Abstract
Background and Aim
Increased oxidative stress is involved in the development of portal hypertension in cirrhosis. Our study aimed to assess the relationship between oxidative stress and hemodynamic parameters in cirrhotic patients.
Methods
Forty-two patients with viral cirrhosis and 24 normal controls were enrolled. Measurements of plasma levels of malondialdehyde (MDA), nitrite/nitrate (NOx), endotoxin, and activities of superoxide dismutase (SOD) were carried out in all subjects. Systemic and splanchnic hemodynamic measurements were carried out in cirrhotic patients.
Results
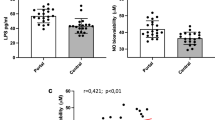
Plasma levels of MDA, endotoxin, and NOx were significantly higher in cirrhotic patients than in normal controls (900 ± 751 versus 226 ± 16 nM, P < 0.01; 62.0 ± 26.0 versus 14.8 ± 4.1 pg/mL, P < 0.01; 50.5 ± 22.6 versus 15.0 ± 9.2 nM, P < 0.01, respectively). Activities of SOD were significantly decreased in cirrhotic patients compared with in normal controls (2.62 ± 0.7 versus 6.8 ± 0.4 U/mL). Further, plasma levels of MDA in cirrhotic patients were significantly positively associated with hepatic venous pressure gradient (HVPG) (r = 0.35; P = 0.025), wedge hepatic venous pressure (WHVP) (r = 0.42; P = 0.007), and hepatic sinusoid resistance (HSR) (r = 0.33; P = 0.033). Plasma MDA levels also correlated positively with plasma endotoxin (r = 0.71, P < 0.001) and NOx (r = 0.55, P < 0.001) levels in the cirrhotic patients. Multiregression analysis showed that the independent and strongest factors to predict HVPG, WHVP, and HSR are plasma levels of NOx, MDA, and endotoxin, respectively.
Conclusion
This study suggests a close interaction among MDA, endotoxin, and NOx and that these substances are also associated with hemodynamic derangement in cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In aerobic life, oxygen is essential in many chemical reactions. During the utilization of the oxygen, oxidants such as aldehydic products of lipid peroxidation are produced [1–3]. At the same time, antioxidants such as superoxide dismutases (SOD) are produced to counteract the generation of oxidants and to protect cells [4]. Increased oxidative stress is defined as an imbalance between the production of oxidants and antioxidants. Increased oxidative stress can stimulate lipid peroxidation reactions and result in DNA damage and posttranslational derangement of proteins [5].
Portal hypertension is a frequent and serious complication of chronic liver diseases. It is initiated by increased intrahepatic resistance and maintained by increased portal inflow [6]. It has been established that increased oxidative stress accelerates the progression of liver fibrosis during chronic liver injury of various etiologies [7]. Oxidative stress also contributes to hepatic endothelial dysfunction by modulating NO bioavailability in the intrahepatic microcirculation [4]. It has also been reported that a reduction in portal pressure and amelioration of hyperdynamic circulation were observed in portal hypertensive and bile-duct-ligated (BDL) rats receiving antioxidants [8–10]. Furthermore, patients receiving acute administration of an antioxidant had attenuation of the postprandial increase in hepatic venous pressure gradient [11]. Theoretically, increased oxidative stress is important for the development of portal hypertension and hyperdynamic circulation in cirrhosis.
Malondialdehyde (MDA), a typical aldehydic product of lipid peroxidation, has been found to be markedly increased in cirrhotic patients [12–15]. Further, chronic endotoxemia is a well-established phenomenon in cirrhotic patients [16]. Increased circulating NO, which positively correlated with endotoxemia, has been observed to be increased in cirrhotic patients [17, 18]. Therefore, the aim of our study was to examine the possible relationships among the increased oxidative stress, increased circulating NO, endotoxemia, and various hemodynamic parameters in cirrhotic patients.
