Abstract
Management of severe refractory functional gastrointestinal disorders (FGIDs) is difficult. Quetiapine, an atypical antipsychotic, may benefit patients by mitigating associated anxiety and sleep disturbances, augmenting the effect of antidepressants, and providing an independent analgesic effect. Outpatient records from a university-based FGID clinic were reviewed, and 21 patients with refractory symptoms who received quetiapine were identified and interviewed. Outcomes included global relief of symptoms, treatment efficacy questionnaire, and change in gastrointestinal (GI) and psychological symptoms. Eleven of 21 patients continued therapy at the time of interview. Six of 11 demonstrated global relief of symptoms, and 9 were satisfied with treatment. The remaining 10 of 21 discontinued therapy because of somnolence and lack of GI benefits. Quetiapine in low doses appeared beneficial in more than half of the adults with severe FGIDs who stayed on treatment. This response in otherwise refractory patients suggests quetiapine might augment the effectiveness of antidepressants in severe FGIDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of irritable bowel syndrome (IBS) and other usually painful functional gastrointestinal disorders (FGIDs) hinges on a biopsychosocial approach. This involves a close practitioner-patient therapeutic alliance, attention to contributing psychosocial factors, and use of peripherally acting agents to target bowel symptoms and centrally acting agents to modulate pain processing [1]. These centrally acting agents have traditionally been antidepressants, particularly tricyclic antidepressants (TCAs), possibly selective serotonin reuptake inhibitors (SSRIs), and more recently selective serotonin and norepinephrine reuptake inhibitors (SNRIs). While their role in the management of FGIDs is fairly well established [2], variable efficacy is achieved often due to non-adherence due to anxiety about taking the medication or associated side effects that may lead to their discontinuation or suboptimal dosage levels.
In recent years, efforts within psychiatry and now in medicine have focused on ‘augmentation therapy.’ This involves potentiating the effect of one centrally acting agent by adding another agent with a different mechanism of action in order to maximize efficacy and minimize side effects. Augmentation therapy may involve combinations of multiple antidepressants or antidepressants with anxiolytics (e.g., buspirone) or atypical anti-psychotics. This strategy, while being successful for the treatment of depression [3], has not yet been formally evaluated for the treatment of FGIDs. However, it is theoretically appealing, especially for patients with severe FGIDs, who account for up to 20% with FGIDs and comprise the majority of patients with FGIDs seen at referral centers, including ours [4]. These patients are often refractory to antidepressant therapy alone, and they often suffer from high levels of associated anxiety and profound sleep disturbances [5, 6] for which antidepressants alone may be inadequate. Since atypical antipsychotics are used within psychiatry for treatment augmentation of depression and also have an anxiolytic and sleep induction benefit in addition to an analgesic benefit [7], the addition of these agents to antidepressants may be a particularly attractive management strategy for patients with refractory FGIDs.
Early in 2006 we began using relatively low doses of 25–100 mg at bedtime of the atypical antipsychotic quetiapine to treat patients with severe FGIDs who: (1) had failed to benefit from antidepressants (including a combination of two antidepressants), (2) reported intolerable side effects to them, (3) exhibited marked anxiety (e.g., post traumatic stress disorder, anxiety disorders, and anxiety related to taking antidepressants), (4) experienced profound sleep disturbances, and/or (5) continued to experience pain despite maximal antidepressant dose. Our clinical observations suggested that this treatment was beneficial. Accordingly, we describe herein the outcome of our series of 21 consecutive adult patients with severe FGIDs who were started on quetiapine therapy after failing to respond to an antidepressant alone.
Methods
This case series included adult patients treated for FGIDs with quetiapine from January 2006 through June 2007 by any of three investigators (D.D., S.D., and C.D.) at the University of North Carolina (UNC) Center for Functional GI and Motility Disorders. The patients studied in this case series are classified as severe based on pain intensity, physician visits, and functional disability. Severity in IBS is analyzed from a broader multidimensional construct that also includes health-related quality of life (QOL), psychosocial impairments, health-care utilization behaviors, and burden of illness [4]. These patients were classified as refractory based on having an adequate trial of centrally acting agents (TCAs, SSRIs, and/or SNRIs) in addition to gut acting agents that failed to control the symptoms. The chart review and telephonic interview were approved by the UNC Institutional Review Board, and informed consent was obtained from all study participants.
