Abstract
Background
Since their discovery, S100 proteins have been associated with diverse diseases of inflammatory, degenerative, or malignant nature. Due to their participation in inflammation, they have also been studied with regard to inflammatory bowel disease (IBD).
Method
To provide a review of available literature, a PubMed, MEDLINE, and Embase-based literature search was performed, using all available nomenclature for each member of the S100 protein family, along with the terms inflammatory bowel disease, ulcerative colitis, Crohn’s disease, or indeterminate colitis.
Result
S100A8/A9, also known as calprotectin, S100A12, or calgranulin C and in a lesser extent S100P, are involved in the pathogenesis, activity, diagnosis, and therapeutic management of IBD. The majority of available literature is focused primarily on S100A8/9, although there is growing evidence on the significance of S100A12. Most studies emphasize the potential merit of S100A8/A9 and S100A12, as markers for differential diagnosis, monitoring of activity, or disease relapse, in IBD. Limitations, regarding the diagnostic utility of these markers, seem to exist and are mainly related to the publication of conflicting results, i.e., for IBD activity, and to the fact that S100A8/A9 and S100A12 are not disease-specific.
Conclusions
Although the existing data link specific S100 proteins with IBD, there are still several drawbacks in the use of these markers for diagnostic purposes. Thus, it seems that further research is mandatory in order to eliminate the impact of confounding factors but also to detect additional associations between S100 proteins and IBD or novel S100 proteins with a closer correlation with IBD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1965, Moore managed to isolate, purify, and characterize a brain-specific soluble protein, S100, which was named after its ability to dissolve in a 100% ammonium sulphate solution [1]. Since then, a variety of similar low-molecular-weight acidic compounds has been identified in vertebrates, thus leading to the constitution of the S100 family of proteins. The members of the S100 protein family are all EF-hand (a helix-loop-a helix), calcium-binding proteins, and with the exception of S100G (calbindin D9 k), which is monomeric, exhibit a dimeric nature. To our knowledge, 25 tissue and cell-specific S100 proteins have been recognized to date in humans (Table 1), of which the vast majority is in a homodimer, heterodimer, or more complex form, which seems to favor target protein binding. These dimerization products participate in calcium-dependent or calcium-independent protein–protein interactions, which take place intra- or extracellularly, via secreted S100 proteins. It is through these interactions that the S100 proteins carry out a series of proposed biological tasks, such as regulation of protein phosphorylation, modulation of enzyme activity, promotion of cell growth, differentiation or apoptosis, participation in Ca++ homeostasis, preservation of cell shape and motility, regulation of coagulation, and induction of proinflammatory/inflammatory responses [2–5]. An example of such interactions is the binding of the S100B, S100A6, 6 S100A8/A9 heterodimer (calprotectin), S100A12 (calgranulin C) [4], or S100P [2] with the receptor for advanced glycation end products (RAGE). In addition, certain S100 proteins, S100A8, S100A9, S100A12, have been identified as molecules released during cell activation and lysis or as damage associated molecular pattern proteins (DAMPs) [3–6]. The implication of the proteins of the S100 family in a wide spectrum of biological processes proved to be quite intriguing hence, triggering intense research towards their involvement in many different malignant [7], degenerative [8], and inflammatory diseases [4, 5]. As a result of this intense quest for evidence, members of the S100 protein family have been linked with both extradigestive and digestive-related disorders, including inflammatory bowel disease (IBD), in a number of studies. In the present review, evidence focusing on the role of S100 proteins in the pathogenesis, activity, diagnosis, and therapeutic management of IBD are classified accordingly, so that studies offering similar results are presented together, in order to facilitate critical comparison and evidence-based conclusion.
The S100 Proteins in Disease
Each member of the S100 family exhibits one or more distinct roles, a feature that facilitates the participation in different pathways and allows the induction of various responses. Hence, a variety of biological responses, mediated by the same S100 protein, may be triggered by a single cause, such as an inflammatory disease or a malignancy. Likewise, a unique, single S100 protein-mediated response could result from various diseases [4, 5]. Moreover, a tissue-specific pattern of expression has been described for a number of S100 family constituents. Tissue specificity is important as it allows the detection of organ or tissue-specific pathology and discriminates between active or inactive states [2, 3]. The above-mentioned characteristics can justify the involvement of the S100 proteins in distinct types of disorders, systemic or isolated, inflammatory, degenerative or malignant.
S100 Proteins and Extradigestive Diseases
Members of the S100 protein family have been implicated in a large number of disorders outside the alimentary tract. Diseases sharing an autoimmune inflammatory background, infections, lung, vascular and dermatological diseases, heart failure, as well as neuropathological states are associated with S100 proteins (Table 1) [4, 5, 8–11]. An either suppressive or promoting role has been documented for S100 proteins, with regard to multiple tumor types, especially of solid nature (Table 1) [7, 12–15]. Based on these findings, it is not surprising that S100 proteins are being determined in various biological fluids (sputum, blood, urine, synovial, pleural [4], cerebrospinal [18], and amniotic fluid [16]) in feces [4] and in biopsy specimens, including placental [16].
