Abstract
Introduction
Several gases are produced through enteric fermentation in the intestinal tract. Carbon dioxide, hydrogen, hydrogen sulfide, and methane are thought to be the most common of these. Recent evidence suggests that methane may not be inert. In this review article, we summarize the findings with methane.
Methods
This is a review article discussing the various component gases in the gastrointestinal tract and their relevance to health and disease. Specific attention was paid to understanding methane.
Results
The majority of these gases are eliminated via flatus or absorbed into systemic circulation and expelled from the lungs. Excessive gas evacuation or retention causes gastrointestinal functional symptoms such as belching, flatulence, bloating, and pain. Between 30 and 62% of healthy subjects produce methane. Methane is produced exclusively through anaerobic fermentation of both endogenous and exogenous carbohydrates by enteric microflora in humans. Methane is not utilized by humans, and analysis of respiratory methane can serve as an indirect measure of methane production. Recent literature suggests that gases such as hydrogen sulfide and methane may have active effects on gut function. In the case of hydrogen sulfide, evidence demonstrates that this gaseous product may be produced by human eukaryotic cells. However, in the case of methane, there is increasing evidence that this gas has both physical and biological effects on gut function. It is now often associated with functional constipation and may have an active role here.
Conclusion
This review of the literature discusses the significance of enteric flora, the biogenesis of methane, and its clinical associations. Furthermore, we examine the evidence for an active role of methane in gastrointestinal motility and the potential applications to future therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human gastrointestinal tract is colonized with up to 1014 microbial cells, while the adult human itself is made up of approximately 1013 eukaryotic cells. Simply stated, the human body harbors ten times more microbial than human cells [1]. The concentration of microorganisms in the colon far outnumbers that of any other segment of the gastrointestinal tract and anaerobic organisms themselves make up the vast majority (>99%) of the colonic microflora [2]. Given the presence of such a diverse microflora, it is likely that these bacteria and their by-products play a key role in the balance between gastrointestinal health and disease.
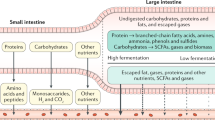
The large intestine houses the anaerobic organisms that, through fermentation, participate in the process of digestion. These microorganisms obtain their energy primarily by breaking down carbohydrates—mainly the undigested polysaccharide fraction of plant cell walls, and some resistant starches. This process results in the generation of short-chain fatty acids, CO2, H2, and CH4 [3]. This creates the potential for large quantities of gas to be produced within the intestinal lumen. For example, up to 12 l of H2 can be produced in 24 h [4]. Clearly, this magnitude of gas production can result in uncomfortable distension of the colon, otherwise known as bloating. Intestinal gas and bloating are among the most frequent gastroenterological complaints. Despite this large volume of gas produced, gaseous distension is not thought to directly cause abdominal pain in healthy individuals.
While intestinal gas is the focus of this paper, humans are capable of forming gases endogenously. Although CO2 is the most obvious, other gases are found and can have a role in the regulation of human biologic systems. These gases have been aptly termed “gasotransmitters” [5]. The first gasotransmitter discovered was nitric oxide (NO). NO, which is released by the endothelial cells of both arteries and veins, plays a key role in controlling vascular tone [6]. Interestingly, abnormalities in NO signaling may be implicated in certain gastrointestinal conditions such as diffuse esophageal spasm and achalasia [7, 8]. H2S is also produced by humans and is found to have physiological activity on smooth muscle [5].
In addition to specialized gas production by humans, a large amount of gases within the human body originate from gut bacteria. Bacteria can clearly be implicated in certain gastrointestinal illnesses, such as infectious diarrhea, small intestinal bacterial overgrowth, and peptic ulcer disease. The by-products of bacterial fermentation include many gases. Methane (CH4) is one of these. CH4 production has been linked to such diseases as constipation predominant irritable bowel syndrome (C-IBS), diverticulosis, and colon cancer [9–12]. These associations have sparked an interest in determining if there exists a causal relationship between CH4 and these clinical entities. This review of the literature will discuss the biogenesis of CH4 in the gut, the relationship of CH4 with certain gastrointestinal disease states, and potential clinical and treatment related implications.