Methods
Study Patients
Between August 1998 and December 2002, 42 viral cirrhotic patients aged 20–80 years who were admitted to the Taipei Veterans General Hospital for diagnosis and evaluation of the severity of their liver disease and portal hypertension were finally enrolled. Diagnosis of cirrhosis was based on the imaging, clinical, and laboratory findings. Diagnosis of hepatitis B virus infection was made in the patients found seropositive for hepatitis B surface antigen (HBsAg; RIA kits, Abbott Laboratories, North Chicago, IL). Diagnosis of hepatitis C virus (HCV) infection was made in patients found to be seropositive for the antibody against HCV by a second-generation enzyme immunoassay (Ortho-Clinical Diagnostics, Johnson-Johnson Company, Bucks, UK). Exclusion criteria included presence of hepatic encephalopathy, presence of hepatocellular carcinoma, history of previous operations for portal hypertension, active infection, history of esophageal variceal bleeding, use of vasoactive drugs within 1 week, use of antioxidants, history of heart diseases or renal disease, history of active alcoholism, and refusal to participate. The etiology of cirrhosis among the 42 enrolled patients was HBV related in 28 patients, HCV related in 8 patients, and HBV and HCV related in 6 patients. The severity of liver cirrhosis was established according to Pugh’s modification of the Child classification and the model for end-stage liver disease (MELD) score [19]. All patients received abdominal ultrasound to assess ascites. The grading of ascites was as suggested by Gines et al. [20]. Briefly, ascites in an amount large enough to result in marked abdominal discomfort was considered as large volume; moderate-volume ascites was considered an amount causing moderate discomfort [20]. Upper gastrointestinal endoscopy was performed in each cirrhotic patient before enrollment. Written informed consent was obtained from each participant.
Hemodynamic Measurements
After overnight fasting, enrolled patients underwent hemodynamic evaluation. Briefly, under local anesthesia, catheterization was performed by 7-F Swan-Ganz thermodilution catheter (Gould, Cupertino, CA) as previously described [21]. By the Seldinger technique, the catheter was inserted percutaneously into the right femoral vein or right internal jugular vein. Then, it was advanced into the right hepatic vein, where the free hepatic venous pressure (FHVP) and wedge hepatic venous pressure (WHVP) were measured by inflation and deflation of the balloon with a multichannel recorder (model 78534C, Hewlett Packard, Palo Alto, CA). The hepatic venous pressure gradient (HVPG) was obtained by subtracting the FHVP from the WHVP. After hepatic vein catheterization, the catheter was turned into the right side of heart and the pulmonary artery for measurements of systemic hemodynamics: right arterial pressure (RAP), mean pulmonary arterial pressure (MPAP), and pulmonary capillary wedge pressure (PCWP). Mean arterial pressure (MAP) and heart rate were recorded with an external vital sign monitor (Dinamap 8100, Critikon, Tampa, FL). Cardiac output (CO) was measured by the thermodilution method. Systemic vascular resistance (SVR; dyne/s/cm5) was calculated according to the following equation: [(MAP - RAP) × 80]/CO. For measurement of hepatic blood flow (HBF), a dosage of 0.5 mg/kg indocyanine green (ICG) was infused via an antecubital vein over a period of 5 min and then a constant rate of 0.48 mg/min was maintained, as previously described [21]. After reaching a steady state of ICG plasma concentration by waiting for 30 min, blood samples were sequentially obtained from the peripheral vein and the hepatic vein at 2 min intervals for 10 min. The hepatic sinusoid resistance (dyne.S.cm−5, HSR) was estimated as HVPG (mmHg) × 80/HBF (L/min) [11]. During the hemodynamic study, peripheral blood was obtained for further laboratory investigations.
Laboratory Investigations
Blood samples were collected to measure plasma endotoxin and NOx (the sum of NO2− and NO3−). A pyrogen-free syringe containing ~75 units of heparin sodium was used. Then, the plasma obtained was stored at −70°C for subsequent analysis within 6 weeks. Enzyme-linked immunosorbent assay (ELISA) kits for measurement of plasma endotoxin and NOx were purchased from Cayman Chemical (Ann Arbor, MI). The serum activity of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALKP), gamma glutamyl transpeptidase (GGT), and total bilirubin (TB) were measured by using a standard autoanalyzer technique (SMAC analyzer, Technicon Instrument Co., Tarrytown, NY).