S100 Proteins and Digestive Diseases
The accumulating evidence linking members of the S100 family with a proinflammatory axis and subsequently with a variety of non-infectious inflammatory diseases has encouraged the study of gastrointestinal-related inflammation with regard to S100 proteins. The vast majority of available studies is focused on three members of the S100 protein family: S100A8, S100A9, and S100A12. There are reports, however, that link other S100 proteins, S100B and S100P, with celiac disease [17] and IBD [18], respectively. S100A8, S100A9, and S100A12, also known as calgranulins, are considered phagocyte-specific, exhibit pro-inflammatory properties, and can be expressed either as homogenous compounds or as heterodimeric molecules (S100A8/S100A9 heterodimer) [4, 19]. In contrast with S100A8 and S100A9, which are found in granulocytes, monocytes, and macrophages, S100A12 is predominantly expressed in granulocytes [4, 5, 19, 20].
Apart from inflammation, a role for certain S100 proteins in alimentary tract-related tumorigenesis has also been suggested. Four S100 proteins, S100A4, S100A8, S100A9, and S100A11, have been linked with both gastric and colorectal cancer [7]. On the other hand, S100A2 [21], S100A3 [22], and S100A7 [21] correlated with gastric, while the S100A6 protein correlated with colorectal cancer [7]. S100 proteins have also been associated with oral [7, 23], esophageal [24], and pancreatic cancer [25], as well as various endocrine intestinal tumors [26]. Thus, the assessment of S100 proteins, carried out in blood, fecal, and tissue specimens, has revealed a field of great interest regarding both bowel inflammation and carcinogenesis. As far as bowel inflammation is concerned, the literature focuses on IBD, implicating S100 proteins in the pathogenesis and activity of Crohn’s disease (CD), ulcerative colitis (UC), and IBD of the unclassified type (IBDU), while at the same time supporting the notion of S100 proteins serving as diagnostic markers or candidates for therapeutic management.
S100 Proteins in IBD
S100 Protein Involvement in IBD Pathogenesis
A genomic dysregulation has already been described in CD, UC, and IBDU and it has been linked with genetic susceptibility for these diseases. According to Lawrance et al. [18], this genetic dysregulation seems to concern S100 genes in IBD. In this study, the global gene expression profiles of inflamed colonic tissue, obtained from UC and CD patients, were examined. An increased expression for S100A9 (M26311) and S100P (×65614) genes in UC and CD specimens was recorded, while an overexpression of S100A11 gene (D38583) was evident only in UC specimens. In another study performed by Srivastava et al. [27], an upregulation of S100 genes in diseased colonic tissue from CD patients was found.
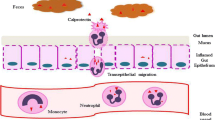
S100-positive accessory cells have been shown to be overrepresented in CD and UC patients [28]. Resection samples, obtained from the terminal ileum of patients with CD, showed a large population of S100-positive cells, proximal to the epithelium of inflamed but otherwise healthy mucosa, adjacent to ulcered areas [29]. The same research group identified S100-positive dendritic cell populations, following a similar distribution pattern, in colectomy specimens from UC patients [30]. Αpart from a predominant presence in the colon, mature dendritic cells, stained for S100 have been detected in mesenteric lymph nodes of CD patients [31]. Interestingly, a decrease in the number of S100-positive nerve fibers in the lamina propria of resected colon from IBD patients has been identified in the study of Kubota et al. [32] probably contributing to disruption of local immunoregulation. Large populations of S100A12-positive granulocytes in UC-induced crypt abscesses, as well as in CD and UC-affected lumen, have also been recognized [33]. According to Leach et al. [34], these recruited granulocytes (mostly neutrophils) along with eosinophils, epithelial cells, or even keratinocytes may release S100 proteins at sites of mucosal inflammation. Moreover, S100A8 and S100A9 expression has been detected in epithelial cells of IBD-inflamed intestine [19]. The accumulation of S100A-8 and S100A9-positive cells has been linked to the excessive presence of free radicals, which in turn induce tissue damage in IBD patients [35].
All the above-mentioned evidence (Fig. 1) seems to underline the importance of S100 proteins’ participation in the intestinal immunity as well as the inflammatory pathways that induce and sustain intestinal injury and dysfunction.