Intestinal Gases
The composition of gastrointestinal gases not only varies between individuals but is also dependent on the site from which it is sampled. For example, the composition of gases in the stomach is actually quite similar to the air we breath [13]. The composition of flatus however, is much more variable (Table 1). According to a study of 20 normal subjects, the primary constituents of flatus are N2 (59%), H2 (20.9%), CO2 (9%), CH4 (7.2%), O2 (3.9%), and H2S (0.00028%). CO2, H2, and N2 are universal constituents in flatus, and all but one subject also had O2 present. CH4 is extremely variable, present in 12/20 subjects and ranging from 0 to 30.3% of the flatus. H2S is present in very small quantities, and like CH4, is not present in all individuals. The excretion rate of flatus in this study was 1.48 ml/min (2.1 l/24 h) [14]. Other investigators have found the volume of flatus to be between 400 and 1,200 ml/day [13].
Greater than 99% of H2 is produced in the large intestine. H2 production is extremely low to undetectable in the small bowel of normal fasting subjects [15]. This is most likely explained by the higher concentration of bacteria in the large bowel compared to the small bowel [2]. Patients with small intestinal bacterial overgrowth (SIBO) however, can have significant production of H2 in the small bowel [16]. CH4 also is not typically a constituent of small-bowel gases [15, 17].
Both H2 and CH4 are thought to be produced exclusively by anaerobic fermentation in the gut [13]. These gases can then traverse the intestinal mucosa and be absorbed into the systemic circulation. Once in the circulation, the only known source of clearance of these two gases is via the lungs [13]. One study found that the volume of H2 present in the bowel of ten normal subjects averaged 0.24 ml/min in the fasting state. This rate sharply increased upon instillation of lactulose, to a mean peak rate of 1.6 ml/min. It was found that 14% of total H2 produced was excreted via the lungs and that breath H2 excretion correlated well with total H2 production [16]. Another much more physiological study of hydrogen production and excretion found that overall 58% of H2 is excreted in the breath. However, this was dependent on production rates, with 65% at lower rates of <200 ml/day and 25% at rates of >500 ml/day [18]. CH4 producers averaged a production rate of 0.45 ml/min of CH4 in gas passed via rectum. Pulmonary CH4 excretion ranged from undetectable to 0.66 ml/min, and 20% of total CH4 produced was excreted via the lungs. Infusion of lactulose into the bowel did not increase CH4 production [17].
H2 and CH4 gases are unique in humans in that they are produced only though microbial fermentation, and once in circulation they are only excreted via the lungs [13, 17]. This can be compared and contrasted to the production and metabolism of H2S. H2S is produced by certain intestinal microorganisms, yet it can also be produced by mammalian cells. It is now well known that vascular tissues have the ability to generate H2S [5]. H2S is potentially toxic to human tissues, yet it possesses important cell signaling properties. Once in circulation, H2S is excreted primarily by the kidneys as free or conjugated sulfate [5]. H2 and CH4 however, are excreted unchanged.
Methane Gas in Humans
At room temperature CH4 is a colorless, odorless, volatile gas. Its characteristic “natural gas odor” is actually an artificial odorant, which is added for safety purposes. It has typically been thought of as an inert gas, aside from the effects of gaseous distention [17]. In humans, CH4 is produced exclusively through anaerobic fermentation of both endogenous and exogenous carbohydrates by enteric microflora [13, 17]. This was clearly shown by Bond et al. via studies on germ-free rats and human infants. CH4 excretion was not detected in germ-free rats until shortly after they were contaminated with feces from a CH4 producing rat [13].
CH4 is never detected in children until 3 years of age. As age increases, so does CH4 production until age 10 when the adult distribution is reached [17]. In a study of pediatric CH4 production, breath CH4 was analyzed in 393 healthy subjects in the Tel-Aviv area from infancy to 59 years old [19]. Similar to findings of the previous study, CH4 production started at 3 years old and averaged 6.4% of 3 and 4 year olds. However, it increased considerably between ages 4 and 8 and then remained stable around 18% until age 14. From age 14 on, the incidence of CH4 production increased sharply to reach that of the adult population (49.4%). Also notable in this study is that in the adolescent and adult groups, significantly more females than males produced CH4.
Early experiments on breath CH4 revealed a familial clustering of CH4 production. One study demonstrated a high concordance for CH4 production between siblings as well as parents and offspring, but not spouses. However, one pair of twins in the study was discordant for CH4 production. Thus, these researchers favored environmental rather than genetic factors as the determinant for CH4 production [17]. However, data in formal twin studies are conflicting. One study of CH4 production in 228 adult Hungarian twins showed similar concordance rates between monozygous and dizygous twins, whether they lived together or apart [20]. These investigators concluded that genetics did indeed play a role in CH4 production, but in a multi-factorial rather than Mendelian inheritance pattern. Yet another study was performed that involved 548 adolescent twins and their families [21]. In stark contrast to the previous study, these researchers found that genetics did not play a significant role, and that shared and unique environmental factors were the main determinants of methanogenicity.
