cells were observed in meningioma tissues. B lymphocytes accounted for less than 0.5% of cells in meningioma specimens (mean = 4 CD20+ cells in 40× field). These findings provide a baseline by which to compare measured values in normal and inflamed gastrointestinal tissues.
Next, B cells were quantified in mucosal specimens of the colon and stomach. Compared with normal colon, there were more B lymphocytes in Crohn’s disease (mean = 78 CD20+ cells per 40× field) and ulcerative colitis (mean = 75 CD20+ cells per 40× field), with an estimated 4% of the cells in the Crohn’s disease and ulcerative colitis samples being B lymphocytes. In contrast, approximately 2% of the cells in the gastritis, normal gastric mucosa, and normal colon samples were B lymphocytes (mean = 41, 33, and 49 CD20+ cells per 40× field, respectively). These results, combined with viral loads described above, suggest that higher EBV viral loads in inflamed tissues reflect, at least in part, an increase in the number of infiltrating B lymphocytes. However, the proportional increase in EBV levels was far higher than the proportional increase in B cell infiltrates for inflamed versus normal mucosa, implying that EBV level is not solely dependent on B cell number. This result suggests that EBV is preferentially localized to inflammatory lesions of the stomach and colon beyond what is expected if EBV were an innocent bystander in random B lymphocytes.
Helicobacter pylori Status
Helicobacter pylori serologic testing had been performed clinically in only a fraction of the gastritis patients, so we performed microscopy to look for evidence of bacteria on the mucosal surface consistent with helicobacter infection. Evidence of infection was found in 5/6 Honduran adult gastritis patients, 9/50 U.S. pediatric gastritis patients, and only 1/33 U.S. patients with concomitant gastric cancer. The latter result is likely an underestimate of the true infection status given the reported loss of bacterial organisms as cancer develops [1]. A more systematic approach to bacterial detection would be required in order to explore association with EBV in normal and inflamed gastric mucosae.
Discussion
This study used state-of-the-art molecular and histochemical methods to examine the prevalence of EBV in gastrointestinal tissues and controls. In healthy individuals, there are estimated to be approximately 1–50 viral genome-carrying cells per million blood cells [9]. In our own series of ten healthy adult blood donors, we measured an EBV viral load of approximately seven EBV DNA copies per million nucleated blood cells. In paraffin-embedded tissue, our battery of six Q-PCR assays confirmed presence of the EBV genome when multiple assays were positive, and the fairly consistent viral loads across multiple positive results helped assure the accuracy of viral DNA measurements.
Since EBV is a ubiquitous virus that infects the majority of humans without major adverse health consequences [2], it is important to distinguish between normal background levels of EBV and abnormally increased levels of EBV that could suggest pathogenicity. This study advances the field by showing that (1) EBV DNA levels vary with the degree of chronic inflammation, (2) EBV is restricted to lymphoid cells in benign gastrointestinal mucosa, (3) active viral replication can occur in lymphoid cells of ulcerative colitis, and (4) EBV DNA levels are disproportionately high in inflamed gastrointestinal mucosa compared to normal mucosa, even after accounting for differences in the numbers of B lymphocytes that are present. Overall, these findings suggest that EBV infection may contribute to the pathogenesis of gastritis and colitis.
It is important to study EBV infection in the stomach because of the potential link to EBV-related gastric cancer. We found that the EBV viral load in normal gastric mucosa is extremely low (<1 EBV DNA copy/100,000 cells), similar to that of whole blood from healthy donors. Likewise, histochemical stains revealed no evidence of EBV infection in normal gastric mucosa and rare EBER-expressing lymphoid cells in a subset of PCR-positive gastritis lesions which we interpret as being related to occasional infected cells in either the scant inflammatory infiltrate or the intravascular compartment. We cannot exclude the possibility that virions swallowed with saliva could have adhered to the surface of gastric mucosa and given rise to the EBV DNA that was measured.
It is important to know if EBV is routinely present in gastritis or whether it enters the mucosa in concert with the onset of EBV-associated neoplasia. Support for early infection comes from Levine et al., who found that EBV serologic titers are elevated prior to diagnosis of EBV-related gastric cancer [32]. Tissue-based studies have been inconsistent on the extent of EBV in gastritis lesions [13, 14, 16, 17, 21, 22]. Yanai et al. [14] reported finding EBV DNA in rare benign epithelial cells by in situ hybridization in three of 20 cases (15%) of chronic atrophic gastritis. A second group supported this finding, while a third group could not confirm the finding even when using similar methods [18, 19]. Zur Hausen et al. [33] suggested that EBV infection was a late event during carcinogenesis based on lack of detectable EBV in intestinal metaplasia or dysplasia. However, others have localized EBER transcripts to dysplastic lesions, suggesting that EBV infection correlates with the onset of neoplastic cell growth [3, 4].
