Abstract
Background
CA19-9 is a tumor marker for pancreatic cancer, cholangiocarcinoma, and other malignancies. However, its sensitivity and specificity is suboptimal in clinical practice, which we hypothesized limits its clinical utility.
Aims
To evaluate the clinical utility and limitations of CA19-9 as a tumor marker.
Methods
We performed a retrospective review of CA19-9 levels (U/ml) in 483 consecutive patients between 2006 and 2008 at two university hospitals. We abstracted clinical, radiographic, and pathological data and final diagnoses. Descriptive and non-parametric analyses were performed.
Results
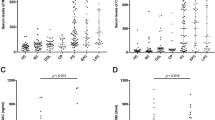
Patients presenting with jaundice had the highest CA19-9 (420) compared to other complaints (<20) (p < 0.01). The indications with the highest CA19-9 had evidence of biliary obstruction (71), liver mass (54), and pancreatic head mass (27) compared to other indications (<15) (p < 0.01). The diagnoses with the highest CA19-9 (p < 0.01) were cholangiocarcinoma (476), pancreatic cancer (161), and choledocholithiasis (138). Using a receiver operator curve to evaluate CA19-9, the area under the curve was 0.7 when evaluating all patients for pancreatic cancer or cholangiocarcinoma or patients with pancreatic head mass for pancreatic cancer.
Conclusions
This study found that for pancreatic cancer and cholangiocarcinoma, CA19-9 had poor clinical utility as a tumor marker and did not change patient management. Elevations in CA19-9 were associated with biliary obstruction based on clinical history, laboratory data, and diagnoses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1979, Koprowski described a monoclonal antibody cultured from cells of colorectal carcinoma that binds to antigenic determinants located on a sialylated Lewis A blood group oligosaccharide; subsequently, he named the antibody serum carbohydrate antigen 19-9 (CA19-9) [1, 2]. Consequently, the 5% of the population that does not express the Lewis antibody are unable to synthesize CA19-9 [3]. Studies on the clinical utility of CA19-9 in diagnosing pancreatic cancer have been small, and meta-analysis has been necessary to extract meaningful results [4]. One meta-analysis of these previous studies showed that the sensitivity for pancreatic cancer using a CA19-9 level of 37 and 100 U/ml was 81 and 68%, respectively, while the specificity using the same levels was 90 and 98%, respectively [5]. Unfortunately, studies have shown that serum CA19-9 levels are also elevated in a broad range of benign and malignant conditions including cholecystitis, choledocholithiasis, hepatitis, cirrhosis, pancreatitis, achalasia, inflammatory bowel disease, rheumatoid arthritis, heavy tea consumption, Sjögren’s syndrome, Hashimoto’s thyroiditis, cholangiocarcinoma, and colorectal, hepatocellular, esophageal, lung, and ovarian carcinomas [6, 7].
Despite the shortcomings, CA19-9 continues to frequently be ordered. Historically, limitations in imaging necessitated the use of tumor markers such as CA19-9 and morbid procedures such as exploratory laparotomy to establish a diagnosis of pancreatic or biliary neoplasia. Significant advances in cross-sectional and endoscopic imaging and sampling since CA19-9 was discovered in 1979 have clearly improved the ability to diagnose diseases of the pancreas and bile ducts in a much less morbid way [8–10]. As such, we hypothesized that the sensitivity and specificity of CA19-9 when used by clinicians in the 21st century is possibly lower than previously described.
Methods
This was a retrospective study from January 2006 to December 2008 performed at the University of Texas Southwestern’s St. Paul University Hospital and Parkland Memorial Hospital, a county hospital affiliated with UT Southwestern. The study was approved by the University of Texas Southwestern Institutional Review Board. First, the laboratory database was queried for any CA19-9 level that was obtained during the study period. Patients were identified this way and counted once regardless of how many CA19-9 levels were drawn for any patient. Both institutions use the immunoenzymatic assay from Beckman Coulter (Ireland) to measure CA19-9 with an upper limit of normal of 55 U/ml. Demographic, clinical, radiographic, and pathological data and final diagnoses were abstracted from electronic and paper medical records.
Descriptive and non-parametric analyses were performed on the data set. Categorical data were reported as number and percentage. A Pearson Chi-square test was used for comparing categorical data. Continuous variables were reported with median and standard deviation. For continuous data, Mann–Whitney U test and Kruskal–Wallis test were used for analyses between groups when appropriate. Receiver operator curves (ROC) were used to evaluate the sensitivity and specificity by determining the area under the curves with 95% confidence intervals. All analyses were performed using PASW Statistics 17.0.2 (SPSS Inc., Chicago, IL).
Results
Demographics
Four hundred and eighty-three consecutive patients were identified, with were 210 men (44%) and 273 women (57%). Men had a non-statistically significant (p = 0.07) higher median CA19-9 level of 26 U/ml (SD 20,000 U/ml), compared to women, who had a median CA19-9 level of 17 U/ml (SD 59,000 U/ml). CA19-9 was distributed differently (p < 0.001) according to ethnicity (Table 1). The median CA19-9 level was highest in Caucasians (38 U/ml), followed by Asians (23 U/ml), African Americans (19 U/ml), and Hispanics (11 U/ml), but it did not correlate with the frequency of patients diagnosed with pancreatic cancer or cholangiocarcinoma (Caucasians 15%, Asian 14%, African Americans 21%, and Hispanics 23%).