CH4 is not utilized by humans, so it is excreted either as flatus, or it traverses the intestinal mucosa and is absorbed into the systemic circulation and excreted unchanged through the lungs. Because approximately 20% of CH4 produced by anaerobic fermentation is thought to be excreted by breath, analysis of respiratory CH4 can serve as an indirect measure of CH4 production rate [17]. This principle forms the basis of the lactulose breath test (LBT). Due to its ease of administration and minimally invasive nature, breath testing has become widely used clinically to aid in diagnosis of certain gastrointestinal conditions and disorders of transit.
When Bond et al. initially studied CH4 production, they incidentally found in one of their subjects that only 9% of CH4 was produced proximal to the splenic flexure [17]. This was the first clue that in normal humans methanogenic flora mainly reside in the distal colon. This notion was supported by Flourie et al. who showed that CH4 production occurred almost exclusively in fecal, as opposed to cecal homogenates [22]. Further confirmation was provided in a study of the distribution of methanogens by analyzing fecal contents and comparing them to right colonic samples collected by pyxigraphy [23]. It was found that in CH4 excretors the methanogen content was higher in the feces than the right colon, representing 12% and 0.003%, respectively.
Numerous studies measuring breath CH4 have been conducted, and it is estimated that approximately 30–62% of healthy adults excrete CH4 [17, 20, 24, 25]. Traditionally, adults have been classified as CH4 producers versus non-producers based on breath CH4 status [13, 15, 17]. However, patients who do not excrete CH4 in the breath can in fact have CH4 present in colonic gas [25]. This has been corroborated through fecal incubation studies demonstrating that the percentage of individuals producing CH4 in the colon (72%) was much higher than the reported range of 30–62%. Breath CH4 positive patients had a methanogen concentration of log10 9.45, whereas breath CH4 negative patients had a concentration of log10 4.91. The investigators estimated that approximately 108 methanogenic organisms per gram dry weight of stool are needed to generate enough CH4 to be detected by breath analysis [11]. These studies suggest that a higher percentage of individuals than previously thought may produce CH4, but only when a certain threshold is reached will CH4 be detectable in the breath.
Methanogenic Flora
The methanogens are a primitive, diverse, group of microorganisms that taxonomically belong to the domain Archaea and the kingdom Euryarchaeota [26]. They are obligate anaerobes that can exist in a number of habitats. They are restricted to a unique form of metabolism in which they must reduce simple substrates to CH4 in order to produce cellular energy [26]. These organisms are extremely fastidious and difficult to culture.
Methanogens have long been studied in ruminant species. A ruminant is a hoofed animal (such as cattle, sheep, and goats) that regurgitates its food, then chews the semi-digested food (known as cud), before it makes its way to the rumen. The rumen is one of the four chambers of the ruminant fore-stomach. It is a complex anaerobic ecosystem in which the feed consumed by the animal undergoes fermentation. Methanogens in the rumen produce CH4 from H2 and CO2 [27]. Ruminant livestock can actually produce 250–500 l of CH4 per day [28]. Production of CH4 accounts for a loss of approximately 6% of the total energy intake of cattle [28]. More recently, much attention has been paid to the potential of CH4 to contribute to climatic change and global warming. Atmospheric CH4 concentrations were stable until about 100 years ago when concentrations began to rise. In 1992, it was estimated CH4 would cause 15–17% of global warming over the next 50 years [28]. In addition to increasing the efficiency of livestock feeds, the global warming phenomenon has bolstered scientific interest in manipulating methanogenic flora of ruminants.
In methanogenic individuals, methanogens range from 107 to 1010 per gram dry weight of feces [29]. The predominant CH4 producing organism in humans is Methanobrevibacter smithii [11, 23, 29]. The diversity of the intestinal microflora has been studied by analyzing the 16S rDNA sequences of three healthy adults. Two out of the three adults produced archaeal products. Despite this, it has been discovered that other microorganisms in the human gut are capable of producing CH4, such as certain Clostridium and Bacteroides species [30].