The plasma activity of superoxide dismutase (SOD) was also measured. Three types of SODs were characterized according to their metal content: copper/zinc (Cu/Zn), manganese (Mn), and iron (Fe). In this study, total plasma SODs (Cu/Zn-, Mn-, and Fe-SOD) activities were measured by using a commercially available ELISA kit (Cayman Chemical, Ann Arbor, MI).
The plasma levels of malondialdehyde (MDA) were measured by a method based on the reaction of thiobarbituric acid with certain products of lipid peroxidation in an acidic environment at increased temperatures, as previous described [22]. The product formed was pink in color, which allowed for spectrophotometric determination. The procedure included addition of 250 μl distilled water, 500 μL 15% trichloroacetic acid (TCA), and 500 μL 0.37% thiobarbituric acid to a 250 μL homogenate. Thiobarbituric acid and TCA solutions were prepared in 0.25 M hydrochloric acid. The samples were heated in a boiling water bath for 10 min. Then, the samples were centrifuged at 4,500 rpm for 10 min after cooling. Absorbance of the sample was measured at 535 nm. MDA concentration was calculated according to the standard curve prepared from 1,1,3,3-tetraethoxypropane.
Healthy Controls
Twenty-four healthy controls who were age- and sex-matched with the cirrhotic patients were enrolled for measurement of general blood tests, plasma levels of MDA, endotoxin, and NOx, and activities of SOD. None of these subjects had a history of major systemic diseases. All subjects agreed to participate in the study and were aware of its content.
Statistical Analysis
Statistical analysis was performed by using the SPSS 15.0 statistical package (SPSS Inc., Chicago, IL). All results are expressed as mean ± standard deviation (SD). Statistical significance of differences between groups was analyzed by using Student’s t-test or the Mann–Whitney U-test. Correlations between continuous parameters were assessed by using Pearson’s correlation analysis. Stepwise multiregression analysis was performed to determine which marker (MDA, endotoxin or NOx) was of greatest and independent significance for prediction of splanchnic hemodynamic parameters. To adjust for the confounding effect of severity of cirrhosis on correlations between plasma markers and splanchnic hemodynamic parameters, partial correlations were run using Child-Pugh and MELD score as the controlling factors. Receiver operating characteristic (ROC) curve analyses were used to determine the diagnostic sensitivity and specificity of MDA, SOD, NO, and endotoxin for distinguishing patients with cirrhosis from healthy subjects. Results were considered statistically significant at a P value of less than 0.05.
Results
Demographic and clinical data are summarized in Table 1. The cirrhotic patients had significant lower levels of serum albumin, hemoglobin, platelet count, and plasma activities of SOD than the healthy controls. Further, significantly higher levels of blood urea nitrogen, total bilirubin, MDA, NOx, and endotoxin were observed in the cirrhotic patients than in the normal controls. The results of hemodynamic parameters for the cirrhotic patients are summarized in Table 2.
In the current study, the plasma levels of MDA correlated positively with HVPG (r = 0.35, P = 0.025) (Fig. 1a), WHVP (r = 0.42, P = 0.007) (Fig. 1b), and HSR (r = 0.33, P = 0.033) (Fig. 1c). Additionally, partial correlations between MDA and hemodynamic parameters were also evaluated after adjusting for the effect of MELD and Child-Pugh scores. Interestingly, partial correlation between MDA and WHVP (r = 0.373, P = 0.018) or between MDA and HVPG (r = 0.34, P = 0.033) was still significant. Meanwhile, a trend of positive correlation was still noted between MDA and HSR (r = 0.31, P = 0.055). Plasma endotoxin level did not correlate with HVPG (r = 0.188, P = 0.227) (Fig. 1d), but had association with WHVP (r = 0.324, P = 0.036) (Fig. 1e) and HSR (r = 0.375, P = 0.015) (Fig. 1f). However, partial correlation of plasma endotoxin with WHVP (r = 0.210, P = 0.193) or HSR (r = 0.30, P = 0.056) was not significant after adjusting for the effect of MELD and Child-Pugh scores. Plasma level of NOx was significantly positively associated with HVPG (r = 0.382, P = 0.013) (Fig. 1g) and WHVP (r = 0.335, P = 0.032) (Fig. 1h) but not HSR (r = 0.220, P = 0.167) (Fig. 1i). The partial correlation of NOx with HVPG was significant (r = 0.34, P = 0.029) but it was insignificant between NOx and WHVP (r = 0.219, P = 0.181) after adjusting for the effect of MELD and Child-Pugh scores.