In the present study, EBV DNA was detected in many gastritis specimens at levels higher than normal gastric mucosa, and rare EBER-expressing lymphocytes were identified as a potential source of the virus. EBER was not seen in benign gastric epithelial cells, a finding that is supported by several other investigators [4, 5, 16, 34–37]. Nor was active viral replication implicated as a source of the viral DNA, since neither BZLF1 nor BMRF1 were expressed in gastritis. While further work is required to more completely understand sources of viral DNA, our findings implicate latently infected lymphocytes as a reservoir. If expressed at all, LMP1 and LMP2A protein must be at a low level or in such rare cells as to be undetected by our immunohistochemical staining procedure.
In terms of geographic variation, the Honduran gastritis tissues were more frequently EBV infected than were the U.S. adult or U.S. pediatric gastritis cases (83, 64 and 30% of cases harboring EBV DNA, respectively). This may be clinically relevant because Hondurans have a very high rate of Helicobacter pylori infection and also a high rate of gastric cancer [38, 39]. Both EBV and Helicobacter pylori are classified as class 1 carcinogens by the World Health Organization, and a substantial fraction of individuals become co-infected by adulthood [40, 41]. These two pathogens could potentially synergize to cause and perpetuate chronic gastritis [21]. For example, if an EBV-infected B cell were activated by exposure to a foreign epitope of Helicobacter pylori, then terminal differentiation of the host B cell would trigger the virus to switch from the latent to lytic phase of its life cycle, thus inciting further inflammation as the immune system recognizes and fights both infections. The resulting cytokine response could trigger infiltration of more lymphocytes, a few of which are EBV infected, thus perpetuating the cycle of infection and inflammation that manifests clinically as chronic gastritis. A proposed model for this process is depicted in Fig. 4. An analogous process could occur in the colon where enteric bacterial pathogens are sources of antigenic stimuli.
A model for synergistic cooperation between Helicobacter pylori and EBV infection in the etiology of gastritis. Helicobacter pylori or similar inflammatory stimulus recruits lymphocytes to the gastric mucosa. If an EBV-infected B cell were activated by exposure to foreign bacterial antigen, then terminal differentiation of the host B-cell would trigger the virus to switch from latent to lytic phases of infection, thus inciting more inflammation as the immune system recognizes and fights active viral infection. The resulting cytokine response triggers infiltration of even more lymphocytes, a few of which are incidentally EBV infected. This inflammation, along with infection of naïve B cells, perpetuates the cycle of infection and inflammation that manifests clinically as chronic gastritis
To date, EBV has not been shown to be involved in colorectal carcinoma [42]. But EBV and other herpes family viruses, CMV and HHV6, have been implicated in the pathogenesis of inflammatory bowel disease [43–50]. Two groups describe finding PCR-amplifiable EBV DNA in inflammatory bowel disease tissues [44, 48]. Yanai et al. [47] localized EBER expression to non-epithelial cells (B lymphocytes and histiocytic cells) in both Crohn’s disease and ulcerative colitis tissues. Gehlert et al. [45] described preferential localization of EBER-expressing cells to zones of active inflammation in both diseases. However, Spieker et al. [43] showed that EBER-expressing lymphocytes were only slightly more prevalent in Crohn’s disease than in chronic appendicitis or diverticulitis, implying no special relation of the virus with Crohn’s colitis. Recent studies have shown that EBV-positive lymphocytes accumulate transmurally in ulcerative colitis, suggesting the colon as a potential site for EBV replication and transmission [43, 45].
Extending previous findings, the present study detected EBV DNA in 55% of Crohn’s disease and 64% ulcerative colitis tissues, with mean viral loads significantly higher in these lesions than in normal colon. Some ulcerative colitis lesions contained abundant EBER-expressing lymphocytes with evidence of active lytic viral replication. Histochemical stains revealed no cell type other than lymphocytes harboring EBV in normal colonic tissue or in any of the other benign tissues that we examined. This implies that infection of benign epithelial cells is very rare or absent.