V. Methane Biogenesis and Competition for Hydrogen
Fermentation of polysaccharides by colonic anaerobic bacteria yields short-chain fatty acids (primarily acetate, propionate, and butyrate), CO2, and H2 [3]. H2 can only be eliminated through three methods: (1) via flatus, (2) absorption into the systemic circulation and subsequent respiratory excretion, and (3) metabolism by colonic microflora (Fig. 1) [4]. Although the first two methods are important, it is now thought that consumption by intestinal microorganisms is the major source of H2 disposal [4]. The methanogens have the ability to significantly decrease this volume of gas by converting 4 mol H2 and 1 mol CO2 to just 1 mol CH4. Reduction of CO2 to CH4 via H2 is accomplished via the following chemical equation [31]:
Methanogens rely on this process as their sole source of energy, with a free energy of −130 kJ/mol CH4 [31]. However, the methanogens are not alone in their need for H2. In order to survive, they must compete for H2 in the gut with the sulfate reducing bacteria (SRB), and to a lesser extent in humans the acetogenic bacteria [18, 32].
The SRB use H2 to reduce sulfate to sulfide (which is rapidly hydrolyzed to H2S) according to the following equation [33]:
As in methanogenesis, 4 mol of H2 are consumed to produce 1 mol of gaseous product, with a free energy of −152.2 kJ/mol, making this a thermodynamically more favorable process that methanogenesis [33, 34].
While less active in humans than methanogenesis and sulfate reduction, acetogenesis has been shown to occur in the colon in individuals who are not highly methanogenic [18, 32]. The acetogenic bacteria reduce CO2 to acetate via molecular H2 according to the following equation [33]:
The free energy of acetogenesis is −95 kJ/mol, making it the least thermodynamically favorable of the three H2 consuming processes in the gut.
Methanogenesis and sulfate reduction form the two major pathways of H2 oxidation in the colon. These two processes are thought to be mutually exclusive in humans [18, 32, 34, 35]. This is evidenced in a study of total H2 excretion by whole body calorimetry and measurement of the activity of methanogens, SRB, and acetogenic bacteria in fecal samples. It was found that only individuals with CH4 excretion in vivo displayed methanogenesis in the feces, whereas the non-methanogenic individuals all showed high levels of sulfate reduction in the feces [18]. In another study, methanogens and SRB were enumerated in 10 CH4 excretors and 9 CH4 non-excretors. It was found that the CH4 excretors harbored more methanogens than the non-excretors and that the non-excretors harbored more SRB than excretors [36]. The important point here is that both methanogens and SRB were present in CH4 excretors and non-excretors, suggesting that competition does exist between the two populations, but the presence of one does not completely exclude the other.
Much effort has been spent in elucidating which H2 consumer dominates the other. Studies of freshwater lake sediments have shown that SRB will out-compete the methanogens as long as sufficient sulfate is available as a substrate [35]. Fecal fermentation experiments have demonstrated that a similar process occurs in the human gut [37]. The rationale behind this theory is twofold. First of all, sulfate reduction is a more thermodynamically favorable process with a ΔGo’ of −152.2 kJ/mol vs. −130 kJ/mol for methanogenesis. Secondly, SRB have a greater affinity for H2 compared to methanogens [34]. This argument was further strengthened by a study in which 6 CH4 excreting subjects were supplemented with sodium sulfate in their diet. Interestingly, three of the subjects had decreased breath CH4 excretion and decreased methanogenic counts in the stool, while sulfate reduction rates in the stool actually increased. The methanogenic counts and breath CH4 recovered after sulfate supplementation was discontinued [35].
In addition to sulfate availability, it has been found that pH is also an important determinant of which route of H2 consumption predominates. Sulfate reduction is optimal at an alkaline pH, whereas methanogenesis favors a neutral pH, and acetogenesis an acidic one [32].
Clinical Associations
A multitude of studies have attempted to link CH4 production to various states (Table 2). This type of research originated decades ago in an intriguing study by Haines et al. that connected breath CH4 positivity to colon cancer [12]. In this study, 80% of subjects with large-bowel cancer had detectable breath CH4, whereas only 40% of subjects without large-bowel disease excreted CH4. This data had some support, but subsequently, multiple studies failed to confirm the association [38, 39].
In recent years, extensive research has been performed in the area of irritable bowel syndrome (IBS) and its relationship to gut ecology. Evidence is accumulating to suggest that the enteric flora indeed plays a pathogenic role in IBS, a disorder which has no clear etiology. A recent study analyzed the fecal microbial genomes of 24 IBS patients and showed that the fecal microbiota is significantly altered in IBS patients [40]. It has also known that infectious agents can, in some patients, serve as a trigger to develop IBS after resolution of an acute episode of gastroenteritis [41].