The study was designed with a mixed-methods approach that involved combining questionnaires and structured interviews (qualitative method) with cross-sectional data collection of participants using structured data abstraction forms (quantitative method) [8]. For each enrolled patient, an investigator not involved in the clinical care (M.G.) of these participants performed a structured chart review of the institution’s electronic medical record. Information on FGID diagnosis, duration of gastrointestinal (GI) symptoms, presence of non-GI symptoms (sleep disturbance, mood disturbance, agitation, delusions, and somatic pain), rationale for starting quetiapine, concurrent treatment with other medications for FGIDs, and information pertaining to concurrent treatment by a psychiatrist or a psychologist was obtained from the medical records.
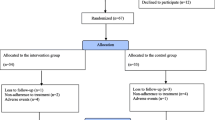
Next, a brief telephone survey of all participants was performed for follow-up assessment. The purpose of the research was introduced to the patients as “We are looking at the effects of quetiapine on bowel symptoms, and I will be asking you a few questions about your GI health and how you are doing in general after being on this medication.” After initially determining whether patients were continuing on quetiapine (group 1) or had discontinued this medication (group 2), groups were asked a series of treatment-specific questions (Fig. 1).
For those patients in group 1 (i.e., those who were still taking quetiapine), a structured interview was conducted to assess: (1) global response to treatment, (2) satisfaction with treatment, and (3) change in gastrointestinal and psychological symptoms. The primary outcome measure was adequate relief, a global response to treatment, which was assessed by asking participants, “In the past 7 days, have you had adequate relief from your symptoms?” As this was a one-time phone interview, the repeated assessment of this variable could not be computed for a complete follow-up assessment of relief. However, this question can operate as a suitable surrogate when viewed in the context of other questions asked during the interview.
Satisfaction with treatment, another global response measure, was assessed using an interview version of the Treatment Efficacy Questionnaire (TEQ), which was adapted from our prior multicenter treatment trial [9]. The TEQ consists of eight items that assess the patient’s experience with the medication on a five-point level of agreement scale that ranges from strongly disagree (1) to strongly agree (5) on statements such as “I am satisfied with the results of my treatment;” “My bowel symptoms have improved as a result of treatment;” “I am engaging in activities that I would not have prior to treatment.” A responder was defined as a subject who scored ≥28 using eight questions on the TEQ (each question was scored as follows: 1, strongly disagree; 2, disagree; 3, neither agree nor disagree; 4, agree; 5, strongly agree) for the eight items on the satisfaction-with-treatment questionnaire (i.e., a score of 28 is a mean/question score of 3.5) [9].
We also assessed several symptomatic secondary endpoints. Gastrointestinal, sleep, and psychological symptoms before and after the use of quetiapine were assessed using symptom-specific questions that compared the present level of functioning to prior functioning using a seven-point Likert-type scale where 1 = “completely better” and 7 = “significantly worse.” All five questions had the same root phrase, “Compared to before you started taking seroquel, how would you rate your symptom,” where symptoms listed were (1) health related to your gastrointestinal condition, (2) abdominal pain, (3) sleep, (4) bowel habits, and (5) mood.
For patients in group 2 (i.e., those who were not on treatment at the time of the assessment), information was obtained via structured interview on the total duration of treatment, the reason for treatment discontinuation, and any changes in health related to their GI condition that patients may have experienced when taking quetiapine. Participants were evaluated using the same seven-point Likert-type scale used in group 1. All participants were asked qualitative questions about side effects and the impact of these side effects on their course of treatment.
As a part of the qualitative research strategy, a case study approach was used that involved a detailed description of the participants, followed by analysis of the data for themes or issues. Data to open-ended questions for patients in group 2, i.e., “reason for stopping the medication,” were not categorized using preset or emergent categories; however, a complete description of the one or more reasons given by the participant was noted.
Results
Patient Sample
Quetiapine was started in 23 patients. Of these, one patient declined to sign the consent, one was not available for telephone interview, and 21 (91%) agreed to fully participate. The demographic and treatment profile of the participants is listed in Table 1. Ninety-one percent were female, 86% were Caucasian, and the mean duration of symptoms was over 7 years. The patients had a primary Rome III diagnosis [10] of functional abdominal pain syndrome (52%), IBS (29%), and other painful functional bowel disorders (19%), including functional chest pain, functional nausea and vomiting, and functional constipation.
Dosing
The initial seroquel doses ranged from 25 to 100 mg: eight took 25 mg, seven 50 mg, and six 100 mg. The dosage varied in 25-mg increments based on clinical need or side effects, and the mean dose was 50 mg (range, 25–100 mg).