We further examined whether the differences in EBV viral load among tissue types merely reflect the proportion of B lymphocytes present in those tissues. After accounting for the number of infiltrating B lymphocytes, the excess EBV DNA found in gastritis samples seems beyond the level expected if the virus were merely a passenger in random infiltrating B lymphocytes. Interestingly, the EBV viral load for ulcerative colitis was greater than for Crohn’s disease, even though the percentage of B lymphocytes (4%) was similar. Ulcerative colitis lesions harbor T helper 2 (Th2) cells producing cytokines (e.g. IL5, IL6) known to be growth factors for EBV-infected lymphocytes [51, 52]. Active viral replication may further contribute to high viral loads. Since viral replication can be inhibited by nucleoside analogs such as gancyclovir [53], it is worth considering whether antiviral agents might diminish mucosal inflammation and reduce risk of carcinogenesis. Other antiviral strategies include unmasking immunogenic viral peptides, infusing EBV-specific cytotoxic T cells, or thwarting downstream effects of viral infection [54, 55]. Vaccination could also be considered as an approach to preventing inflammatory sequelae.
A limitation of our study is the confounding variables of two geographic sites, two age groups, and presence or absence of concomitant cancer. Further exploration of EBV and other elements of the virome are required to understand the epidemiology of virus-related inflammation and pre-neoplasia in the gastrointestinal tract [56, 57]. Of special interest is the “field effect” by which cancer seems to arise in mucosa that already has some molecular fingerprints of malignancy [58]. Indeed, viral infection could represent one factor among the accumulating epigenetic and genetic changes along the pathways of carcinogenesis. Once cancer develops, malignant cells could potentially secrete factors influencing the function of surrounding tissues. In this regard, it is interesting to note our consistent detection of all six segments of the EBV genome in gastritis lesions located adjacent to EBV-related gastric cancer. A possible source of PCR template is inter-cellular transmission of DNA in exosomes or other microvesicles [59, 60].
In summary, our work shows that EBV levels are higher in inflamed gastrointestinal mucosa compared to normal counterparts. By histochemical analysis, latent and lytic EBV infection was localized to lymphocytes but not to benign epithelial cells of inflamed gastric and colonic mucosa. Further work is warranted to understand the role of EBV in pathogenesis of gastritis and colitis and to explore how antiviral strategies could ameliorate inflammation and associated cancer predisposition.
References
Correa P, Piazuelo MB, Camargo MC. The future of gastric cancer prevention. Gastric Cancer. 2004;7:9–16.
Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn. 2008;10:279–292.
Imai S, Koizumi S, Sugiura M, et al. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci USA. 1994;91:9131–9135.
Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996;27:20–27.
Zhang Y, Molot R. Severe gastritis secondary to Epstein-Barr viral infection. Unusual presentation of infectious mononucleosis and associated diffuse lymphoid hyperplasia in gastric mucosa. Arch Pathol Lab Med. 2003;127:478–480.
Owens SR, Walls A, Krasinskas AM, Rund CR. Epstein-barr virus gastritis: rare or rarely sampled? A case report. Int J Surg Pathol. 2011;19:196–198.
Toll AD, Malik S, Tuluc M. Ulcerative gastritis secondary to Epstein-Barr viral infection. Dig Dis Sci. 2010;55:218–219.
Kitayama Y, Honda S, Sugimura H. Epstein-Barr virus-related gastric pseudolymphoma in infectious mononucleosis. Gastrointest Endosc. 2000;52:290–291.
Babcock GJ, Decker LL, Freeman RB, Thorley-Lawson DA. Epstein-barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J Exp Med. 1999;190:567–576.
Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726–4735.
Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer—a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417.
Schneider BG, Gulley ML, Eagan P, Bravo JC, Mera R, Geradts J. Loss of p16/CDKN2A tumor suppressor protein in gastric adenocarcinoma is associated with Epstein-Barr virus and anatomic location in the body of the stomach. Hum Pathol. 2000;31:45–50.
Arikawa J, Tokunaga M, Tashiro Y, et al. Epstein-Barr virus-positive multiple early gastric cancers and dysplastic lesions: a case report. Pathol Int. 1997;47:730–734.
Yanai H, Takada K, Shimizu N, Mizugaki Y, Tada M, Okita K. Epstein-Barr virus infection in non-carcinomatous gastric epithelium. J Pathol. 1997;183:293–298.
Arikawa J, Tokunaga M, Satoh E, Tanaka S, Land CE. Morphological characteristics of Epstein-Barr virus-related early gastric carcinoma: a case-control study. Pathol Int. 1997;47:360–367.
Hungermann D, Muller S, Spieker T, Lisner R, Niedobitek G, Herbst H. Low prevalence of latently Epstein-Barr virus-infected cells in chronic gastritis. Microsc Res Tech. 2001;53:409–413.
