Abstract
Background
The prevalence of celiac disease among type 1 diabetes mellitus (T1DM) patients is 5–10 times higher than in the general population. Thus, evaluation of celiac serology is indicated at diagnosis of T1DM and on follow up.
Aim
This study was prompted by the observation that elevated anti-TTG antibody levels in diabetic children may spontaneously normalize despite continued consumption of gluten. The objective of the study was to investigate the prevalence of this phenomenon and associated factors.
Materials and Methods
The files of all children diagnosed with type 1 diabetes mellitus from 2003–2009 at a tertiary pediatric medical center were reviewed for those with elevated serum levels of anti-TTG antibody. Clinical, medical, laboratory, and treatment data were collected. Findings were compared between patients diagnosed with celiac disease and patients with initially elevated anti-TTG antibody levels that spontaneously normalized.
Results
Forty-eight of the 738 patients with type 1 diabetes attending our center (6.5%) had elevated anti-TTG antibody blood levels. Celiac disease was diagnosed in 23, and anti-TTG antibody levels normalized in 17 (35.4%), all of whom consumed gluten. At one-year follow-up, there was no significant difference between the groups in HbA1c level or change in anthropometric measurements.
Conclusion
Physicians treating children with type 1 diabetes and mildly elevated anti-TTG antibody levels might consider 12-month serologic follow-up on a gluten-containing diet rather than immediate duodenal biopsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is a common, immune-mediated enteropathy characterized clinically by sensitivity to gluten, a peptide found in wheat, barley, and rye [1]. Its development is thought to arise as a result of inherited predisposition. As part of the immune response, there is an increase in specific serological markers in the serum, including anti-tissue transglutaminase (TTG) antibody [2]. The clinical picture is diverse, ranging from no symptoms to severe malabsorption. Non-gastrointestinal symptoms include iron deficiency anemia, short stature, increased prevalence of autoimmune diseases, neurologic manifestations, and liver and bone disease [2–4]. Studies have shown that approximately 20% of asymptomatic patients with positive serology and typical intestinal biopsy will become symptomatic within seven years [5]. Left untreated, CD places patients at increased risk of gastrointestinal malignancy, for example non-Hodgkin lymphoma, cancers of the mouth, pharynx, and esophagus [6], and increased overall mortality [7]. These complications support the need for early diagnosis and the common practice of excluding gluten-containing foods from the diet, even for asymptomatic cases.
The most widely accepted screening tool for CD is serology for levels of immunoglobulin A (IgA) anti-TTG antibodies [8]. Positive findings are followed by small-bowel biopsy. Although small-bowel biopsy is regarded as the best means of CD diagnosis [8, 9], high levels of anti-TTG antibody may predict positive biopsy findings [10], and patients with only minor findings on biopsy may still benefit from a gluten-free diet [11]. Serology is also important for monitoring compliance with treatment [12].
The prevalence of CD in the general population ranges from 1 in 70 to 1 in 200 throughout the world [13, 14]. It is even higher in family members of patients with CD, patients with genetic conditions such as Down, Turner, and Williams syndromes, and patients with other autoimmune diseases. CD has been found to occur 5–10 times more often in children and adults with type 1 diabetes mellitus (T1DM) than in the general population [8, 15, 16], although the reason for this association is still unclear. Both T1DM and CD result from a complex interplay between genetic susceptibility and environmental exposure, and both are associated with the major histocompatibility complex class II antigen DQ2, encoded by the alleles DQA1*501 and DQB1*201, indicative of a common genetic basis for disease expression [17, 18]. Recent studies have also revealed non-HLA loci common to the two diseases [17, 19]. Because most cases of CD in patients with T1DM are asymptomatic and detected by serologic screening [20], the International Society of Pediatric Diabetes recommends that all children diagnosed with T1DM be screened for CD at the time of diagnosis and then annually for the first five years and every second year thereafter [21].