As an extension of this particular theme, there is also evidence that small intestinal bacterial overgrowth (SIBO) may play a role in the pathophysiology of IBS. It has now been shown in a series of studies that IBS patients often have an abnormal LBT suggesting the presence of SIBO, and that systemic treatment with neomycin can often normalize the breath test and result in IBS symptom improvement [42].
Although these studies are a paradigm shift and have endured their fair share of criticism, they have indeed challenged the way we understand IBS and further solidified the evidence for the pathogenic role of an altered gut flora [43]. More recently, CH4 gas and its relationship to intestinal transit have been investigated. Research has shown us that CH4 production is more common in constipating conditions such as encopresis and diverticulosis, and much less frequent in predominantly diarrheal conditions such as inflammatory bowel disease [9, 11, 25, 44, 45]. It has also been shown in children with chronic constipation that colonic transit time is significantly prolonged in CH4 producers compared to non-producers [46]. Two studies have demonstrated slower transit times in CH4-producing adults. The first measured mouth-to-cecum transit times of healthy subjects by lactulose hydrogen breath test. They found that breath CH4 producers had a significantly longer transit time (111 min) compared to their non-producing counterparts (68 min) [24]. In the second study, whole gut transit was evaluated. They found that breath CH4 producers had a mean transit time of 84.6 h, versus 48.6 h in non-producers [47]. However, investigators in both studies only felt this was associated, not cause-and-effect.
Similar results are now seen in IBS. Approximately one-third of IBS patients are of the constipation predominant variety [48]. A series of studies have now confirmed that among IBS patients, CH4 on LBT is almost universally associated with the C-IBS subtype and that treatment and elimination of CH4 on LBT can significantly improve the symptom of constipation [9, 42, 49]. One particular study has shown that the degree of breath CH4 production in IBS patients correlates with the severity of constipation, and inversely correlates with stool frequency and severity of diarrhea [10].
Evidence has accumulated to support an association between CH4 and intestinal transit. However, none of the previously discussed studies provide any strong evidence towards a clear cause-and-effect relationship. Does CH4 play an active role in altering motility, or is it merely a marker of transit? One possibility is that methanogenic organisms may favor proliferation in an environment of slower transit. This notion is supported by studies showing that treatment with laxatives and bowel cleansing can eliminate CH4 production in some patients [38, 44, 45]. Another possibility is that methanogens may alter the quantity of other substances in the gut by competing for a common substrate, such as H2. For example, if methanogenic organisms were to out-compete SRB in the gut, concentrations of H2S (a known bioactive gasotransmitter) would decrease, producing a subsequent effect. Because H2S may be potentially toxic to colonic epithelium, methanogenic organisms could in this manner indirectly influence the colonic environment [50]. No studies have yet examined this possibility.
A third possibility is that CH4 gas itself may directly affect intestinal transit. One study consisting of two experiments supports this concept of CH4 being involved in the regulation of intestinal motor function [51]. In one experiment, CH4 gas was infused into the fistulated small intestine of a canine model and a radiolabel was used to measure transit. CH4 produced a slowing of transit in all dogs by an average of 59% compared to room air. In a second experiment, the contractile activity of guinea pig ileum in response to brush strokes was significantly augmented when exposed to CH4 gas compared to room air. In this paper, it was hypothesized that CH4 slows small intestinal contractile activity by augmenting small-bowel contractile activity, possibly through non-propulsive segmental contractions. This fascinating study lends credibility to the notion that CH4 may be a bioactive molecule, but further research in human subjects is needed to substantiate this hypothesis.
One potential confounding factor is that the previously mentioned study only examined small-bowel activity, whereas methanogenic organisms tend to populate the left colon [17, 22, 23]. One possibility is that if CH4 is indeed a bioactive molecule, then generation in the colon could trigger a reflex pathway causing upstream activity in the small bowel. In the canine study, CH4 infused in the distal small intestine slowed transit in the proximal intestine [51]. Also, a study of lactulose breath testing has shown two patterns of CH4 production. One type is a late rise in CH4 approximately 6 h after lactulose. The other type is an early rise in CH4 levels starting approximately 90 min after lactulose [24]. This early rise suggests that there is some methanogenic activity in the small bowel. One study demonstrated that some clostridial and bacteroides species are capable of producing CH4, which could potentially explain this early activity [30].
