Abstract
Background
Interleukin-33 (IL-33) is a novel member of the IL-1 family of cytokines, and it is closely related to IL-18, one of the best characterized members of the IL-1 family. It’s been demonstrated that elevated levels of IL-18 are involved in a wide variety of tumors, especially in gastric cancer.
Aims
The purpose of this study was to determine the correlations between serum IL-33 levels and the clinicopathologic features in gastric cancer patients.
Methods
Serum samples were collected from 68 patients with gastric cancer and 57 controls. Serum IL-33 levels were measured by ELISA. Classical tumor markers of CEA and CA19-9 levels were routinely detected by chemiluminescence immunoassay. Western blot analysis was used to detect IL-33 expression in gastric cancer tissue samples and cell lines. The relationship between serum levels of IL-33 and clinical characteristics in patients was analyzed.
Results
IL-33 levels in the serum of gastric cancer patients were significantly elevated in comparison with that of healthy volunteers. Furthermore, higher serum levels of IL-33 in gastric cancer patients were found to correlate with several poor prognostic factors like depth of invasion, distant metastasis and advanced stage (stage III/IV). On the other hand, serum IL-33 levels did not correlate with CEA and CA19-9. The expression of IL-33 protein was upregulated in carcinoma tissues in comparison with matched normal tissues, and no statistically significant difference was found between the four gastric cancer cell lines and human gastric epithelial cell line GES-1.
Conclusions
Serum IL-33 may be a useful biomarker for predicting the prognosis of gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric carcinoma is the second most diagnosed malignancy in the world, and it is a major cause of cancer-related death [1, 2]. It is generally recognized that there is immune dysfunction in cancer patients. Several experiments showed that levels of serum cytokines such as IL-6, IL-10 and IL-18 were significantly higher in patients with solid or hematopoietic tumors than that of healthy people, and associated with a worse prognosis [3–8].
IL-33, identified by Carriere and colleagues [9], is a novel member of the IL-1 family of cytokines. It is now regarded as the 11th member in the IL-1 family [10], which includes IL-1α, IL-1β and IL-18. IL-33 was first cloned from canine vasospastic arteries induced by subarachnoid haemorrhage, and was found to be expressed in cultured smooth muscle cells in response to IL-1β and interferon-γ [11]. IL-33 is produced as a 30-kDa precursor protein and is cleaved by caspase-1 to produce a mature, secreted 18-kDa form [10]. In the IL-1 family, IL-33 is most closely related to IL-18, one of the best characterized members of the IL-1 family [12]. Elevated levels of serum IL-18 have been demonstrated to relate to a wide variety of tumors [13–15], especially to gastric cancer [6–8]. It has been reported that IL-33 was significantly increased in the lung tissues of K-ras TG mice, which is related to cancer development [16]. And some researchers observed abundant expression of IL-33 in endothelial cells in several distinct human tumors and also in cancer cell lines [17, 18]. However, it is not clear to date whether serum levels of IL-33 are associated with the clinical significance of patients with cancer. Therefore, in the present study, we focused on serum levels of IL-33 in patients with gastric cancer using ELISA to investigate the relationship between serum levels of IL-33 and clinical significance. We also detected the expression of IL-33 protein in gastric cancer cell lines and tissues.
Materials and Methods
Patients and Blood Samples
Sixty-eight patients who underwent resection of gastric carcinoma in the department of surgery, Ruijin Hospital between October 2007 and March 2008 were enrolled in this study. These patients were 26 females and 42 males from 29 to 80 years of age (59 ± 11, mean ± SD). All patients were classified according to UICC stage classification. The number of patients at each tumor stage was described as follows: stage I, n = 18; stage II, n = 21; stage III, n = 23; and stage IV, n = 6. Fifty-seven healthy volunteers with a mean age of 56 years (range, 24–72; 20 females and 37 males) were recruited as controls. The healthy volunteers had no history of gastrointestinal complaints. Patients who were unable or unwilling to give informed consent or who had undergone chemotherapy or radiation therapy before surgery were excluded, as were patients and controls with rheumatic disease, acute infection or other types of cancer. A 10-ml blood sample was drawn from each subject prior to surgery, spun, aliquot, and stored at −80°C until testing. Overall survival time was calculated as the time in months from the date of diagnosis to death (for non-censored events) or to the most recent contact/visit (for censored events) as of August 31st, 2010. All investigations described in this study were done after informed consent and in accordance with the Institutional Review Board and the Ethics Committee of Ruijin Hospital (Shanghai, P.R. China).