S100 Proteins as Diagnostic Markers in IBD
Members of the S100 protein family have been isolated from blood, intestinal mucosa, and feces of IBD patients, in several studies. The most promising results, however, seem to originate from the assessment of three S100 proteins: S100A8, S100A9, and S100A12. The diagnostic utility of these S100 proteins has been studied extensively leading to results that could either justify or raise reasonable doubt regarding such a use.
The Case in Favor
Serum and Mucosal Determinations
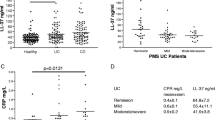
A correlation between IBD and upregulated serum levels of calprotectin was identified in a study performed in 1995 by Lugering et al. [36] in CD patients. In a case–control study by Leach et al., serum and mucosal calprotectin and S100A12 were assayed in 39 children with IBD, 16 with celiac disease (disease controls), and 33 non-IBD controls, all recruited prospectively. Both serum and mucosal calprotectin as well as S100A12 were upregulated in UC (p < 0.01) and CD (p < 0.001) patients compared to non-IBD controls. Patients with IBDU seemed to exhibit higher levels of mucosal (p < 0.001) but not serum calprotectin or S100A12 compared to non-IBD controls. Nevertheless, an overexpression of S100A8/A9 and S100A12 was also recorded in celiac disease-affected mucosa [34]. Foell et al. [33] reported similar results in 40 adult CD patients who exhibited increased S100A12 serum levels, irrespective of disease activity while Kapsoritakis et al. [37] showed that the significantly elevated serum S100A12 levels in CD and UC could discriminate IBD from irritable bowel syndrome (IBS) patients.
Fecal Studies
The levels of S100A12 in serum have been shown to correlate significantly (p < 0.05) with fecal S100A12, in 12 children with pancolitis, as shown in the study of de Jong et al. In the same study, the elevated fecal S100A12 levels recorded in 23 IBD patients (22 with active CD and one with active UC) could discriminate between subjects with IBD at diagnosis and healthy controls, with a sensitivity of 96% and a specificity of 92%, when a cut-off of 10 mg/kg for fecal S100A12 was used [38]. Kaiser et al. reported similar results regarding fecal S100A12 in a set of 32 CD, 27 UC, 24 IBS patients, 88 patients with infectious gastroenteritis, and 24 healthy individuals. Fecal S100A12 levels were significantly elevated in patients with either active or inactive IBD (p < 0.001 in all cases) and bacterial gastroenteritis but not in patients with IBS or viral gastroenteritis, compared to healthy controls. Moreover, fecal S100A12 could distinguish patients with active IBD from healthy controls with a sensitivity of 81% for CD, 91% for UC, and a specificity of 100% for both diseases. Likewise, fecal S100A12 levels could differentiate IBS from active IBD with a 86% sensitivity and 96% specificity. The difference, however, in fecal S100A12 levels between patients with active IBD and bacterial enteritis did not prove to be significant in this study [39]. The utility of S100A12 levels in feces, as a diagnostic marker for IBD, was also confirmed by Sidler et al. in a study group consisting of 30 CD, one UC, and 30 children or adolescents with various other disorders. The reported sensitivity, specificity, positive and negative predictive values for fecal S100A12, at a 10 mg/kg cut-off were 97% in all cases [40]. Consistent with these results were the findings of Foell et al. [41] who reported an upregulation of fecal S100A12 in IBD patients. Statistically significant elevated calprotectin levels in fecal specimens from IBD patients have been recorded in numerous studies where either children [40, 41, 43–45] or adults [44, 46, 47] were recruited. Interestingly, a single fecal calprotectin estimation could predict abnormal small bowel radiology in the study of Dolwani et al. [48]. Moreover, elevated calprotectin levels in feces have been shown to differentiate IBD from IBS or discriminate IBD patients from healthy subjects [40, 49–53]. In a meta-analysis of prospective studies performed by von Roon et al., which included a total of 5,983 patients, the diagnostic precision of fecal calprotectin for IBD was higher in children than in adults at a cut-off level of 100 μg/g and 50 μg/g respectively. Overall sensitivity and specificity for IBD diagnosis was 95 and 91%, respectively [44].
The Case Against
Serum and Mucosal Studies
Notwithstanding the fact that S100A12 and S100A8/A9 emerged as a promising marker for IBD diagnosis, confounding factors must be taken into account.
As mentioned above, in patients with IBDU, the levels of serum S100A8/A9 or S100A12 were comparable to non-IBD controls while, in the same study, an overexpression of S100A8/A9 and S100A12 was also detected in the mucosa of patients with celiac disease [34]. Increased expression of S100A8, S100A9, and S100A12 in H. pylori-inflamed gastric mucosa has been detected in the study of Leach et al. [54] and may be a confounding factor when S100 proteins are being determined.