Clinical Data
A chief complaint was stated in the note of 479 out of the 483 patients (Table 1). This included abdominal pain (38%), jaundice (7%), increased abdominal girth (6%), and other complaints (i.e., edema, weakness, shortness of breath, musculoskeletal pain, gastrointestinal bleeding, and neurological changes; 42%). Each chief complaint had a large variance in CA19-9 levels, but jaundiced patients had the highest CA19-9 level (420 U/ml), which was significant compared to the other chief complaints (p < 0.001).
Serum CA19-9 was obtained for indications included in Table 1. Prior to measuring CA19-9, all patients already had imaging including abdominal ultrasound, CT or MRI. The most common indications were to evaluate an unknown primary malignancy (27%), pancreatic mass (24%), liver mass (12%), and biliary obstruction (11%). Median CA19-9 was highest in patients with biliary obstruction (71 U/ml) followed by patients with liver masses (54 U/ml), and pancreatic masses (27 U/ml), and each indication had a different distribution of CA19-9 (p = 0.001). Fifty-three percent of patients with pancreas head masses and 10% of patients with liver masses had a final diagnosis of pancreatic adenocarcinoma or cholangiocarcinoma. After reviewing the records of the 129 patients with unknown primary malignancies, CA19-9 was neither helpful in establishing the primary etiology of an unknown primary malignancy nor did it result in additional pancreatic or biliary diagnostic testing to elucidate a primary malignancy in a single patient.
Other Laboratory Data
Serum laboratory values were available for 95% of the patients. AST, ALT, total bilirubin, alkaline phosphatase, albumin, creatinine, hemoglobin, white blood cell count, platelets, anti-nuclear antibody, and glucose were extracted from chart review. Laboratory data was then dichotomized based on the upper limit of normal for serum CA19-9 (Table 2). Patients with an elevated CA19-9 consistently had higher AST, ALT, total bilirubin, and alkaline phosphatase compared to patients without elevated serum CA19-9 (p < 0.001). Additionally, a different distribution of serum albumin values including a lower median and larger standard deviation was noted in patients with elevated CA19-9 (p < 0.001). The distribution of hemoglobin, white blood cell count, platelets, and glucose was not statistically different between the two groups. Anti-nuclear antibody titers were available for 25 patients without any statistical difference (p = 0.8) between the two groups.
Final Diagnoses
The final diagnoses in 448 out of 483 (93%) patients included infectious, biliary, and biopsy-proven malignant processes (Table 3). The most common diagnoses included pancreatic adenocarcinoma (14%), unknown primary malignancy (7%), and pancreatitis (7%). The distribution of CA19-9 amongst the different disease processes varied widely (p < 0.001). The final diagnoses associated with the highest median serum CA19-9 levels were cholangiocarcinoma (476 U/ml), primary biliary cirrhosis or primary sclerosing cholangitis (170 U/ml), pancreatic cancer (161 U/ml), choledocholithiasis (128 U/ml), colon cancer (102 U/ml), and hepatocellular carcinoma (74 U/ml). The subset of 143 patients who carried one of these six diagnoses were dichotomized based on serum CA19-9 elevated higher or lower than the assay’s upper limit of normal to analyze the serum laboratory values in these patients. An elevated serum AST, ALT, total bilirubin, and alkaline phosphatase were all again associated with an elevated CA19-9 (all p < 0.01) in this subset of patients.
Receiver Operative Curve Analysis
An ROC was constructed to determine the efficiency of CA19-9 in diagnosing biliary and pancreatic malignancy (Fig. 1). The area under the curve (AUC) was 0.67 (95% CI 0.59–0.75) for biopsy-proven cholangiocarcinoma or pancreatic cancer. The AUC in diagnosing biopsy-proven pancreatic adenocarcinoma in patients with a known pancreatic head mass was 0.66 (95% CI 0.55–0.76). The sensitivities and specificities at different CA19-9 levels used by previous study groups are represented in tabular format in Table 4.