This study was prompted by our observation that elevated levels of anti-TTG antibody in diabetic children may spontaneously normalize, despite continued consumption of gluten. The objectives of this study were threefold:
-
1.
to investigate the prevalence and extent of spontaneous normalization of TTG antibody levels in patients with T1DM;
-
2.
to characterize children with T1DM and spontaneous normalization of anti-TTG antibody levels in terms of demographics, growth, and metabolic control; and
-
3.
to compare changes in anthropometric measurements and metabolic control between patients with T1DM and CD who are adherent or nonadherent to a gluten-free diet.
Methods
Patients and Setting
The files of all patients diagnosed with T1DM at the Institute of Endocrinology and Diabetes of a tertiary pediatric medical center from December 2003 to December 2009 were reviewed for those with elevated anti-TTG antibody serum levels. For all patients identified, the following data were collected:
-
clinical—weight, height, and body mass index (BMI) at the first elevation of serum anti-TTG antibody and one year later, in addition to sex, age at diagnosis, other autoimmune diseases, and family history of CD, T1DM, and other autoimmune diseases;
-
laboratory—serum levels of anti-TTG antibody, hemoglobin A1c (HbA1c) concentration at the first elevation of serum anti-TTG antibody level and one year later, and serum levels of anti-endomysial antibody (EMA) and IgA; and
-
intervention and treatment—findings on small-intestine biopsies and adherence to a gluten-free diet, as assessed at each visit by a trained dietitian as part of the routine practice at our institution.
The study was approved by our institutional review board.
Techniques
Anthropometrics
In the Diabetes Clinic, weight and height are measured for all patients at first finding of an elevated anti-TTG antibody level (see the section “Serology”) and annually thereafter. Height is measured with a commercial Harpenden–Holtain stadiometer, and weight (in light clothing) with standard calibrated scales. BMI is calculated as weight in kilograms divided by height in meters squared. For this study, anthropometric measurements were converted to age-specific and sex-specific Z scores based on the WHO growth reference of 2007 [22].
HbA1c
Capillary HbA1c is measured on a regular basis for all patients with T1DM by use of an automated immunochemical technique (DCA 2000; Bayer Diagnostics, Tarrytown, NY, USA; reference range: 4.3–5.8%).
Serology
Anti-TTG antibody values were available for all patients because it is departmental practice to perform annual CD serology studies for patients with T1DM, starting from diagnosis [21, 23]. Patients with positive values are referred to the Gastroenterology Clinic for a repeat of the test, and for further verification by EMA test. This study included all patients whose initial TTG levels were elevated. Anti-TTG antibody is analyzed by use of anti-human TTG antibody (hu-TTG) IgA ELISA (IgA conjugate; IMMCO Diagnostics, Buffalo, NY, USA); anti-EMA is analyzed by use of the immunofluoresence Anti-EMA Test system (IMMCO). Findings of >20 EU/ml for TTG antibody and 1:2.5 for EMA were regarded as positive.
Biopsy
For patients with suspected CD, biopsy samples from the small intestine are routinely taken from the second part of the duodenum (four specimens) and from the duodenal bulb (two specimens) and classified according to the modified Marsh–Oberhuber classification.
Data Analysis
Patients with elevated TTG antibody levels were divided into two groups: Group 1 consisted of patients diagnosed with CD by accepted criteria and confirmed by biopsy [9]. Group 1 was further divided into those who were adherent (group 1a) or nonadherent (group 1b) to a gluten-free diet. Group 2 consisted of patients for whom TTG levels normalized spontaneously despite their continued consumption of a gluten-containing diet. In this group, patients who still had an elevated anti-TTG antibody level at the first gastroenterology visit were referred for biopsy, whereas those for whom levels had already normalized were observed only.