Hirano A, Yanai H, Shimizu N, et al. Evaluation of epstein-barr virus DNA load in gastric mucosa with chronic atrophic gastritis using a real-time quantitative PCR assay. Int J Gastrointest Cancer. 2003;34:87–94.
Fukayama M, Chong JM, Uozaki H. Pathology and molecular pathology of Epstein-Barr virus-associated gastric carcinoma. Curr Top Microbiol Immunol. 2001;258:91–102.
Jing X, Nakamura Y, Nakamura M, et al. Detection of Epstein-Barr virus DNA in gastric carcinoma with lymphoid stroma. Viral Immunol. 1997;10:49–58.
Zur Hausen A, van Rees BP, van Beek J et al. Epstein-Barr virus in gastric carcinomas and gastric stump carcinomas: a late event in gastric carcinogenesis. J Clin Pathol. 2004;57:487–491.
Shukla SK, Prasad KN, Tripathi A, et al. Epstein-Barr virus DNA load and its association with Helicobacter pylori infection in gastroduodenal diseases. Braz J Infect Dis. 2011;15:583–590.
Minoura-Etoh J, Gotoh K, Sato R, et al. Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein-Barr virus (EBV) in gastric epithelial cells latently infected with EBV. J Med Microbiol. 2006;55:905–911.
Kojima Y, Mogaki M, Takagawa R, et al. A case of lymphoepithelioma-like carcinoma of the colon with ulcerative colitis. J Gastroenterol. 2007;42:181–185.
Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003;24:584–588.
Vaughan JH, Nguyen MD, Valbracht JR, Patrick K, Rhodes GH. Epstein-Barr virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. J Clin Invest. 1995;95:1316–1327.
Harley JB, Harley IT, Guthridge JM, James JA. The curiously suspicious: a role for Epstein-Barr virus in lupus. Lupus. 2006;15:768–777.
Lunemann JD, Munz C. Epstein-Barr virus and multiple sclerosis. Curr Neurol Neurosci Rep. 2007;7:253–258.
Swanson-Mungerson M, Longnecker R. Epstein-Barr virus latent membrane protein 2A and autoimmunity. Trends Immunol. 2007;28:213–218.
Ryan JL, Fan H, Glaser SL, Schichman SA, Raab-Traub N, Gulley ML. Epstein-Barr virus quantization by real-time PCR targeting multiple gene segments: a novel approach to screen for the virus in paraffin-embedded tissue and plasma. J Mol Diagn. 2004;6:378–385.
Ling PD, Vilchez RA, Keitel WA, et al. Epstein-Barr virus DNA loads in adult human immunodeficiency virus type 1-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2003;37:1244–1249.
Glaser SL, Gulley ML, Borowitz MJ, et al. Inter- and intra-observer reliability of Epstein-Barr virus detection in Hodgkin lymphoma using histochemical procedures. Leuk Lymphoma. 2004;45:489–497.
Levine PH, Stemmermann G, Lennette ET, Hildesheim A, Shibata D, Nomura A. Elevated antibody titers to Epstein-Barr virus prior to the diagnosis of Epstein-Barr-virus-associated gastric adenocarcinoma. Int J Cancer.1995;60:642–644.
van Beek J, van Zur Hausen A, Klein Kranenbarg E, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664–670.
Oda K, Koda K, Takiguchi N, Nunomura M, Seike K, Miyazaki M. Detection of Epstein-Barr virus in gastric carcinoma cells and surrounding lymphocytes. Gastric Cancer. 2003;6:173–178.
Luqmani YA, Linjawi SO, Shousha S. Detection of Epstein-Barr virus in gastrectomy specimens. Oncol Rep. 2001;8:995–999.
Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma. Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer. 1994;73:2239–2249.
Yuen ST, Chung LP, Leung SY, Luk IS, Chan SY, Ho J. In situ detection of Epstein-Barr virus in gastric and colorectal adenocarcinomas. Am J Surg Pathol. 1994;18:1158–1163.
Morgan DR, Dominguez RL, Keku TO, et al. Gastric cancer and the high combination prevalence of host cytokine genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol Hepatol. 2006;4:1103–1111.
Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378:507–514.
Cho HJ, Kim JY, Yoo J, Lee SS. Gastric carcinoma with lymphoid stroma: incidence of EBV and Helicobacter pylori infection. Appl Immunohistochem Mol Morphol. 2003;11:149–152.
Normark S, Nilsson C, Normark BH, Hornef MW. Persistent infection with Helicobacter pylori and the development of gastric cancer. Adv Cancer Res. 2003;90:63–89.