Studies in Feces
The excess in fecal S100A12 levels may not be merely attributed to IBD since, besides bacterial gastroenteritis [39], colorectal cancer and advanced adenoma are also potential causes of such an increase and for that capable of leading to misinterpretations [42]. Elevated fecal calprotectin levels were also found in subjects suffering from bacterial or viral gastroenteritis [40], acute uncomplicated diverticulitis or symptomatic diverticular disease [55], celiac disease, food intolerance, non-steroidal anti-inflammatory drug (NSAID) enteropathy and immunodeficiency [56], as well as in patients with colorectal cancer or adenomatous polyps [42, 57, 58] and in those who had undergone pelvic radiation [59]. Significant positive correlations were also found between fecal calprotectin and age [60, 61], obesity, physical inactivity, and an inverse relationship with vegetable consumption and fiber intake [61].
These observations may raise skepticism regarding the potential use of calgranulins for diagnostic purposes in IBD, as they cannot be regarded as disease-specific, rather reflecting mucosal inflammation or destruction.
S100 Proteins as Markers of Disease Activity
The intriguing idea of using the ubiquitous members of S100 protein family calgranulin C and calprotectin for diagnosing and discriminating IBD from other pathologic entities has encouraged the intensive study of these proteins in IBD patients. It was not long until a new hypothesis emerged, pointing towards a possible correlation between calprotectin or calgranulin C and IBD activity, which in turn could encourage the use of these proteins as novel markers of disease activity. The results, however, originating from disease activity-related studies proved to be quite controversial.
The Case in Favor
Serum and Mucosal Studies
In the study of Foell et al., a significant difference in S100A12 serum levels between active and inactive CD as well as between active and inactive UC was recorded (p < 0.01, p < 0.001, respectively). S100A12 serum levels, however, were comparable between patients with inactive UC and healthy controls. Serum S100A12 levels have been found to correlate with endoscopic and histological scores (p < 0.01 and p < 0.001, respectively). Steroid treatment did not seem to affect S100A12 serum levels while therapy with infliximab led to a decrease in S100A12 levels [33]. Another serum study performed in 54 CD, 56 CD, and 81 IBS adult patients showed that although elevated serum S100A12 levels correlated with C reactive protein (CRP) and serum amyloid A (SAA), they could not discriminate between active and inactive states of IBD [37]. In a pediatric population, the association between S100 proteins and IBD activity was confirmed only for serum and mucosal calprotectin, which proved to correlate well with both CRP and pediatric Crohn’s disease activity index (PCDAI) (p < 0.05, in both cases) but not with either a modified disease activity index or albumin, platelet count and disease location [34].
Fecal Studies
In an original contribution by de Jong et al., fecal S100A12 levels were found to correlate significantly with CRP (p < 0.001), platelet count (p < 0.0012), and albumin (p < 0.005), in children with either non-continuous colonic CD or CD isolated beyond the ileocecal valve. Children with CD “pancolitis”, however, exhibited levels of fecal S100A12, which correlated with PCDAI (p < 0.0012), erythrocyte sedimentation rate (ESR) (p < 0.05) and platelets (p < 0.00250) but not with CRP or albumin. A significant decrease in fecal S100A12 (p < 0.05) was also recorded for CD in remission [38]. Kaiser et al. on the other hand, managed to associate fecal S100A12 levels with histological inflammation score, ESR, CRP, platelet and white blood cell count, hematocrit and hemoglobin in IBD adult patients. Although a significant correlation between fecal S100A12 and colitis activity index (CAI) in UC was recorded, no such correlation became evident between S100A12 and Crohn’s disease activity index (CDAI), in CD patients. Fecal calprotectin was also higher in active compared to inactive IBD, but did not seem to perform equally when disease location was taken into account. Unlike calprotectin, fecal calgranulin C performed equally well, regardless of disease location [39].
Differences in fecal calprotectin levels between patients with active and inactive IBD were recorded in a number of studies both in adults [47, 62–65] and children [41, 66]. A significant correlation between fecal calprotectin and the Crohn’s Disease endoscopic index of severity or the CDAI was observed in the study of Sipponen et al. [65]. Fagerberg et al. managed to correlate the magnitude of calprotectin excretion in feces with the extent and severity of macroscopically as well as microscopically detected inflammation in IBD children. A significant difference between symptomatic and asymptomatic IBD children was also recorded (p < 0.001) [43]. In another study recruiting IBD adults, positive associations between fecal calprotectin and CRP or activity indices were found (CDAI and Mayo Disease Activity Index) [67]. Similarly, the Rachmilewitz endoscopic activity index for UC and the Simple Endoscopic Score for Crohn’s disease (SES-CD) were shown to correlate more closely with fecal calprotectin than with CRP, blood leucocytes, or conventional indices (CAI or CDAI) [68, 69].