Statistics
Statistical analysis was performed with SPSS 17.0 (Chicago, IL, USA). The χ 2 test was used to estimate sex distribution. Differences between groups were analyzed by use of the two-tailed Student t test for independent samples. Anti-TTG antibody values were recorded as times the upper limit of normal (ULN) were exceeded. The data were plotted on a receiver operator characteristic (ROC) curve and the cutoff point yielding 100% specificity for diagnosing celiac disease was selected. Positive and negative predictive values for repeated (follow-up) TTG and EMA testing compared with the biopsy results were calculated. To compare means of more than two samples, one-way analysis of variance (ANOVA) was used. P values <0.05 were regarded as significant.
Results
Patient Groups
We reviewed the files of all 738 patients with T1DM who attended our center during the study period and were tested for anti-TTG antibody levels within four months of diagnosis and yearly thereafter (Fig. 1). Data were missing for another 77 patients with T1DM, and these were excluded from the study.
Forty-eight patients (6.5%) were found to have elevated anti-TTG antibody levels; of these, 23 were diagnosed with CD by intestinal biopsy (Marsh III) (group 1). Anti-TTG antibody levels for another 17 patients (35.4%) normalized on a gluten-containing diet during follow-up (group 2). Ten of these underwent duodenal biopsy that was found to be normal (Marsh 0), and seven did not have biopsies because by the time their biopsy was scheduled anti-TTG antibody levels normalized. The background characteristics of groups 1 and 2 are shown in Table 1. The other eight patients with high anti-TTG antibody levels were excluded from the analysis because of latent CD (elevated consecutive TTG tests but normal biopsy findings (n = 2) or initiation of a gluten-free diet on the basis of positive serum findings on consecutive tests, without performing a biopsy (n = 6)).
Analysis of adherence to treatment in group 1 yielded 13 patients (56.5%) who maintained a gluten-free diet, confirmed by the dietician’s reports and a decline in serological TTG antibody values.
Serology
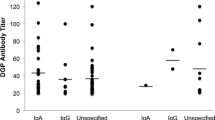
There was statistically significant difference in serum anti-TTG antibody levels between groups 1 and 2 at diagnosis (Fig. 2) (group 1, median × 3.3 ULN (range 1.4–15.1); group 2, median × 1.6 ULN (range 1.1–3) (P = 0.006)). All patients were followed periodically for anti-TTG antibody levels, and the median time to normalization in group 2 was six months (range 2–12). The anti-TTG antibody level did not normalize in any of the patients with CD who did not adhere to treatment (group 1b, median × 2.57 ULN (range 2–7.5)). In all but one patient in group 2, anti-TTG antibody values remained normal until the end of the follow-up period (median follow-up after normalization, 3.7 years (range 0.8–5.4 years)).
EMA findings were positive for all 23 patients with CD (group 1) and for 10/17 patients (59%) whose TTG values normalized (group 2) (P = 0.004). Spontaneous normalization of the EMA value and normalization of the TTG value were observed for seven of the EMA-positive patients in group 2.
In ROC curve analysis, anti-TTG antibody values that were 3 × ULN had 100% specificity for diagnosis of CD and 61% sensitivity. On follow-up testing, the positive predictive value was 92% for anti-TTG antibody and 82.1% for EMA, and the negative predictive value was 100% for both.
HbA1c
There was no significant difference in HbA1c concentration at diagnosis between the groups. Group 1 had a median value of 7.3% (range 6.1–11.16) and group 2 had a median value of 8% (range 6.1–14.9; P = 0.1). Similarly, after 1 year, taking adherence to treatment into account, group 1a (with GFD) had a median value of 7.1% (range 6–8.8), group 1b (without GFD) had a median value of 7.6% (range 6.7–11.5), and group 2 had a median value of 7.37% (range 6.5–14.4) (P = 0.39).
Growth
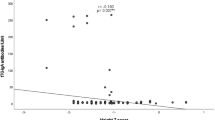
Table 2 shows the Z scores for weight, height, and BMI at diagnosis and after 1 year, and Fig. 3 shows the change in Z score for BMI from baseline to one year. There were no significant differences among groups 1a, 1b, and 2 for any of the data.