Detection of Serum Levels of IL-33 and Tumor Markers
Serum IL-33 levels were determined using ELISA kits (R&D Systems) according to the manufacturer’s instructions. Briefly, undiluted serum samples were reacted with a monoclonal antibody that recognized an epitope of human IL-33. The clinicopathologic parameters studied for prognostic value were depth of invasion, lymph node metastasis, distant metastasis and tumor stages. Classical tumor markers of CEA and CA19-9 were routinely measured by chemiluminescence immunoassay (CLIA, Abbott Architect i2000) in patients with gastric cancer at our hospital before operation.
Tissue Samples
For Western blot analysis, seven representative fragments of fresh tumor and matched normal tissues at least 5 cm away from malignant tissues were taken within 1 h after surgery. They were immediately snap-frozen in liquid nitrogen, and then stored at −80°C until processing.
Cell Culture
Gastric cancer cell lines MKN28, SGC7901, MKN45 and AGS were all preserved in our laboratory, and human gastric epithelial cell line GES-1 was obtained from American Type Culture Collection. Cells were cultured in advanced Dulbecco’s modified eagle medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum and 100 U/ml of penicillin–streptomycin. The cells were incubated at 37°C in a humidified incubator with 5% CO2 in air. Media were changed three times weekly.
Western Blot Analysis
Cells or tissue samples were lysed in radio immuno precipitation assay (RIPA) lysis buffer (Beyotime), and PMSF (1 mM, final concentration) was added after several minutes. Protein samples were electrophoresed on 10% denaturing sodium dodecylsulfate gels, and transferred to PVDF membrane (Millipore). IL-33 antibody (Abcam) followed by HRP-goat anti-rabbit antibody (Kangcheng Co.), and mouse anti-β-actin antibody (Sigma) followed by HRP-goat anti-mouse antibody (Kangcheng Co.) were added on the membrane, respectively. IL-33 and β-actin were visualized by ECL detection (Amersham Pharmacia Biotech) and analyzed by gray scale scanning quantitation.
Statistical Analysis
Data analysis was performed using the SPSS 13.0 statistical software package. Data are presented as mean ± standard deviation (SD). Comparisons were performed using the nonparametric Mann–Whitney U-test for unpaired samples and paired t-test for paired samples. The correlation between the levels of IL-33 and the levels of CEA or CA19-9 in gastric cancer patients were analyzed by Spearman’s coefficient analysis. Analysis of receiver operating characteristic (ROC) was performed to calculate the cut-off value. Survival curve was calculated according to the Kaplan–Meier method and P-value was evaluated by the log-rank test for censored survival data. Tests with P < 0.05 were considered to be statistically significant and all statistical tests were two-sided.
Results
Western Blot Analysis of IL-33 Expression
To investigate the expression of IL-33 protein in gastric cancer, we examined gastric cancer cell lines by Western blot, using human gastric epithelial cell line GES-1 as control. Meanwhile, we also examined seven gastric cancer tissue samples, using matched normal tissues as control. IL-33 protein expression was detected in all cell lines, and no statistically significant difference was found between the four gastric cancer cell lines and human gastric epithelial cell line (Fig. 1a). But the average of IL-33 expression levels (IL-33/β-actin ratio) was higher in tumors than that of controls (P < 0.05; Fig. 1b, c).