The Case Against
Fecal calprotectin levels seem to fluctuate as a result of different disease location in CD [39]. Besides, in a pediatric population, it was shown that, in active CD, fecal calprotectin seemed to be predominantly released from sites of colonic inflammation [41]. Interesting were the results originating from a study focusing on corticosteroid-treated IBD children. In some cases, although clinical remission was evident, fecal calprotectin did not reach values within normal range, while in others, cessation of corticosteroid therapy led to increased calprotectin levels within 4 weeks [70]. Abnormal fecal calprotectin values were detected even in adults with inactive disease [64]. In a few studies, greater fecal calprotectin values were recorded in IBD patients in clinical remission, compared to those with active IBD [61, 65]. Increased fecal calprotectin levels were also found in CD patients who had undergone ileocolonic resection and entered clinical remission status [71]. Even the association of calgranulin C and calprotectin with other markers of inflammation has been denied. In the study of Sidler et al., fecal S100A12 did not correlate with ESR, CRP, platelet count, or serum albumin in CD children. Fecal calprotectin correlated only with albumin (p = 0.03) while no association whatsoever between fecal S100A12 or S100A8/A9 and PCDAI was found [40].
S100 Proteins Predicting Disease Relapse
Among the symptomatic IBD cases, in the aforementioned study of Fagerberg et al., there were two patients with significantly elevated calprotectin levels, along with small bowel inflammation alone, while in the asymptomatic group, an increase in fecal calprotectin was observed in patients with mild microscopic inflammation (p < 0.004). According to the authors, the increased fecal calprotectin in these asymptomatic patients could be related to disease relapses [43]. In addition, in a study performed earlier, Tibble et al. [72] reported that IBD patients in clinical remission who exhibited elevated fecal calprotectin levels had a 13-fold increased risk of relapse during a year follow-up period. Costa et al. [73] confirmed these results and underlined the significance of fecal calprotectin as a predictive marker of relapse, especially in UC patients (14-fold risk, against a two-fold risk for CD patients). Consistent with these observations were reports on a positive association between fecal calprotectin and relapse in UC and CD [74, 75]. Similar were the results reported in two separate studies, showing that fecal calprotectin levels could identify high risk of relapse among CD patients who had undergone ileocecal resection [76, 77]. In the same way, elevated fecal calprotectin levels have been associated with pouchitis and pouchitis-related scores (Objective Pouchitis Score, Pouch Disease Activity Index, endoscopical and histological inflammation scores), in UC patients [76, 78, 79]. All these variations along with the fluctuations of calprotectin and calgranulin C with respect to several IBD-related characteristics, are summarized in Table 2.
S100 Proteins as Candidates for Therapeutic Management
The intense scientific research on S100 proteins has revealed a wide range of possibilities for novel S100-oriented therapeutic interventions.
In the study of Hofmann et al. [80], inhibition of the interaction of S100A12 with RAGE reduced inflammation in a murine model of colitis. Moreover, the S100A8/A9 heterodimer, which is upregulated in IBD, has been shown to bind to human colonic tumor cells, via carboxylated glycan- and RAGE-mediated binding, thus promoting cell proliferation. In the same study, the administration of an anti-carboxylate glycan antibody reduced chronic colonic inflammation as well as the associated tumorigenesis in mice [81]. In another study, however, the treatment of colon cancer cells with different concentrations of human S100A8/A9, instead of resulting in proliferation, led to increased cell death [82], which was attributed to a RAGE-independent pathway [83]. This S100-related, anti-oncogenic potential was demonstrated in two separate studies, where an increased number of S100-positive dendritic cells was associated with a lower TNM score and a better prognosis, in patients with colorectal cancer [84, 85]. Whether the overrepresentation of S100-positive cells and S100A8/A9 in IBD serve as a defense mechanism against inflammation-induced tumorigenesis or not, is a matter open for discussion.
Another interaction with a therapeutic potential is that of cromolyn, which has been shown to bind S100P, preventing the activation of RAGE and subsequently tumor growth, while increasing at the same time the sensitization of pancreatic cancer cells in gemcitabine [86]. This observation may be of importance since the S100P seems to be implicated in IBD pathogenesis, too [18].
Finally, there is the paradigm of S100A1’s implication in chronic functional heart failure. This well-established association has led to rescue strategies, including recombinant AAV6-S100A1 gene therapy, which may serve as a model for future interventions in IBD [9].
Critical Assessment of S100 Protein Family in Clinical Practice
In view of the role of S100 proteins in IBD, it seems that especially calprotectin and calgranulin C can be useful markers for diagnosis (Fig. 2), as well as for activity surveillance of IBD. There are issues, however, that need to be considered.