Recent data has linked CH4 gas to serotonin (5-hydroxytryptamine). Serotonin is a neurotransmitter which, among many functions, participates in peristaltic control of the gut [52]. In fact, the majority of serotonin in the human body is found in the gastrointestinal tract, produced mainly by the enterochromaffin cells [53]. It is now known that diarrhea-predominant IBS patients have elevated postprandial serum serotonin levels compared to controls [54]. A recent study has addressed the role of serotonin in CH4-producing IBS patients [55]. In four of 18 IBS subjects who produced CH4 on LBT, postprandial serotonin levels were reduced compared to the H2 producing subjects. Although there is no other evidence in the gastrointestinal literature that CH4 influences serotonin levels, there is some pertinent data in the anesthesia literature. A study found that halogenated methane is able to inhibit the pulmonary uptake of serotonin in rat lung [56]. An atmosphere of 100% pure CH4 however, showed no effect at all on serotonin uptake. Although it has been shown that aberrations exist in both CH4 and serotonin in IBS, it is still unclear whether these two molecules work together to influence gut motor function. Further research in this area is necessary.
Manipulations of Methanogenic Flora and Therapeutic Implications
If CH4 gas does indeed directly or indirectly contribute to colonic pathophysiology, then attempting to manipulate the methanogenic flora in the setting of intestinal diseases could be a viable therapeutic option. Significant research in ruminants has been conducted on CH4 manipulation. Reduction of methanogenesis in ruminants is utilized to increase the efficiency of feeds and to minimize the contribution of CH4 to global warming [28]. Strategies, such as feeding of salts, use of ionophore antibiotics, amount and type of feed intake, forage processing, and lipid addition to feeds are often used [28, 57]. It has also been demonstrated that the HMG-CoA reductase inhibitors, mevastatin and lovastatin, specifically inhibit in vitro growth of Methanobrevibacter strains isolated from the rumen without inhibiting other fermentative bacteria [58].
Although the methanogenic flora is not specifically targeted in clinical practice, research has identified certain inhibitors of methanogenesis in humans. Two studies have shown that bowel cleansing prior to colonoscopy or surgery can inhibit CH4 production [38, 45]. This is thought to be due to a colonic purging phenomenon. Also, human bile has been incubated with methanogenic feces and was found to inhibit methanogenesis in a dose-dependent manner [59]. Antibiotics can eliminate CH4 production as well. Peled et al. abolished breath CH4 excretion in three of eight subjects using antibiotics gentamycin and cephazolin [45]. Pimentel et al. were able to eliminate breath CH4 in five of five CH4 producers with oral neomycin therapy [49]. Antibiotic therapy, however, is non-specific and eradicates other gut organisms along with the methanogenic ones.
If methanogenesis were to be intentionally manipulated for clinical use, ideally a therapy specific to CH4-producing microorganisms would be desired in order to spare the remaining gut flora. The ionophore antibiotics used in ruminants are non-specific, although they inhibit Gram positives more than the Gram negatives [57]. The finding that certain HMG-CoA reductase inhibitors can selectively inhibit methanogens in vitro is interesting and deserves further study. Another possible approach to reducing CH4 production would be to somehow shift the dominant H2 consuming organism in the gut away from methanogens and towards another organism, such as SRB. Unfortunately, this could be somewhat detrimental as there is data suggesting the product of sulfate reduction, H2S, may be toxic to colonic epithelium [50]. Another possibility would be to skew the balance towards acetogenic microorganisms. This concept has been discussed somewhat in the veterinary literature but the main obstacle remains overcoming the bioenergetics that make methanogenesis a more favorable process that acetogenesis [28].
Conclusion
It has become apparent that the gut microflora plays an integral role in the balance between intestinal health and disease. Enteric bacteria are responsible for the fermentation of carbohydrates, yielding H2, H2O, and CH4. Although this is an essential step in the digestive process, the emission of large quantities of these gases can potentially augment abdominal symptoms in patients with functional abdominal pain. CH4 gas in the gut is produced strictly by methanogenic archaea, which compete for hydrogen primarily with the SRB. These processes serve to significantly reduce the volume of gas in the colon. It is still unclear, however, which process out-competes the other.
IBS patients have also been found to have an altered gut microflora and a number of studies have now linked IBS to SIBO. Recent data are showing that methanogenicity is more common in constipating conditions and that methane production on LBT is strongly associated with the constipation predominant subtype of IBS. Moreover, treatment with non-absorbable antibiotics has been found to eliminate CH4 on LBT and improve symptoms of constipation.
Evidence is now accumulating to suggest that methanogenic organisms and their gaseous by-products may actively participate in control of intestinal motor function, as opposed to being a surrogate marker for enteric function. Clearly, further investigation in this area is required. If confirmed, however, CH4 gas would be named as another gasotransmitter, and could be potentially targeted for future therapeutics.