Discussion
In this study, serum CA19-9 level was found to have a lower sensitivity and specificity for diagnosing pancreatic cancer or cholangiocarcinoma than what has been previously reported. The receiver operating curve showed that CA19-9 was unreliable regardless of the cut-off used for sensitivity or specificity based on an AUC of 0.67, which had not been described in previous studies. The sensitivity for diagnosing cholangiocarcinoma or pancreatic cancer was 64 and 59% using cut-off levels of 40 and 100 U/ml, which is lower than that previously described. We found similar results for specificity, which were 69 and 82%, respectively. While the reason for CA19-9 was not explicitly stated in the medical records, there is an underlying assumption that the clinician ordering the test was concerned about pancreatic or biliary malignancy. In patients with a pancreatic head mass on imaging, which could be considered a particular subset of patients with a higher pre-test probability for pancreatic neoplasia, pancreatic cancer was ultimately diagnosed in 53% of patients. In this subset, CA19-9 had a sensitivity of 60 and 57% and specificity of 81 and 86% using a cut-off level of 40 and 100 U/ml, respectively. In patients with known pancreatic head masses, CA19-9 had an AUC of 0.66. Interestingly, the poor sensitivity and specificity of CA19-9 was also reflected in the fact that the level did not change patient management in a single patient reviewed. In patients with unknown primary malignancies, the CA19-9 level was not used as a test to confirm, exclude, or guide diagnostic studies for neoplasms of a pancreatic or biliary origin. Invasive tests were sometimes performed to establish a diagnosis regardless of the CA19-9 level. In all of the patients reviewed, the CA19-9 was not explicitly indicated as either a diagnostic or adjunct test leading to additional testing or procedures. Conversely, the patient’s symptoms, cross-sectional imaging, and endoscopic findings were frequently referred to in notes when clinicians decided to pursue additional diagnostic studies and procedures.
Our study also showed that CA19-9 variation across ethnic backgrounds may exist, which has not been previously reported, and did not correlate with the frequency of malignancy; that is, while Caucasians had a higher CA19-9 level they were not more likely to be diagnosed with neoplasia compared to the other ethnic groups. On the other hand, African Americans and Hispanics had very low median CA19-9 levels, which could indicate that it is even less helpful as a diagnostic tool in these groups. Biological variability beyond the absence of Lewis antibody may need to be considered when interpreting a CA19-9 level.
Our study confirms the association between biliary obstruction and elevated CA19-9 [11]. However, those patients with evidence of biliary obstruction with elevations of AST, ALT, and bilirubinemia would have received an appropriate hepatic, pancreatic, and biliary evaluation regardless of the CA19-9 level. Actually, even patients with non-hepatitis B/C cirrhosis, cholangiocarcinoma, primary biliary cirrhosis, primary sclerosing cholangitis, pancreatic cancer, choledocholithiasis, colon cancer, or hepatocellular cancer with an elevation of CA19-9 had elevations in AST, ALT, bilirubin, and alkaline phosphatase. More importantly, patients with the aforementioned diagnoses had normal liver enzymes and bilirubin when CA19-9 was not elevated. This suggests that biliary obstruction, which produced the highest CA19-9 levels, determines the CA19-9 elevation irrespective of whether the obstruction is benign or malignant.
The strengths of our study include the large number of patients, biopsy-proven diagnoses of malignancy, patient heterogeneity (a university hospital and a municipal hospital), and the inclusion of demographic, clinical, pathological, and radiographic data. The retrospective nature of the study makes it impossible to establish a cause for all CA19-9 elevations, especially when dealing with benign disease.
In conclusion, serum CA19-9 is a poor diagnostic tumor marker for pancreatic cancer and cholangiocarcinoma. Furthermore, multiple confounding variables including gender, ethnicity, medical history, and biliary obstruction make interpretation of CA19-9 levels extremely difficult. With the availability of and advances in imaging technology, the diagnostic role of CA19-9 must be questioned as it was imaging and not CA19-9 that physicians frequently cited when recommending additional testing. In our review, CA19-9 did not aid in the diagnosis of malignancy, did not add to the impression of biliary obstruction, and did not alter management. Therefore, the role of CA19-9 in the 21st century is of questionable significance. Clinicians should carefully consider their goals when ordering this test.
References
Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigen detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–972.
Itkowitz SH, Kim YS. New carbohydrate tumor markers. Gastroenterology. 1986;90:491–494.
Tempero MA, Uchida E, Takasaki H, et al. Relationship of carbohydrate antigen and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501–5503.
Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–270.
Steinberg W. The clinical utility of the CA19-9 tumor-associated antigen. Am J Gastroenterol. 1990;85:350–355.
Andriulli A, Gindro T, Plantino P, et al. Prospective evaluation of the diagnostic efficacy of CA19-9 assay as a marker for gastrointestinal cancers. Digestion. 1986;33:26–33.
Uygur-Bayramicli O, Dabak R, Orbay E, et al. Type 2 diabetes mellitus and CA19-9 levels. World J Gastroenterol. 2007;13:5537–5539.
Mertz HR, Sechopoulos P, Delbeke D, et al. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc. 2000;52:367–371.
Sreenarasimhaiah J. Efficacy of endoscopic ultrasound in characterizing mass lesions in chronic pancreatitis. J Clin Gastroenterol. 2008;42:81–85.
Mishra G, Conway JD. Endoscopic ultrasound in the evaluation of radiologic abnormalities of the liver and biliary tree. Curr Gastroenterol Rep. 2009;11:150–154.
Mann DV, Edwards R, Ho S, et al. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474–479.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Tang, Sj., Sreenarasimhaiah, J. et al. The Clinical Utility and Limitations of Serum Carbohydrate Antigen (CA19-9) as a Diagnostic Tool for Pancreatic Cancer and Cholangiocarcinoma. Dig Dis Sci 56, 2491–2496 (2011). https://doi.org/10.1007/s10620-011-1709-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1709-8