Expression of interleukin-33 (IL-33) in gastric cancer cell lines and tissue samples. a Western blot analysis of IL-33 protein levels in gastric cancer cell lines MKN28, SGC7901, MKN45, AGS and human gastric epithelial cell line GES-1. b Western blot analysis of IL-33 protein levels in gastric cancer tissue samples (T1–T7) and matched normal gastric tissue samples (N1–N7). c IL-33 expression in tissue samples is presented relative to that of β-actin (*P < 0.05)
Analysis of Serum IL-33 Levels
Serum IL-33 levels were analyzed in 68 gastric cancer patients and 57 normal controls. No age or gender differences were found between gastric patients and control group. By adding the recombinant human IL-33 protein at concentrations ranging from 31.25 pg/well (100 μl) to 2 ng/well (100 μl), we established a seven point standard curve for estimating the concentration of IL-33 in sera of the patients (R2 = 0.996). The serum concentrations of IL-33 in patients ranged from 25.3 pg/ml to 87.6 pg/ml. The mean concentration of serum IL-33 in gastric cancer patients (43.3 ± 10.6 pg/ml) was significantly higher than that in healthy volunteers (36.3 ± 4.9 pg/ml, range 29.8–53.0 pg/ml, P < 0.001; Fig. 2). When the cut-off value of serum IL-33 was set at 39.6 pg/ml, its sensitivity and specificity for gastric cancer were 68 and 79%, respectively.
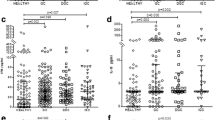
Correlations Between Serum IL-33 Levels and Clinicopathologic Features of the Patients
In Table 1, the relationship between serum IL-33 levels and clinicopathologic findings in all patients was listed. Serum IL-33 was associated with factors related to disease progression, such as depth of invasion (P = 0.034) and presence of distant metastases (P = 0.036; Fig. 3a). Although the IL-33 levels in patients with stage I disease did not differ from normal controls (P > 0.05), the levels in patients with stage II, III and IV disease were significantly higher compared with healthy volunteers (P < 0.05). In addition, serum IL-33 levels increased significantly in accordance with the progression of UICC stage classification (P = 0.019; Fig. 3b). In these gastric patients, no significant difference was found in serum IL-33 levels according to age (P = 0.745), gender (P = 0.295), and node status (P = 0.119). Furthermore, we were unable to demonstrate a correlation between IL-33 concentrations and serum CEA concentrations (correlation coefficient, 0.062; P = 0.615; Fig. 3c) or CA19-9 concentrations (correlation coefficient, 0.101; P = 0.412; Fig. 3d) (data not shown).
Correlations between serum IL-33 levels (pg/ml) and clinical features of gastric cancer patients. a Serum IL-33 levels in gastric cancer patients with metastasis or not. Means are shown by horizontal bars (P = 0.036). b Serum IL-33 levels in patients with gastric cancer at different stages compared with healthy volunteers. c Correlation of serum CEA levels and serum IL-33 levels (correlation coefficient, 0.062; P = 0.615). d Correlation of serum CA19-9 levels and serum IL-33 levels (correlation coefficient, 0.101; P = 0.412)
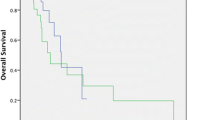
Correlation of Serum IL-33 Levels with Survival Rate
The patients with gastric cancer were followed for a median of 30.0 months (range, 7.5–34.0 months) after enrollment. Two subgroups were divided according to the cut-off value of IL-33 calculated on receiver operating characteristic (ROC) analysis. We compared the survival curves between the two subgroups. The mean survival time in the group with higher level of serum IL-33 (>39.6 pg/ml) was 26.7 ± 2.7 months, and that in the group with lower level of serum IL-33 was 30.8 ± 1.4 months. Kaplan–Meier survival curves and log-rank statistics showed that the serum IL-33 levels could be used as a prognostic biomarker for gastric cancer patients (P = 0.040; Fig. 4).