References
Savage D. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi:10.1146/annurev.mi.31.100177.000543.
Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984;86:174–193.
Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet. 1983;1:1206–1209. doi:10.1016/S0140-6736(83)92478-9.
Strocchi A, Levitt MD. Maintaining intestinal H2 balance: credit the colonic bacteria. Gastroenterology. 1992;102:1424–1426.
Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous transmitter? FASEB. 2002;16:1792–1798. doi:10.1096/fj.02-0211hyp.
Ignarro LJ, Buga GM, Wood KS, et al. Endothelium-derived relaxing factor and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi:10.1073/pnas.84.24.9265.
Murray JA, Ledlow A, Launspach J, et al. The effects of recombinant human hemoglobin on esophageal motor functions in humans. Gastroenterology. 1995;109:1241–1248. doi:10.1016/0016-5085(95)90584-7.
Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest. 1993;23:724–728. doi:10.1111/j.1365-2362.1993.tb01292.x.
Pimentel M, Mayer AG, Park S, et al. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86–92. doi:10.1023/A:1021738515885.
Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–841. doi:10.1111/j.1572-0241.2007.01072.x.
Weaver GA, Krause JA, Miller TL, et al. Incidence of methanogenic bacteria in a sigmoidoscopy population: an association of methanogenic bacteria and diverticulosis. Gut. 1986;27:698–704. doi:10.1136/gut.27.6.698.
Haines A, Metz G, Dilawari J, et al. Breath-methane in patients with cancer of the large bowel. Lancet. 1977;2:481–483. doi:10.1016/S0140-6736(77)91605-1.
Levitt MD, Bond JH. Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921–929.
Kirk E. The quantity and composition of human colonic flatus. Gastroenterology. 1949;12:782–794.
Levitt MD, Ingelfinger FJ. Hydrogen and methane production in man. Ann N Y Acad Sci. 1968;150:75–81. doi:10.1111/j.1749-6632.1968.tb19033.x.
Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281:122–127.
Bond JH, Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971;133:572–588. doi:10.1084/jem.133.3.572.
Christl SU, Murgatroyd PR, Gibson GR, et al. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269–1277.
Peled Y, Gilat T, Liberman E, et al. The development of methane production in childhood and adolescence. J Pediatr Gastroenterol Nutr. 1985;4:575–579. doi:10.1097/00005176-198508000-00013.
Flatz G, Czeizel A, Metneki J, et al. Pulmonary hydrogen and methane excretion following ingestion of an unabsorbable carbohydrate: a study of twins. J Pediatr Gastroenterol Nutr. 1985;4:936–941. doi:10.1097/00005176-198512000-00014.
Florin TH, Zhu G, Kirk KM, et al. Shared and unique environmental factors determine the ecology of methanogens in humans and rats. Am J Gastroenterol. 2000;95:2872–2879. doi:10.1111/j.1572-0241.2000.02319.x.
Flourie B, Etanchaud F, Florent C, et al. Comparative study of hydrogen and methane production in the human colon using caecal and faecal homogenates. Gut. 1990;31:684–685. doi:10.1136/gut.31.6.684.
Pochart P, Lemann F, Flourie B, et al. Pyxigraphic sampling to enumerate methanogens and anaerobes in the right colon of healthy humans. Gastroenterology. 1993;105:1281–1285.
Cloarec D, Bornet F, Gouilloud S, et al. Breath hydrogen response to lactulose in healthy subjects: relationship to methane producing status. Gut. 1990;31:300–304. doi:10.1136/gut.31.3.300.
McKay LF, Eastwood MA, Brydon WG. Methane excretion in man—a study of breath, flatus, and faeces. Gut. 1985;26:69–74. doi:10.1136/gut.26.1.69.
Jones WJ, Nagle DP, Whitman WB. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987;51:135–177.
Hungate RE. Symposium: selected topics in microbial ecology. I. Microbial ecology of the rumen. Bacteriol Rev. 1960;24:353–356.
Johnson KA, Johnson DE. Methane emissions from cattle. J Anim Sci. 1995;73:2483–2492.
Miller TL, Wolin MJ. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol. 1982;131:14–18. doi:10.1007/BF00451492.
McKay LF, Holbrook WP, Eastwood MA. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Pathol Microbiol Immunol Scand. 1982;90:257–260.
Blaut M. Metabolism of methanogens. Antonie Van Leeuwenhoek. 1994;66:187–208. doi:10.1007/BF00871639.
Gibson GR, Cummings JH, Macfarlane GT, et al. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990;31:679–683. doi:10.1136/gut.31.6.679.