Correlation of plasma levels of malondialdehyde (MDA) with a hepatic venous pressure gradient (HVPG), b wedge hepatic venous pressure (WHVP), and c hepatic sinusoid resistance (HSR). Correlation of plasma levels of endotoxin with d HVPG, e WHVP, and f HSR. Correlation of plasma levels of nitrite/nitrate (NOx) with g HVPG, h WHVP, and i HSR
Further, stepwise multiregression analysis was performed to identify which factor (from MDA, endotoxin, NO, Child-Pugh score, and MELD score) independently predicted the three hemodynamic parameters in cirrhotic patients. We found that plasma MDA level is the most significantly independent factor to predict the level of WHVP (standardized coefficients β = 0.42, P = 0.007). Furthermore, plasma NO level is the only significantly independent factor to predict the level of HVPG (β = 0.382, P = 0.013). In addition, plasma endotoxin level is the only significantly independent factor to predict the level of HSR (β = 0.375, P = 0.015).
The plasma levels of MDA were also significantly correlated with the plasma levels of endotoxin and NOx (r = 0.71, P < 0.001; r = 0.55, P < 0.001, respectively) (Fig. 2a, b). Additionally, a significant positive correlation was noted between the plasma levels of NOx and endotoxin in the cirrhotic patients (r = 0.59, P < 0.001) (Fig. 2c). However, the plasma levels of MDA did not correlate with other measured hemodynamic parameters, including CO, SVR, and MAP. There was no significant correlation between plasma MDA level and age (r = –0.14, P = 0.38) or body mass index (r = −0.15, P = 0.34) of the cirrhotic patients. In contrast, the activities of SOD had no significant correlation with any of the hemodynamic parameters, clinical data, or plasma levels of endotoxin and NOx.
In addition, plasma MDA, endotoxin, and NOx levels increased with the severity of liver cirrhosis (Table 3). Plasma MDA, endotoxin, and NOx levels were significantly higher in patients with Child B + C cirrhosis than in those with Child A cirrhosis (Table 3). The cirrhotic patients with high (≥14) MELD scores had significantly higher plasma MDA and endotoxin levels than those with low (<14) MELD scores (Table 3). Further, cirrhotic patients with ascites had significantly higher endotoxin and NO levels than those without ascites (Table 3). Plasma MDA in cirrhotic patients with ascites was only slightly elevated compared with those without ascites (P = 0.19).
The distributions of plasma levels of MDA, endotoxin, and NOx in both studied groups are shown as Fig. 3. The ROC curve analyses are shown in Fig. 4 (sensitivity versus 1-specificity). The cutoff values of plasma MDA, endotoxin, and NOx to separate cirrhotic patients from healthy subjects were 426 nM, 21.5 pg/mL, and 27.5 nM, respectively.
Discussion
It has been shown that systemic overproduction of NO contributes to peripheral vasodilatation and hyperdynamic circulation in cirrhosis [18]. Endotoxin, the lipopolysaccharide component of the cell wall of Gram-negative bacteria, stimulates NO synthesis [23]. In cirrhotic patients, elevated plasma levels of endotoxin have been found to be associated with increased circulating NO [17]. Similarly, a significant positive correlation was noted between the increased plasma level of endotoxin and NO in the cirrhotic patients of this study.
In our study, plasma levels of MDA were positively correlated with plasma levels of endotoxin in the patients with cirrhosis. It has been shown that cirrhotic patients exhibit increased translocation of gut-derived endotoxin, which can reach portal and systemic circulation due to increased intestinal permeability [17, 24]. In carbon tetrachloride-induced cirrhotic rats, an increase in the generation of oxygen free radicals may damage intestinal mucosa and increase production of intestinal MDA [25]. The increased oxidative-stress-related damage of enterocytes in cirrhotic rats leads to increased intestinal permeability [25]. Accordingly, the increase in intestinal oxidative stress and MDA may aggravate the preexistent endotoxemia in cirrhosis. Further, in bile-duct-ligated (BDL) rats, increased bacterial translocation was also associated with increased intestinal mucosal concentrations of MDA, and both intestinal mucosal MDA and bacterial translocation were reduced by chronic administration of antioxidants [26]. On the other hand, rats receiving injection of endotoxin exhibited increased production of MDA in various tissues, including liver [27–29]. Taken together, MDA and endotoxin were caught in a vicious circle.