As far as the optimal type of sample, blood or fecal, used for calprotectin and calgranulin C determination is concerned, the following must be taken into account. Research, as well as in-hospital testing, tend to benefit from the use of simpler techniques (sampling, storing, testing requirements) and for that reason, serum has been considered to be a “friendlier” sample for both research and routine determinations. Limitations, however, regarding the use of serum S100 protein determinations in IBD do exist and are mainly due to the small number of available studies, especially in adults [33, 36, 37] and the large number of diseases that could result in an upregulation of S100 proteins in serum [4]. Feces, on the other hand, are in direct contact with the intestinal mucosa and despite the fact that the determination of calprotectin or calgranulin C requires the use of enzyme-linked immunosorbent assay (ELISA) techniques, a stability of fecal specimens is acceptable for a 7 or 10-day period at room temperature [38, 87, 88], thus allowing individual shipment of fecal samples, even by custom mail [88]. Since new fecal rapid tests have emerged which tend to exhibit remarkable sensitivity and negative predictive value, in discriminating IBD from IBS (100% for fecal calprotectin rapid test), it is anticipated that this simplified determination will add up to the efficiency of in-patient diagnostic testing and monitoring for IBD [50].
Confounding factors such as infectious gastroenteritis, celiac disease, biological treatment for concomitant diseases, i.e., rheumatoid arthritis or Sjögren’s syndrome and pelvic radiation for different types of cancer could influence both calprotectin and calgranulin C levels, thus reducing diagnostic utility in IBD. The impact of perianal disease (abscess and fistula) on calprotectin or calgranulin C levels should also be examined. A closer look at the levels of calgranulins in treated IBD patients is also mandatory, as it is not yet clear whether persisting high concentrations represent a potential flaw or a determinant of potential disease flare-up. What should also be kept in mind is that intestinal tumors have been shown to upregulate S100 proteins, calprotectin and calgranulin C included [7, 44], hence a careful interpretation is vital so that the recorded upregulation is not wrongfully attributed to an inflammatory process, thus delaying tumor diagnosis.
Based on the above-mentioned evidence, to rely solely on calprotectin or calgranulin C determination, following a single “best” laboratory test pattern can prove misleading. Perhaps it is safer to apply a combined determination of markers, as it has been suggested for colorectal cancer diagnosis [42], in order to increase diagnostic efficiency in all IBD types, irrespective of disease activity or location.
Whether calprotectin or calgranulin C could replace other traditional markers such as CRP or ESR is a question yet to be answered, although both have been shown to exhibit a higher diagnostic efficiency than CRP and ESR [40, 89].
It is possible that in the near future, calprotectin and calgranulin C may serve as essential components in a novel, updated activity index for UC and CD. A similar task could be carried out with an emphasis on the prediction of disease relapse [43, 72–79] or even the need for surgical intervention, i.e., colectomy in severe UC [90].
Another possible link that should be examined is that between calprotectin and stenosis, since it has been shown that calprotectin may facilitate fibroblast apoptosis [82]. This might mean that in the actively diseased ileum where S100A8/A9 is not overrepresented, fibroblasts may carry out fibrotic tasks, which are not subject to a S100A8/A9 apoptotic control, thus leading to a greater risk for stenosis, in the ileum, compared to the colon.
Further studies are needed in order to clarify whether S100 proteins could serve as reliable diagnostic or prognostic markers for IBD-related tumorigenesis.
There is also the intriguing notion that S100-positive cells and S100 proteins, which can be present in excess in IBD, comprise a line of defense against malignant cells [84, 85] and that this beneficial effect could be amplified by known pharmaceutical compounds [86]. Perhaps a modified S100A8/A9 molecule incapable of interacting with RAGE, but capable of carrying out RAGE-independent tasks could retain colonic inflammation while reducing at the same time the risk for colorectal cancer in IBD patients.
Conclusions
Taken together, all data designate the importance of S100 proteins in IBD. There is no doubt that this intense S100 protein-focused quest for evidence with regard to IBD has been fruitful. Research on the other hand should always be targeted towards the direction of new, perhaps even more promising markers. Ideally, a member of S100 protein family exhibiting better tissue-specific properties with regard to intestinal mucosa could serve as an efficient research tool and reliable diagnostic marker but also as a promising target for more effective and selective therapies.
References
Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commmun. 1965;19:739–744.
Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–214.
Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551.
Foell D, Frosch M, Sorg C, et al. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344:3751.
Foell D, Wittkowski H, Vogl T, et al. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37.
Leclerc E, Fritz G, Weibel M, et al. S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J Biol Chem. 2007;282:31317–31331.
Salama I, Malone PS, Mihaimeed F, et al. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364.
Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res. 2007;85:1373–1380.
Pleger ST, Most P, Boucher M, et al. Stable myocardial-specific AAV6–S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515.
Eckert RL, Broome AM, Ruse M, et al. S100 proteins in the epidermis. J Invest Dermatol. 2004;123:23–33.