Clinical Response for Patients Who Remained on Treatment (Group 1)
Eleven of the 21 patients (52.3%) were continuing quetiapine treatment at the time of the interview. The mean dose was 50 mg (range, 25–100 mg), and mean duration on treatment at the time of interview was 145 days (range, 28–450 days). All 11 patients were also receiving antidepressants: 7 with duloxetine, 2 with desipramine, and 2 with amitriptyline.
Of these 11 patients, 6 (54%) reported adequate relief of symptoms, our primary outcome measure for clinical response. Furthermore, with regard to the TEQ, another global endpoint measure, 9/11 (82%) were responders with a combined score of ≥28. In terms of the specific items, nine were satisfied with the results of their treatment, nine were engaging in more activities, nine were coping better and reported improved symptoms, and four found the treatment to be more helpful than expected (Table 2). Finally, in terms of secondary endpoints, compared to before initiating treatment, 4 of the 11 patients (36.4%) reported overall improvement (“significantly” or “completely” better), and no patient reported worsening of symptoms. Three of 11 reported improvement in abdominal pain, 7 of 11 reported improved sleep and mood, and 2 of 11 reported improvement in bowel habits. However, considering the refractory nature of symptoms in this population, symptoms getting “sometimes better” can also be clinically meaningful (Table 3).
Clinical Response for Patients Who Discontinued Treatment (Group 2)
Ten of the 21 patients (47.6%) stopped the treatment after being on it for different lengths of time (Table 4). Although the mean duration on quetiapine was 90 days (range, 1–330 days), three stopped within 3 days of starting because of somnolence, lack of perceived GI benefit, and dizziness. Table 4 also lists the reasons for discontinuing medication, the most common being somnolence or lack of perceived benefit. With regard to the clinical response, three reported feeling somewhat or significantly better, five reported no change, and two reported feeling somewhat worse when on treatment. Notably six patients (60%) had weight gain, but only one patient discontinued for that reason.
Overall Response
The design of this study did not permit us to calculate intention to treat numbers; however, overall 6 of 21 (28.4%) started on quetiapine showed adequate response, which was our primary endpoint, and 9 of 21 (42.8%) were defined as responders based on overall score on TEQ. However, a compliers-only analysis yielded an adequate response rate of 6 of 11 (54.5%) and responder rate of 9 of 11 (81.8%). These results need to be considered not in the context of a clinical trial, but as an observational study of a cohort of severe refractory FGID patients who were previously unresponsive to all treatments.
Discussion
The IBS and other FGIDs exist across a wide spectrum of severity [11]. While the majority of patients with FGID have mild to moderate symptoms, on the opposite end are the 20% of patients who suffer from severe FGIDs characterized by higher levels of pain, poorer health-related QOL, more pronounced illness behavior, higher health-care utilization, and more psychosocial (e.g., maladaptive thought processes and history of abuse [12, 13]) and psychiatric (e.g., anxiety and depression [14]) comorbidities. These patients usually require a multimodal approach in which behavioral and psychotropic agents are added [10, 15]. Our rationale for using antidepressants in patients with severe FGIDs includes treatment of comorbid psychiatric distress, central antinociception, peripheral motility, and analgesic effects [2]. Still, many of these patients, especially the ones seen at our referral center, fail to respond to antidepressants alone because of lack of efficacy and/or lack of compliance that stems from anxiety about taking these drugs or side effects associated with them. Accordingly, we have had interest in exploring newer treatment options.
Atypical antipsychotics have gained wide acceptance for treatment of bipolar disorder and schizophrenia because of their efficacy and low toxicity. They can also be beneficial in lower dosages in FGIDs because of their analgesic properties (alone or in synergism with antidepressants [7]) and their sedative and anxiolytic effects. To our knowledge, this is the first study to assess the use of quetiapine for severe FGIDs in a clinical setting.
This retrospective review of our experience suggests that quetiapine may be helpful in over 50% of the patients who stayed on medication: 6 of 11 patients had adequate relief of symptoms, the primary measure of response used in most treatment trials of patients with FGIDs. Also, 9 of 11 (81.8%) were defined as responders based on response to the TEQ, another global measure of response used in our previous research. Notably, most of these patients had experienced chronic symptoms unresponsive to all medications for years. Thus, that over 50% of those who remained on the medication responded positively is quite notable. Additionally, these patients experienced marked improvement in their overall functioning, coping skills, sleep, and mood.