Discussion
IL-33 is a recently described member of the IL-1 family that signals through the IL-1 receptor-related protein ST2 [10]. The ST2 gene encodes three isoforms of ST2 protein including ST2L, which can serve as a decoy receptor for IL-33 [19, 20]. The activity of soluble IL-33 does not show T helper 1 (Th1)-associated proinflammatory characteristics, in contrast to other closely related family members [21, 22]. On the other hand, stimulation of cells with IL-33 or binding of IL-33 to the ST2L on mast cells leads to the production of IL-5 and IL-13 and increases serum immunoglobulin (Ig) levels, which are typical of Th2-driven hyper-responsiveness [10, 23, 24]. Malignant tumors often impair host immune responses, and Th2 type cytokines have been shown to down-regulate tumor specific immune response by inhibiting tumor antigen presentation [25, 26]. Thus exogenous IL-33 seems to act more as an immunoregulatory cytokine that participates in the control of Th2 immunity rather than as a proinflammatory cytokine [18]. Recently several researches suggested that IL-33 may be involved in tumorigenesis and the development of vascular diseases [27]. However, to our knowledge, there are no publications on the clinical characteristics of IL-33 in gastric cancer.
Expression of IL-33 has been observed in various organs including stomach, lung, and intestine of patients with Crohn’s disease, as well as in cells including epithelial cells, fibroblasts, endothelial cells, pancreatic cancer cells and activated macrophages [9, 10]. In this study, the expression of IL-33 protein was detected in four gastric cancer cell lines and gastric carcinoma tissues by Western blotting. We found that IL-33 was upregulated in gastric cancer patients in comparison with matched normal tissues. And we found statistically there was no significant difference between the four gastric cancer cell lines and GES-1, which suggested that expression of IL-33 may be modulated by local environment, or IL-33 also could be produced by other cells responding to the gastric cancer cells in tumor.
IL-33 is most closely related to IL-18 of the IL-1 family [12]. Stimulation through both TLRs and NLRs are all required for processing and release of IL-18 and IL-33 from normal monocytes [28]. Both precursor forms of IL-18 and IL-33 are cleaved by caspase-1 to generate mature and biologically active cytokines [10]. Serum IL-18 levels have been found to be markedly up-regulated in gastric cancer patients [6–8], which indicate that there may be a close relation between serum IL-33 and tumor. However, the IL-18 levels in patients with stage IV disease did not differ from normal controls [29]. The results of our study showed that serum levels of IL-33 in patients with gastric cancer were significantly higher than that of healthy people, which preliminarily confirmed the hypothesis. And the levels of IL-33 in patients with stages II, III and IV disease were significantly higher than that of healthy volunteers, which suggests that serum IL-33 levels may have a closer correlation with gastric cancer than IL-18 levels.
Furthermore, mature IL-33 was found to signal via the IL-1R/TLR family member ST2, activating NF-κB and mitogen-activated protein kinases in a mast cell line [24, 30]. Recent evidence demonstrates that activation of NF-κB contributes to the development of several types of human cancer [31, 32]. These findings also suggest that serum IL-33 may be related to progression of the cancer. So in the present study, we investigated serum levels of IL-33 in gastric cancer patients, and analyzed potential correlations between serum IL-33 levels and certain clinical characteristics of the tumor. The results showed that elevated serum levels of IL-33 correlated with several poor prognostic factors like depth of invasion, distant metastasis and advanced stage.
In addition, we were unable to demonstrate a correlation between IL-33 concentrations and serum CEA or CA19-9 concentrations. In other words, elevated serum IL-33 level was found to be an independent prognostic indicator, and this lack of correlation suggested that IL-33 and CEA or CA19-9 might provide complementary information.
In conclusion, our data suggest that serum IL-33 may be a useful biomarker for predicting the prognosis of gastric cancer. Prospective studies in a larger population should be carried out to confirm the findings.
References
Pisani P, Parkin DM, Ferlay J. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993;55:891–903.
Terry MB, Gaudet MM, Gammon MD. The epidemiology of gastric cancer. Semin Radiat Oncol. 2002;12:111–127.
Ikeguchi M, Hatada T, Yamamoto M, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. 2009;12:95–100.
Seguchi T, Yokokawa K, Sugao H, Nakano E, Sonoda T, Okuyama A. Interleukin-6 activity in urine and serum in patients with bladder carcinoma. J Urol. 1992;148:791–794.
Fortis C, Foppoli M, Gianotti L, et al. Increased interleukin-10 serum levels in patients with solid tumors. Cancer Lett. 1996;104:1–5.