Wong NA, Herbst H, Herrmann K, et al. Epstein-Barr virus infection in colorectal neoplasms associated with inflammatory bowel disease: detection of the virus in lymphomas but not in adenocarcinomas. J Pathol. 2003;201:312–318.
Spieker T, Herbst H. Distribution and phenotype of Epstein-Barr virus-infected cells in inflammatory bowel disease. Am J Pathol. 2000;157:51–57.
Wakefield AJ, Fox JD, Sawyerr AM, et al. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn’s disease using the nested polymerase chain reaction. J Med Virol. 1992;38:183–190.
Gehlert T, Devergne O, Niedobitek G. Epstein-Barr virus (EBV) infection and expression of the interleukin-12 family member EBV-induced gene 3 (EBI3) in chronic inflammatory bowel disease. J Med Virol. 2004;73:432–438.
Ruther U, Nunnensiek C, Muller HA, Bader H, May U, Jipp P. Interferon alpha (IFN alpha 2a) therapy for herpes virus-associated inflammatory bowel disease (ulcerative colitis and Crohn’s disease). Hepatogastroenterology. 1998;45:691–699.
Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K. Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol. 1999;94:1582–1586.
Marszalek A, Marciniak R, Szkaradkiewicz A, et al. Inflammatory bowel disease—is there something new in the immunological background? Folia Histochem Cytobiol. 2011;49:357–362.
Maher MM, Nassar MI. Acute cytomegalovirus infection is a risk factor in refractory and complicated inflammatory bowel disease. Dig Dis Sci. 2009;54:2456–2462.
Sipponen T, Turunen U, Lautenschlager I, Nieminen U, Arola J, Halme L. Human herpesvirus 6 and cytomegalovirus in ileocolonic mucosa in inflammatory bowel disease. Scand J Gastroenterol. 2011;46:1324–1333.
Baumann MA, Paul CC. Interleukin-5 is an autocrine growth factor for Epstein-Barr virus-transformed B lymphocytes. Blood. 1992;79:1763–1767.
Tosato G, Tanner J, Jones KD, Revel M, Pike SE. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990;64:3033–3041.
Mauser A, Holley-Guthrie E, Zanation A, et al. The Epstein-Barr virus immediate-early protein BZLF1 induces expression of E2F–1 and other proteins involved in cell cycle progression in primary keratinocytes and gastric carcinoma cells. J Virol. 2002;76:12543–12552.
Israel BF, Kenney SC. Virally targeted therapies for EBV-associated malignancies. Oncogene. 2003;22:5122–5130.
Kenney S, Theodore E. Woodward award: development of novel, EBV-targeted therapies for EBV-positive tumors. Trans Am Clin Climatol Assoc. 2006;117:55–74.
Foxman EF, Iwasaki A. Genome-virome interactions: examining the role of common viral infections in complex disease. Nat Rev Microbiol. 2011;9:254–264.
Young VB. The intestinal microbiota in health and disease. Curr Opin Gastroenterol. 2012;28:63–69.
Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci. 2009;39:331–337.
Meckes DG Jr, Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85:12844–12854.
Pegtel DM, van de Garde MD, Middeldorp JM. Viral miRNAs exploiting the endosomal-exosomal pathway for intercellular cross-talk and immune evasion. Biochim Biophys Acta. 1809;2011:715–721.
Acknowledgments
The authors thank Sandra Elmore for technical assistance. This study was funded by the University of North Carolina Department of Pathology and Laboratory Medicine with support from the National Institutes of Health: Environmental Pathology Training Grant (T32-ES07017) supporting graduate studies of Julie L. Ryan, PhD, MPH, Cancer Epidemiology Award (K07CA125588) and funds for the UNC Center for Gastrointestinal Biology and Disease (P30 DK 034987) supporting Douglas R. Morgan, MD MPH, and the Alliance for Clinical Trials in Oncology (U10 CA031946), Clinical Translational Science Award (U54 RR024383), and Innovative Technologies for Molecular Analysis of Cancer (R21 CA155543) award supporting Margaret L. Gulley, MD.
Conflict of interest
MLG is a consultant for McKesson, Abbott Laboratories, and Roche Molecular Systems, and serves on the clinical advisory board of Generation Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryan, J.L., Shen, YJ., Morgan, D.R. et al. Epstein-Barr Virus Infection Is Common in Inflamed Gastrointestinal Mucosa. Dig Dis Sci 57, 1887–1898 (2012). https://doi.org/10.1007/s10620-012-2116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2116-5