Changes in Z scores for BMI from baseline to 1 year for group 1a (diagnosed with CD, gluten-free diet), group 1b (diagnosed with CD, gluten-containing diet), and group 2 (anti-TTG antibody levels normalized on a gluten-containing diet). The difference among the groups was not statistically significant. CD, celiac disease; TTG, tissue transglutaminase (antibodies); GFD, gluten-free diet
Discussion
The major finding of this study is that elevated serum anti-TTG antibody levels in patients with newly diagnosed T1DM will normalize in 35% of cases within one year, even on a gluten-containing diet. For some patients (10/17 in our study), EMA levels normalize also. These changes are apparently unrelated to either HbA1c concentration or anthropometric measurements, which were similar to those for patients with a diagnosis of CD, irrespective of their adherence to a gluten-free diet.
In agreement with earlier studies showing that children with T1DM have higher prevalence of CD and elevated anti-TTG antibody levels compared with the general population [2, 24–28], we observed 4% occurrence of CD and 6.5% TTG antibody positivity among our patients diagnosed with T1DM.
Our search of the literature yielded no studies demonstrating normalization of anti-TTG antibody, and sometimes EMA, in patients with T1DM. However, one group reported 49% transient anti-TTG antibody positivity in patients genetically susceptible to CD who were tested periodically during the first 4–7 years of life [29], with no relation to T1DM. They suggested that at least for young children at risk, a regulatory immune response extinguishes the incipient CD. Another study of patients with a genetic susceptibility to CD found that anti-TTG antibody blood levels varied over time, and a higher titer predicted abnormal biopsy findings. The authors proposed that to reduce the frequency of unnecessary (normal) diagnostic biopsies for CD, the threshold for screening-identified cases should be set higher than for clinically identified cases [30].
At the time of TTG determination, none of our patients had decelerated growth or signs of malnourishment, similar to most reports in the literature [20, 24, 31, 32]. Furthermore, at 1 year there was no difference in anthropometric measurements or metabolic control between patients with CD or with anti-TTG antibody normalization and between patients who adhered to a gluten-free diet or those who did not. Although the patients with CD who did not adhere to treatment had a 1.1% higher mean HbA1c value at 1 year than the adherent subgroup, the difference was not statistically significant. Findings in the literature on the effect of a gluten-free diet on symptoms and growth in children with T1DM and CD are inconsistent, with some suggesting a clinical benefit [2, 33, 34] and others not [15, 35].
The lack of effect of a gluten-free diet on anthropometric data in our study might be attributable to the relatively short follow-up time. One year may not be sufficient to reflect these changes, especially for patients who were mostly diagnosed by screening and were not malnourished at diagnosis. Nevertheless, in a recent study on the advantages of screening children with T1DM for CD serology, there was no difference in the mean decrease in anti-TTG antibody levels between patients who were on a gluten-free diet and those who were not, and no difference in symptoms, anthropometrics, or metabolic control over a two-year period [36]. However, the authors did not report on spontaneous normalization of antibodies. Accordingly, a recent multicenter prospective study of patients with T1DM observed that height and weight were lower in those with CD than in those without CD at both presentation and five years later [37].
Thus, taken together, our findings of an absence of stunting or malnutrition at diagnosis, and similar anthropometrics and metabolic control at 1 year in patients with and without CD and patients with CD on a gluten-free or gluten-containing diet, and the substantial rate of normalization of anti-TTG antibody levels, suggest that, at least in the presence of anti-TTG antibody values up to three times the upper normal limit, repeated serologic testing of patients on a gluten-containing diet is preferred over immediate intestinal biopsy. Furthermore, treatment with a gluten-free diet should be delayed until the findings are definite, so that spontaneous normalization of the high levels of anti-TTG antibodies is not misinterpreted. Factors that can guide the decision to perform an early intestinal biopsy study are family history of CD, which is more likely in patients with CD according to our data and the literature [38], in addition to findings of a high antibody titer and EMA positivity. However, clinicians should take into account that there is an overlap of TTG values between patients with CD and patients for whom anti-TTG antibody values eventually normalize: Some patients with CD may have rather low values of TTG, with our without co-normalization of EMA level, whereas some patients without CD may have high values.