Giovannoni G. Multiple sclerosis cerebrospinal fluid biomarkers. Dis Markers. 2006;22:187–196.
Yao R, Lopez-Beltran A, Maclennan GT, Montironi R, Eble JN, Cheng L. Expression of S100 protein family members in the pathogenesis of bladder tumors. Anticancer Res. 2007;27:3051–3058.
Hancq S, Salmon I, Brotchi J, et al. S100A5: a marker of recurrence in WHO grade I meningiomas. Neuropathol Appl Neurobiol. 2004;30:178–187.
Pierce A, Barron N, Linehan R, et al. Identification of a novel, functional role for S100A13 in invasive lung cancer cell lines. Eur J Cancer. 2008;44:151–159.
Landriscina M, Schinzari G, Di Leonardo G, et al. S100A13, a new marker of angiogenesis in human astrocytic gliomas. J Neurooncol. 2006;80:251–259.
Michetti F, Gazzolo D. S100B testing in pregnancy. Clin Chim Acta. 2003;335:1–7.
Esposito G, Cirillo C, Sarnelli G, et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology. 2007;133:918–925.
Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet. 2001;10:445–456.
Roth J, Vogl T, Sorg C, et al. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158.
Vogl T, Propper C, Hartmann M, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–25296.
El-Rifai W, Moskaluk CA, Abdrabbo MK, et al. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6826.
Liu J, Li X, Dong GL, et al. In silico analysis and verification of S100 gene expression in gastric cancer. BMC Cancer. 2008;8:261.
Sapkota D, Bruland O, Bøe OE, et al. Expression profile of the S100 gene family members in oral squamous cell carcinomas. J Oral Pathol Med. 2008;37:607–615.
Ji J, Zhao L, Wang X, et al. Differential expression of S100 gene family in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2004;130:480–486.
Rodriguez JA, Li M, Yao Q, et al. Gene overexpression in pancreatic adenocarcinoma: diagnostic and therapeutic implications. World J Surg. 2005;29:297–305.
Capella C, Riva C, Rindi G, et al. Endocrine tumors of the duodenum and upper jejunum. A study of 33 cases with clinico-pathological characteristics and hormone content. Hepatogastroenterology. 1990;37:247–252.
Srivastava MD, Kulaylat MN. Gene expression profiles of late colonic Crohn’s disease. J Med. 2004;35:233–255.
Rugtveit J, Nilsen EM, Bakka A, et al. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112:1493–1505.
Sarsfield P, Jones DB, Wright DH. Accessory cells in Crohn’s disease of the terminal ileum. Histopathology. 1996;28:213–219.
Waraich T, Sarsfield P, Wright DH. The accessory cell populations in ulcerative colitis: a comparison between the colon and appendix in colitis and acute appendicitis. Hum Pathol. 1997;28:297–303.
Verstege MI, ten Kate FJ, Reinartz SM, et al. Dendritic cell populations in colon and mesenteric lymph nodes of patients with Crohn’s disease. J Histochem Cytochem. 2008;56:233–241.
Kubota Y, Petras RE, Ottaway CA, et al. Colonic vasoactive intestinal peptide nerves in inflammatory bowel disease. Gastroenterology. 1992;102:1242–1251.
Foell D, Kucharzik T, Kraft M, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853.
Leach ST, Yang Z, Messina I, et al. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1321–1331.
Rugtveit J, Haraldsen G, Hogasen AK, et al. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14 + L1 + monocyte derived cells. Gut. 1995;37:367–373.
Lügering N, Stoll R, Kucharzik T, et al. Immunohistochemical distribution and serum levels of the Ca(2 +)-binding proteins MRP8, MRP14 and their heterodimeric form MRP8/14 in Crohn’s disease. Digestion. 1995;56:406–414.
Kapsoritakis AN, Georgoulias PA, Manolakis AC, et al. Serum S100A12, a marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2008;57:A138.
de Jong NS, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn’s disease. Inflamm Bowel Dis. 2006;12:566–572.
Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56:1706–1713.
Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflamm Bowel Dis. 2008;14:359–366.
Foell D, Wittkowski H, Ren Z, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–192.
Karl J, Wild N, Tacke M, et al. Improved diagnosis of colorectal cancer using a combination of fecal occult blood and novel fecal protein markers. Clin Gastroenterol Hepatol. 2008;6:1122–1128.
Fagerberg UL, Lööf L, Lindholm J, et al. Fecal calprotectin: a quantitative marker of colonic inflammation in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;45:414–420.
von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–813.
Joishy M, Davies I, Ahmed M, et al. Fecal calprotectin and lactoferrin as noninvasive markers of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:48–54.
D’Incà R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–437.
Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169.
Dolwani S, Metzner M, Wassell JJ, et al. Diagnostic accuracy of faecal calprotectin estimation in prediction of abnormal small bowel radiology. Aliment Pharmacol Ther. 2004;20:615–621.