The remaining 49% (10 of 21) stopped taking the medication, approximately half of them in less than 6–8 weeks, thus precluding our ability to determine a possible treatment response. The main reason for discontinuation, including for two patients who stopped almost immediately, was somnolence. Because mild to moderate somnolence associated with quetiapine usually resolves in 1–2 weeks [16], a future goal would be to further counsel patients that this should resolve with time.
Importantly, since the intent was to obtain synergism using these two different classes of drugs, all patients on quetiapine were also treated with an antidepressant. The most common antidepressant used was duloxetine, an SNRI with analgesic properties that has been shown to reduce somatic pain severity [17]. Quetiapine may act synergistically with these antidepressant agents through a different mechanism of action that includes strong antagonistic binding to histamine H1, alpha 1 adrenergic, and serotonergic 5 HT 2A receptors, and low antagonistic affinity for the dopamine D2 and D1 receptors [18].
There are several putative reasons for achieving benefit with quetiapine when added to an antidepressant among patients with refractory symptoms. First, in IBS the prevalence of anxiety disorders approaches 40% [19, 20]; sexual and physical abuse as well as PTSD are also quite common [12, 21]. Quetiapine at low doses has been shown to reduce social anxiety symptoms [22, 23] via its 5HT antagonism [24], independently from its sedative effects [16]. Additionally, PTSD-type symptoms, such as re-experiencing phenomena, agitation, nightmares, and hyper-arousal, are reported to be particularly responsive to quetiapine [25].
Second, over 50% of patients with severe FGIDs have impaired sleep quality characterized by reduced slow-wave sleep activity and sleep fragmentation [6]. Nocturnal awakening and unrefreshing sleep are the most commonly reported sleep problems and are related to the intensity of GI symptoms [26]. Through its antihistaminergic, antiadrenergic, and possibly antidopaminergic properties, quetiapine induces sleep without adversely affecting sleep architecture [27]. As a result, even at doses as low as 25 mg, patients experience increases in the total sleep time, sleep efficiency, and subjective sleep quality [28].
Third, quetiapine may have independent analgesic effects on the central modulation of chronic visceral pain. In animal studies, clozapine and olanzapine, which are related atypical antipsychotics, have shown analgesic effects [29]. In 90% of the clinical studies reviewed by Fishbain et al., atypical antipsychotics, particularly tiapride, olanzapine, and quetiapine, were effective analgesic agents for treating lower back pain, headaches, fibromyalgia, and cancer-related pain [7]. An improvement in pain symptoms has also been reported in a case series with doses up to 200 mg/day [30], and also successful treatment of underlying psychiatric disorders may raise the pain threshold.
Finally, there is emerging data from other medical diseases to show the benefit of this class of drugs for functional visceral and somatic symptoms. Olanzapine has shown benefit for treating nausea and vomiting in cancer patients [31], and quetiapine for fibromyalgia [30, 32], which is commonly associated with IBS [33].
We chose quetiapine over other atypical antipsychotics because its side-effect profile appears to be relatively safe, particularly in lower dosages. The incidence of extrapyramidal side effects and effects on QT interval with quetiapine across the entire dose range (50–750 mg/day) are no different from placebo [34]. The most commonly reported side effects in placebo-controlled clinical trials have been dry mouth, sedation, somnolence, dizziness, abnormal liver function tests, dyspepsia, lethargy, and metabolic syndromes (weight gain, diabetes, or hypercholesterolemia) [35].
From our experience, a major component of FGID treatment is the establishment of an effective physician-patient relationship. This is especially important when using atypical antipsychotics to treat patients who have often been told that their problems are “all in their head.” Thus, the rationale of using these agents must be adequately explained [36]. For example, we found that if patients are told that their treatment would involve dosages at one-tenth of that used for schizophrenia or bipolar disorder and that it was helpful for sleep, they were more willing to accept a trial of the medication. Often the sleep benefit and anxiety reduction experienced within the first few days helped patients to stay on the medication long enough to achieve improvement in bowel symptoms after several weeks.