This study was limited by its retrospective design and relatively small number of patients in each group (although the overall sample size was large). The lack of between-group differences in anthropometric measurements and metabolic control during follow-up indicated that a one-year delay in establishing diagnosis has no deleterious effects on these. Nevertheless, the study may still have been underpowered for observing some changes. Second, HLA testing was not performed routinely, and it could be of diagnostic assistance for subjects who do not have intestinal biopsies. However, the HLA data would affect the diagnosed cases only, and would not limit the high percentage of patients with T1DM who eventually lost their anti-TTG antibodies on a gluten-containing diet. Last, our serologic follow-up was limited to the period of the study (up to 6 years). Some authors may argue that although CD is more common at diabetes diagnosis and in the first five years after diagnosis [39], it may develop later in some cases.
In conclusion, our study shows that high anti-TTG antibody levels in children diagnosed with T1DM spontaneously normalize in approximately one-third of cases. We suggest that in asymptomatic children with T1DM and mildly elevated TTG levels, physicians should consider serologic follow-up on a gluten-containing diet for at least 12 months rather than immediate duodenal biopsy.
References
Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188.
Narula P, Porter L, Langton J, et al. Gastrointestinal symptoms in children with type 1 diabetes screened for celiac disease. Pediatrics. 2009;124:e489–e495.
Hartman C, Hino B, Lerner A, et al. Bone quantitative ultrasound and bone mineral density in children with celiac disease. J Pediatr Gastroenterol Nutr. 2004;39:504–510.
Shamir R. Advances in celiac disease. Gastroenterol Clin N Am. 2003;32:931–947.
Maki M, Mustalahti K, Kokkonen J, et al. Prevalence of celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524.
Holmes GK, Prior P, Lane MR, Pope D, Allan RN. Malignancy in coeliac disease-effect of a gluten free diet. Gut. 1989;30:333–338.
Shamir R, Hernell O, Leshno M. Cost-effectiveness analysis of screening for celiac disease in the adult population. Med Decis Mak. 2006;26:282–293.
Kagnoff MF. AGA institute medical position statement on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1977–1980.
Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American society for pediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19.
Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572–577.
Kurppa K, Ashorn M, Iltanen S, et al. Celiac disease without villous atrophy in children: a prospective study. J Pediatr. 2010;157:373–380.
Dieterich W, Laag E, Schopper H, et al. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–1321.
Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–1933.
Shamir R, Lerner A, Shinar E, et al. The use of a single serological marker underestimates the prevalence of celiac disease in Israel: a study of blood donors. Am J Gastroenterol. 2002;97:2589–2594.
Saukkonen T, Vaisanen S, Akerblom HK, Savilahti E. Coeliac disease in children and adolescents with type 1 diabetes: a study of growth, glycaemic control, and experiences of families. Acta Paediatr. 2002;91:297–302.
Collin P, Kaukinen K, Valimaki M, Salmi J. Endocrinological disorders and celiac disease. Endocr Rev. 2002;23:464–483.
Smyth DJ, Plagnol V, Walker NM, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–2777.
Barker JM, Liu E. Celiac disease: pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr. 2008;55:349–365.
Sumnik Z, Cinek O, Bratanic N, et al. Risk of celiac disease in children with type 1 diabetes is modified by positivity for HLA-DQB1*02-DQA1*05 and TNF-308A. Diabetes Care. 2006;29:858–863.
Telega G, Bennet TR, Werlin S. Emerging new clinical patterns in the presentation of celiac disease. Arch Pediatr Adolesc Med. 2008;162:164–168.