Thauer RK, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180.
Gibson GR, Macfarlane GT, Cummings JH. Sulfate reducing bacteria and hydrogen metabolism in the human large intestine. Gut. 1993;34:437–439. doi:10.1136/gut.34.4.437.
Christl SU, Gibson GR, Cummings JH. Role of dietary sulphate in the regulation of methanogenesis in the human large intestine. Gut. 1992;33:1234–1238. doi:10.1136/gut.33.9.1234.
Pochart P, Dore J, Lemann F, et al. Interrelations between populations of methanogenic archaea and sulfate-reducing bacteria in the human colon. FEMS Microbiol Lett. 1992;98:225–228.
Gibson GR, Cummings JH, Marfarlane GT. Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine. J Appl Bacteriol. 1988;65:241–247.
Karlin DA, Jones RD, Stroehlein JR, et al. Breath methane excretion in patients with unresected colorectal cancer. J Natl Cancer Inst. 1982;69:573–576.
Kashtan H, Rabau M, Peled Y, et al. Methane production in patients with colorectal carcinoma. Isr J Med Sci. 1989;25:614–616.
Kassinen A, Krogius-Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi:10.1053/j.gastro.2007.04.005.
Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome—a meta-analysis. Am J Gastroenterol. 2006;101:1894–1899. doi:10.1111/j.1572-0241.2006.00654.x.
Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419.
William HL. Lactulose breath testing, bacterial overgrowth, and IBS: just a lot of hot air? Gastroenterology. 2003;125:1898–1900.
Fiedorek SC, Pumphrey CL, Casteel HB. Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 1990;10:473–477. doi:10.1097/00005176-199005000-00010.
Peled Y, Weinberg D, Hallak A, et al. Factors affecting methane production in Humans. Dig Dis Sci. 1987;32:267–71. doi:10.1007/BF01297052.
Soares AC, Lederman HM, Fagundes-Neto U, et al. Breath methane associated with slow colonic transit time in children with chronic constipation. J Clin Gastroenterol. 2005;39:512–515. doi:10.1097/01.mcg.0000165665.94777.bd.
Stephen AM, Wiggins HS, Englyst HN, et al. The effect of age, sex and level of dietary fibre from wheat on large-bowel function in thirty healthy subjects. Br J Nutr. 1986;56:349–361. doi:10.1079/BJN19860116.
Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128:580–589. doi:10.1053/j.gastro.2004.12.006.
Pimentel M, Chatterjee S, Chow EJ, et al. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that Is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006;51:1297–1301. doi:10.1007/s10620-006-9104-6.
Pitcher MC, Cummings JH. Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut. 1996;39:1–4. doi:10.1136/gut.39.1.1.
Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089–G1095. doi:10.1152/ajpgi.00574.2004.
Bulbring E, Lin RCY. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis, the local production of 5-hydroxytryptamine and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958;140:381–407.
Bertaccini G. Tissue 5-hydroxytryptamine and urinary 5-hydroxyindoleacetic acid after partial or total removal of the gastrointestinal tract in the rat. J Physiol. 1960;153:239–249.
Bearcroft CP, Perret D, Farthing MJG. Postprandial 5-hydroxytryptaminein diarrhea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42:42–46.
Pimentel M, Kong Y, Park S. IBS subjects with methane on lactulose breath test have lower postprandial serotonin levels than subjects with hydrogen. Dig Dis Sci. 2004;49:84–87. doi:10.1023/B:DDAS.0000011607.24171.c0.
Hede AR, Andersson L, Post C. Effect of a homologous series of halogenated methanes on pulmonary uptake of 5-hydroxytryptamine in isolated perfused rat lung. Acta Pharmacol Toxicol (Copenh). 1985;57:291–296.
Wolin MJ. Fermentation in the rumen and human large intestine. Science. 1981;213:1463–1468. doi:10.1126/science.7280665.
Miller TL, Wolin MJ. Inhibition of growth of methane-producing bacteria of the ruminant forestomach by hydroxymethylglutaryl SCoA reductase inhibitors. J Dairy Sci. 2001;84:1445–1448.
Florin THJ, Woods HJ. Inhibition of methanogenesis by human bile. Gut. 1995;37:418–421. doi:10.1136/gut.37.3.418.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahakian, A.B., Jee, SR. & Pimentel, M. Methane and the Gastrointestinal Tract. Dig Dis Sci 55, 2135–2143 (2010). https://doi.org/10.1007/s10620-009-1012-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-1012-0