Meanwhile, a significant correlation between plasma MDA and NO levels in the cirrhotic patients was observed in this study. It has been demonstrated that overproduction of NO increased lipid peroxidation and oxidative stress in cells [30, 31]. Theoretically, increased circulating NO may also lead to the increased plasma levels of MDA in our cirrhotic patients. In brief, the current study suggests interactions among endotoxin, NO, and MDA in cirrhosis.
Interestingly, plasma MDA levels were noted to have positive associations with HVPG and WHVP in the cirrhotic patients in this study. It has been demonstrated that MDA inhibits the endothelium-dependent vasorelaxation to acetylcholine in the tail artery of normal rats [32]. Additionally, NO also plays a major key role in the development of hyperdynamic circulation and portal hypertension in cirrhosis [18]. Multiregression analysis showed that the level of plasma MDA was an independent factor to predict the value of WHVP, whereas the level of plasma NOx was the strongest variable to predict HVPG. Therefore, the significant association of plasma MDA levels and HVPG may be caused by the interaction between NO and MDA in cirrhotic patients in this study.
On the other hand, plasma MDA levels further correlated with HSR in cirrhotic patients in our study. It is conceivable that oxidative stress may play a contributory role in enhanced intrahepatic resistance in cirrhosis [4]. A previous study demonstrated that the cirrhotic patients with high plasma MDA levels had marked postprandial elevation of portal pressure, which represented increased intrahepatic resistance [11]. That study also showed that acute administration of high doses of ascorbic acid reduced the plasma MDA levels and blunted the postprandial increase in portal pressure of cirrhotic patients [11]. Moreover, a concordant increase of hepatic and plasma MDA was demonstrated in cirrhotic rats [33]. Accordingly, the elevation of calculated hepatic sinusoid resistance (HSR), which can estimate the increased intrahepatic resistance indirectly, may be explained by the increased plasma level of MDA in cirrhotic patients of our study. Nevertheless, endotoxemia, which can enhance release of MDA in cirrhosis, has been also found to increase intrahepatic resistance and subsequently elevate portal pressure [16, 25, 34, 35]. In this study, multiregression analysis demonstrated that the plasma level of endotoxin was the strongest factor to predict HSR. Therefore, the significant association between plasma MDA and HSR may be the result of close interaction between endotoxin and MDA in these cirrhotic patients.
Increased oxidative stress, which is indicated by increased oxidants and decreased antioxidants, has been observed in cirrhotic patients with various etiologies [12–15, 36–38]. Our study found significantly higher plasma levels of oxidant MDA and significantly lower plasma activities of antioxidant SOD in the patients with viral cirrhosis than in the healthy controls. This result further supports the existence of increased oxidative stress in cirrhotic patients [37, 38]. Oxidative stress not only contributes to the derangement of hemodynamics mentioned above in cirrhosis, but also promotes liver fibrosis [7]. In BDL rats, the increased plasma MDA level was found to correlate positively to collagen deposition in liver [33]. Further, it was also reported that higher MDA level was associated with more advanced stage of liver fibrosis in patients with chronic liver disease [39]. Moreover, our study found that the cirrhotic patients with Child B + C or high (≥14) MELD score had significantly higher plasma MDA levels. Similarly, a previous study showed that plasma MDA levels were positively correlated with Child-Pugh scores of cirrhotic patients [36]. These results indicate that plasma MDA levels in cirrhotic patients might increase in concordance with the progression of the severity of cirrhosis and fibrosis.
In this study, plasma MDA was associated with the hemodynamic parameters and also the severity of cirrhosis. The previous study had demonstrated that the severity of cirrhosis correlated with HVPG [19]. To avoid the confounding effect of the severity of cirrhosis on the association between plasma MDA and hemodynamic parameters, we performed partial correlation analysis using Child-Pugh score and MELD score as controlling factors. Interestingly, significant associations were still noted between plasma MDA and hemodynamic changes (HVPG and WHVP). These results suggested that these associations of MDA with hemodynamic derangement may be independent of the severity of cirrhosis. However, the sample size of this study was relatively small, so a contribution of the severity of cirrhosis to the association between MDA and hemodynamic changes could not be totally excluded.