Kang JS, Bae SY, Kim HR, et al. Interleukin-18 increases metastasis and immune escape of stomach cancer via the downregulation of CD70 and maintenance of CD44. Carcinogenesis. 2009;30:1987–1996.
Thong-Ngam D, Tangkijvanich P, Lerknimitr R, Mahachai V, Theamboonlers A, Poovorawan Y. Diagnostic role of serum interleukin-18 in gastric cancer patients. World J Gastroenterol. 2006;12:4473–4477.
Haghshenas MR, Hosseini SV, Mahmoudi M, Saberi-Firozi M, Farjadian S, Ghaderi A. IL-18 serum level and IL-18 promoter gene polymorphism in Iranian patients with gastrointestinal cancers. J Gastroenterol Hepatol. 2009;24:1119–1122.
Carriere V, Roussel L, Ortega N, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287.
Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490.
Onda H, Kasuya H, Takakura K, et al. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1999;19:1279–1288.
Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499.
Park H, Byun D, Kim TS, et al. Enhanced IL-18 expression in common skin tumors. Immunol Lett. 2001;79:215–219.
Eissa SA, Zaki SA, El-Maghraby SM, Kadry DY. Importance of serum IL-18 and RANTES as markers for breast carcinoma progression. J Egypt Natl Canc Inst. 2005;17:51–55.
Merendino RA, Gangemi S, Ruello A, et al. Serum levels of interleukin-18 and sICAM-1 in patients affected by breast cancer: preliminary considerations. Int J Biol Markers. 2001;16:126–129.
Lee S, Kang J, Cho M, et al. Profiling of transcripts and proteins modulated by K-ras oncogene in the lung tissues of K-ras transgenic mice by omics approaches. Int J Oncol. 2009;34:161–172.
Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:e3331.
Masamune A, Watanabe T, Kikuta K, Satoh K, Kanno A, Shimosegawa T. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G821–G832.
Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840.
Trajkovic V, Sweet MJ, Xu D. T1/ST2–an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004;15:87–95.
Gayle MA, Slack JL, Bonnert TP, et al. Cloning of a putative ligand for the T1/ST2 receptor. J Biol Chem. 1996;271:5784–5789.
Kumar S, Minnich MD, Young PR. ST2/T1 protein functionally binds to two secreted proteins from Balb/c 3T3 and human umbilical vein endothelial cells but does not bind interleukin 1. J Biol Chem. 1995;270:27905–27913.
Ali S, Huber M, Kollewe C, Bischoff SC, Falk W, Martin MU. IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc Natl Acad Sci USA. 2007;104:18660–18665.
Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555.
De Vita F, Orditura M, Galizia G, et al. Serum interleukin-10 levels in patients with advanced gastrointestinal malignancies. Cancer. 1999;86:1936–1943.
Sharma A, Rajappa M, Saxena A, Sharma M. Cytokine profile in Indian women with cervical intraepithelial neoplasia and cancer cervix. Int J Gynecol Cancer. 2007;17:879–885.
Choi YS, Choi HJ, Min JK, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117–3126.
Becker CE, O’Neill LA. Inflammasomes in inflammatory disorders: the role of TLRs and their interactions with NLRs. Semin Immunopathol. 2007;29:239–248.
Kawabata T, Ichikura T, Majima T, et al. Preoperative serum interleukin-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer. 2001;92:2050–2055.
Iikura M, Suto H, Kajiwara N, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978.
Salminen A, Kaarniranta K. Glycolysis links p53 function with NF-kappaB signaling: impact on cancer and aging process. J Cell Physiol. 2010;224:1–6.
Mancino A, Lawrence T. Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res. 2010;16:784–789.
Acknowledgments
We thank Zhongyin Yang, Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pinghu Sun and Qiwen Ben contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, P., Ben, Q., Tu, S. et al. Serum Interleukin-33 Levels in Patients with Gastric Cancer. Dig Dis Sci 56, 3596–3601 (2011). https://doi.org/10.1007/s10620-011-1760-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1760-5