Kordonouri O, Maguire AM, Knip M, et al. ISPAD clinical practice consensus guidelines 2006–2007. Other complications and associated conditions. Pediatr Diabetes. 2007;8:171–176.
WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World’s Children and Adolescents. Geneva: WHO; 2009 (http://www.who.int/growthref/tools/en/).
Kordonouri O, Maguire AM, Knip M, et al. Other complications and associated conditions with diabetes in children and adolescents. Pediatr Diabetes. 2009;10:204–210.
Karavanaki K, Kakleas K, Paschali E, et al. Screening for associated autoimmunity in children and adolescents with type 1 diabetes mellitus (T1DM). Horm Res. 2009;71:201–206.
Lampasona V, Bonfanti R, Bazzigaluppi E, et al. Antibodies to tissue transglutaminase C in type I diabetes. Diabetologia. 1999;42:1195–1198.
Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143–148.
Kordonouri O, Dieterich W, Schuppan D, et al. Autoantibodies to tissue transglutaminase are sensitive serological parameters for detecting silent coeliac disease in patients with type 1 diabetes mellitus. Diabet Med. 2000;17:441–444.
Djuric Z, Stamenkovic H, Stankovic T, et al. Celiac disease prevalence in children and adolescents with type 1 diabetes from Serbia. Pediatr Int. 2010;52:579–583.
Simell S, Hoppu S, Hekkala A, et al. Fate of five celiac disease-associated antibodies during normal diet in genetically at-risk children observed from birth in a natural history study. Am J Gastroenterol. 2007;102:2026–2035.
Liu E, Bao F, Barriga K, et al. Fluctuating transglutaminase autoantibodies are related to histologic features of celiac disease. Clin Gastroenterol Hepatol. 2003;1:356–362.
Sud S, Marcon M, Assor E, Palmert MR, Daneman D, Mahmud FH. Celiac disease and pediatric type 1 diabetes: diagnostic and treatment dilemmas. Int J Pediatr Endocrinol. 2010;2010. doi:10.1155/2010/161285.
Gillett PM, Gillett HR, Israel DM, et al. High prevalence of celiac disease in patients with type 1 diabetes detected by antibodies to endomysium and tissue transglutaminase. Can J Gastroenterol. 2001;15:297–301.
Mohn A, Cerruto M, Iafusco D, et al. Celiac disease in children and adolescents with type I diabetes: importance of hypoglycemia. J Pediatr Gastroenterol Nutr. 2001;32:37–40.
Hansen D, Brock-Jacobsen B, Lund E, et al. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening-detected celiac disease: a population-based screening study with 2 years’ follow-up. Diabetes Care. 2006;29:2452–2456.
Rami B, Sumnik Z, Schober E, et al. Screening detected celiac disease in children with type 1 diabetes mellitus: effect on the clinical course (a case control study). J Pediatr Gastroenterol Nutr. 2005;41:317–321.
Simmons JH, Klingensmith GJ, McFann K, et al. Celiac autoimmunity in children with type 1 diabetes: a 2-year follow-up. J Pediatr. 2011;158:276–281.
Fröhlich-Reiterer EE, Kaspers S, Hofer S, et al. Anthropometry, metabolic control, and follow-up in children and adolescents with type 1 diabetes mellitus and biopsy-proven celiac disease. J Pediatrics. 2011;158:589–593.
Rubio-Tapia A, Van Dyke CT, Lahr BD, et al. Predictors of family risk for celiac disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:983–987.
Larsson K, Carlsson A, Cederwall E, et al. Annual screening detects celiac disease in children with type 1 diabetes. Pediatr Diabetes. 2008;9:354–359.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waisbourd-Zinman, O., Hojsak, I., Rosenbach, Y. et al. Spontaneous Normalization of Anti-Tissue Transglutaminase Antibody Levels Is Common in Children with Type 1 Diabetes Mellitus. Dig Dis Sci 57, 1314–1320 (2012). https://doi.org/10.1007/s10620-011-2016-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-2016-0