Further, investigators have tried to find more noninvasive biomarkers for cirrhotic patients for years [40]. We observed good abilities of plasma MDA, endotoxin, and NOx levels to distinguish cirrhotic patients from healthy subjects, with good sensitivities and specificities by ROC analysis. Additionally, these parameters also were found to be elevated in concordance with the severity of cirrhosis. Thereby, it is possible that plasma levels of MDA, endotoxin, and NOx could be evaluated as candidate biomarkers for initial and long-term assessment of liver cirrhosis in our clinical practice.
In the present study, we found significantly decreased plasma SOD activity in cirrhotic patients as compared with normal volunteers. This result is similar to the finding of a previous study [13]. Further, Nalini et al. [13] found that alcoholic cirrhotic patients had significantly lower SOD activities in the hemolysate than those in nonalcoholic cirrhotic ones. In contrast, another previous study demonstrated higher serum SOD activity in alcoholic cirrhotic patients than in normal subjects [41]. Moreover, SOD is the enzyme to catalyze O ·−2 to hydrogen peroxide (H2O2) [4]. Hydrogen peroxide has been demonstrated to be an endothelium-derived hyperpolarizing factor (EDHF) in human mesenteric arteries [42] and therefore may induce splanchnic vasodilatation which would contribute to cirrhotic portal hypertension [18]. However, the release of EDHF was inhibited by NO [43], which is significantly elevated in cirrhotic systemic circulation [17]. This may explain why no significant correlation was observed between SOD and hemodynamic parameters in cirrhotic patients in this study.
In conclusion, systemic oxidative stress was significantly increased in patients with viral cirrhosis. This study suggests close interaction among increased endogenous oxidant MDA, endotoxin, and NOx and that these substances are associated with hemodynamic derangement in cirrhotic patients.
References
Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645.
Cave AC, Brewer AC, Narayanapanicker A, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728.
Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–284.
Rodriguez-Vilarrupla A, Bosch J, Garcia-Pagan JC. Potential role of antioxidants in the treatment of portal hypertension. J Hepatol. 2007;46:193–197.
Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110.
Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32:141–156.
Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306.
Marley R, Holt S, Fernando B, et al. Lipoic acid prevents development of the hyperdynamic circulation in anesthetized rats with biliary cirrhosis. Hepatology. 1999;29:1358–1363.
Fernando B, Marley R, Holt S, et al. N-acetylcysteine prevents development of the hyperdynamic circulation in the portal hypertensive rat. Hepatology. 1998;28:689–694.
Yang YY, Lee KC, Huang YT, et al. Effects of N-acetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J Hepatol. 2008;49:25–33.
Hernandez-Guerra M, Garcia-Pagan JC, Turnes J, et al. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43:485–491.
Aboutwerat A, Pemberton PW, Smith A, et al. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta. 2003;1637:142–150.
Nalini G, Hariprasad C, Narayanan VA. Oxidative stress in alcoholic liver disease. Indian J Med Res. 1999;110:200–203.
Jain SK, Pemberton PW, Smith A, et al. Oxidative stress in chronic hepatitis C: Not just a feature of late stage disease. J Hepatol. 2002;36:805–811.
Clot P, Tabone M, Arico S, Albano E. Monitoring oxidative damage in patients with liver cirrhosis and different daily alcohol intake. Gut. 1994;35:1637–1643.
Goulis J, Patch D, Burroughs AK. Bacterial infection in the pathogenesis of variceal bleeding. Lancet. 1999;353:139–142.
Guarner C, Soriano G, Tomas A, et al. Increased serum nitrite and nitrate levels in patients with cirrhosis: Relationship to endotoxemia. Hepatology. 1993;18:1139–1143.
Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: From the patient to the molecule. Hepatology. 2006;43:S121–S131.
Wang YW, Hou TI, Yang YY, et al. Correlation and comparison of the model for end-stage liver disease, portal pressure, and serum sodium or outcome prediction in patients with liver cirrhosis. J Clin Gastroenterol. 2007;41:706–712.
Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654.
Lin HC, Tsai YT, Lee FY, et al. Hemodynamic evaluation of octreotide in patients with hepatitis B-related cirrhosis. Gastroenterology. 1992;103:229–234.
Yesilova Z, Yaman H, Oktenli C, et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:850–855.
Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012.
Toh Y, Korenaga D, Maekawa S, et al. Assessing the permeability of the gastrointestinal mucosa after oral administration of phenolsulfonphthalein. Hepatogastroenterology. 1997;44:1147–1151.
Ramachandran A, Prabhu R, Thomas S, Reddy JB, Pulimood A, Balasubramanian KA. Intestinal mucosal alterations in experimental cirrhosis in the rat: Role of oxygen free radicals. Hepatology. 2002;35:622–629.
Schimpl G, Pesendorfer P, Steinwender G, Feierl G, Ratschek M, Hollwarth ME. Allopurinol and glutamine attenuate bacterial translocation in chronic portal hypertensive and common bile duct ligated growing rats. Gut. 1996;39:48–53.
Yoshikawa T, Takano H, Takahashi S, Ichikawa H, Kondo M. Changes in tissue antioxidant enzyme activities and lipid peroxides in endotoxin-induced multiple organ failure. Circ Shock. 1994;42:53–58.
Portoles MT, Ainaga MJ, Pagani R. The induction of lipid peroxidation by E. coli lipopolysaccharide on rat hepatocytes as an important factor in the etiology of endotoxic liver damage. Biochim Biophys Acta. 1993;1158:287–292.
Dogru-Abbasoglu S, Parildar-Karpuzoglu H, Balkan J, Aykac-Toker G, Uysal M. Nitrotyrosine formation and heme oxygenase-1 expression in endotoxemic cirrhotic rats. Arch Med Res. 2007;38:28–33.
Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18:195–199.
Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487.
Rodriguez-Martinez MA, Martinez-Orgado J, Salaices M, Marin J. Impairment of acetylcholine relaxations by malondialdehyde, a marker of lipid peroxidation. J Vasc Res. 1996;33:463–470.
Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med. 1996;20:351–359.
Miller AM, Masrorpour M, Klaus C, Zhang JX. LPS exacerbates endothelin-1 induced activation of cytosolic phospholipase A2 and thromboxane A2 production from Kupffer cells of the prefibrotic rat liver. J Hepatol. 2007;46:276–285.
Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: Implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233–240.
Bianchi G, Marchesini G, Fabbri A, Ronchi M, Chianese R, Grossi G. Lipoperoxide plasma levels in patients with liver cirrhosis. Hepatogastroenterology. 1997;44:784–788.
Trevisani F, Caraceni P, Simoncini M, et al. Evidence of oxidative imbalance in long-term liver transplant patients. Dig Liver Dis. 2002;34:279–284.
Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun. 1998;247:166–170.
Paradis V, Mathurin P, Kollinger M, et al. In situ detection of lipid peroxidation in chronic hepatitis C: Correlation with pathological features. J Clin Pathol. 1997;50:401–406.
Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851.
Szuster-Ciesielska A, Daniluk J, Kandefer-Szerszen M. Oxidative stress in the blood of patients with alcohol-related liver cirrhosis. Med Sci Monit. 2002;8:CR419–CR424.
Matoba T, Shimokawa H, Kubota H, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290:909–913.
Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347.
Acknowledgments
The authors gratefully acknowledge Peng Chi-Yi and Judy Huang for their excellent technical assistance. This work was supported by grants NSC96-2314-B-075-018-MY3 and NSC96-2314-B-075-035 from the National Science Council of the Republic of China, Taiwan and grants V96C1-005 and VGH96B1-001 from the Taipei Veterans General Hospital, Taipei, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kuei-Chuan Lee and Ying-Ying Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, KC., Yang, YY., Wang, YW. et al. Increased Plasma Malondialdehyde in Patients with Viral Cirrhosis and Its Relationships to Plasma Nitric Oxide, Endotoxin, and Portal Pressure. Dig Dis Sci 55, 2077–2085 (2010). https://doi.org/10.1007/s10620-009-0990-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-0990-2