Schoepfer AM, Trummler M, Seeholzer P, et al. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14:32–39.
Otten CM, Kok L, Witteman BJ, et al. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275–1280.
Limburg PJ, Ahlquist DA, Sanborn WJ, et al. Faecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am J Gastroenterol. 2000;95:2831–2837.
Caroccio A, Iacono G, Cottone M, et al. Diagnostic accuracy of faecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49:861–867.
Costa F, Mumolo MG, Bellini M, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liv Dis. 2003;35:642–647.
Leach ST, Mitchell HM, Geczy CL, et al. S100 calgranulin proteins S100A8, S100A9 and S100A12 are expressed in the inflamed gastric mucosa of Helicobacter pylori-infected children. Can J Gastroenterol. 2008;22:461–464.
Tursi A, Brandimarte G, Elisei W, et al. Faecal calprotectin in colonic diverticular disease: a case-control study. Int J Colorectal Dis. 2009;24:49–55.
Bremner A, Roked S, Robinson R, et al. Faecal calprotectin in children with chronic gastrointestinal symptoms. Acta Paediatr. 2005;94:1855–1858.
Tibble J, Sigthorsson G, Foster R, et al. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut. 2001;49:402–408.
Hoff G, Grotmol T, Thiis-Evensen E, et al. Testing for faecal calprotectin (PhiCal) in the Norwegian Colorectal Cancer Prevention trial on flexible sigmoidoscopy screening: comparison with an immunochemical test for occult blood (FlexSure OBT). Gut. 2004;53:1329–1333.
Wedlake L, McGough C, Hackett C, et al. Can biological markers act as non-invasive, sensitive indicators of radiation-induced effects in the gastrointestinal mucosa? Aliment Pharmacol Ther. 2008;27:980–987.
Reinders CA, Jonkers D, Janson EA, et al. Rectal nitric oxide and fecal calprotectin in inflammatory bowel disease. Scand J Gastroenterol. 2007;42:1151–1157.
Poullis A, Foster R, Shetty A, et al. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:279–284.
Langhorst J, Elsenbruch S, Mueller T, et al. Comparison of 4 neutrophil-derived proteins in feces as indicators of disease activity in ulcerative colitis. Inflamm Bowel Dis. 2005;11:1085–1091.
Sipponen T, Savilahti E, Kolho KL, et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46.
Sipponen T, Savilahti E, Kärkkäinen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398.
Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229.
Canani RB, Terrin G, Rapacciuolo L, et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40:547–553.
Vieira A, Fang CB, Rolim EG, et al. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221.
Schoepfer AM, Beglinger C, Straumann A, et al. Ulcerative colitis: Correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leucocytes. Inflamm Bowel Dis. 2009;15:1851–1858.
Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leucocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169.
Kolho KL, Raivio T, Lindahl H, et al. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41:720–725.
Scarpa M, D’Incà R, Basso D, et al. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum. 2007;50:861–869.
Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22.
Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364–368.
D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014.
Gisbert JP, Bermejo F, Perez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–1198.
Orlando A, Modesto I, Castiglione F, et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci. 2006;10:17–22.
Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96:663–674.
Thomas P, Rihani H, Røseth A, et al. Assessment of ileal pouch inflammation by single-stool calprotectin assay. Dis Colon Rectum. 2000;43:214–220.
Johnson MW, Maestranzi S, Duffy AM, et al. Faecal calprotectin: a noninvasive diagnostic tool and marker of severity in pouchitis. Eur J Gastroenterol Hepatol. 2008;20:174–179.
Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901.
Turovskaya O, Foell D, Sinha P, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis. 2008;29:2035–2043.
Ghavami S, Kerkhoff C, Los M, et al. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76:169–175.
Ghavami S, Kerkhoff C, Chazin WJ, et al. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIABLO and Omi/HtrA2. Biochim Biophys Acta. 2008;1783:297–311.
Nakayama Y, Inoue Y, Minagawa N, et al. Relationships between S-100 protein-positive cells and clinicopathological factors in patients with colorectal cancer. Anticancer Res. 2003;23:4423–4426.
Nagorsen D, Voigt S, Berg E, et al. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med. 2007;5:62.
Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–1818.
Røseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27:793–798.
Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171–177.
Xiang JY, Ouyang Q, Li GD, et al. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53–57.
Ho GT, Lee HM, Brydon G, et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673–678.
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manolakis, A.C., Kapsoritakis, A.N., Tiaka, E.K. et al. Calprotectin, Calgranulin C, and Other Members of the S100 Protein Family in Inflammatory Bowel Disease. Dig Dis Sci 56, 1601–1611 (2011). https://doi.org/10.1007/s10620-010-1494-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1494-9