The major limitation of this study is its retrospective case series design, and therefore it should be considered as a pilot study. The results cannot provide sufficient evidence to recommend this treatment routinely for patients with severe refractory FGIDs. Nevertheless, the results form a basis for conducting a well-designed prospective study. While there is no comparison group to control for any placebo effects or therapeutic benefit derived from patient-centered care, most in this group (18 of 21) were already under our care and had proven to be refractory to treatment. These are patients who were dissatisfied with all previous treatments and had low expectation for a clinical response; thus, we believe that a placebo response would have been lower than the standard placebo rate in most studies. The observational design of this study did not allow us to calculate the number who declined treatment with quetiapine. Another limitation is that retrospective recall of symptoms over long periods of time is inherently subject to bias that may be influenced by coming to a major referral center for treatment of refractory FGIDs.
In conclusion, in this experience over one-half of patients with severe refractory FGIDs who stayed on quetiapine to augment the effects of an antidepressant were benefited. A more effective patient-physician relationship, closer follow-up, and careful dose readjustments might help reduce non-adherence. Considering that these patients had severe refractory symptoms, this response rate is notable and warrants additional studies, preferably a controlled clinical trial.
References
Creed F, Levy R, Bradley L, et al. Psychosocial aspects of functional gastrointestinal disorders. In: Drossman DA, Corazziari E, Delvaux M, et al., eds. Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates Inc.; 2006:295–368.
Thiwan SM, Drossman DA. Treatment of functional GI disorders with psychotropic medicines: a review of evidence with a practical approach. Gastroenterol Hepatol. 2006;2:678–688.
Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–1252. doi:10.1056/NEJMoa052964.
Lembo A, Ameen V, Drossman DA. Irritable bowel syndrome: toward an understanding of severity. Clin Gastroenterol Hepatol. 2005;3:717–725. doi:10.1016/S1542-3565(05)00157-6.
Lydiard RB. Irritable bowel syndrome, anxiety, and depression: what are the links? J Clin Psychiatry. 2001;62(Supp 8):38–45. discussion 46–47.
Rotem AY, Sperber AD, Krugliak P, Freidman B, Tal A, Tarasiuk A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003;26:747–752.
Fishbain DA, Cutler RB, Lewis J, Cole B, Rosomoff RS, Rosomoff HL. Do the second-generation “atypical neuroleptics” have analgesic properties? A structured evidence-based review. Pain Med. 2004;5(4):359–365. doi:10.1111/j.1526-4637.2004.04054.x.
Creswell JW. Research Design. Qualitative, Quantitative and Mixed Methods Approaches. 2nd ed. California: Sage Publications; 2003.
Drossman DA, Toner BB, Whitehead WE, et al. Cognitive behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19–31. doi:10.1016/S0016-5085(03)00669-3.
Drossman DA. The functional gastrointestinal disorders and the Rome III process. In: Drossman DA, Corazziari E, Delvaux M, et al., eds. Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates Inc.; 2006:1–29.
Longstreth G, Thompson W, Chey W, Houghton L, et al. Functional bowel disorders. In: Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, et al., eds. Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates Inc.; 2006:487–555.
Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med. 1995;123(10):782–794.
Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134(2):396–404. doi:10.1053/j.gastro.2007.11.011.
Whitehead WE, Palsson O, Jones KR. Systematic review of comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi:10.1053/gast.2002.32392.
Drossman DA, Whitehead WE, Toner BB, et al. What determines severity among patients with painful functional bowel disorders? Am J Gastroenterol. 2000;95(4):974–980. doi:10.1111/j.1572-0241.2000.01936.x.
Hirschfeld RM, Weisler RH, Raines SR, Macfadden W, BOLDER Study Group. Quetiapine in the treatment of anxiety in patients with bipolar 1 and bipolar 2 depression: a secondary analysis from a randomized, double blind, placebo-controlled study. J Clin Psychiatry. 2006;67:355–362.
Goldstein DJ, Lu Y, Detke MJ, Hudson J, Iyengar S, Demitrack MA. Effects of duloxetine on painful physical symptoms associated with depression. Psychosomatics. 2004;45(1):17–28. doi:10.1176/appi.psy.45.1.17.
Tarazi FI, Zhang K, Baldessarini RJ. Long term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology (Berl). 2002;161(3):263–270. doi:10.1007/s00213-002-1016-3.
Creed F, Guthurie E, Ratcliffe J, et al. Does psychosocial treatment help only those patients with severe irritable bowel syndrome who also have a concurrent psychiatric disorder? Aust N Z J Psychiatry. 2005;39:807–815. doi:10.1111/j.1440-1614.2005.01686.x.
Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–1659. doi:10.1053/j.gastro.2003.09.028.
Drossman DA, Li Z, Leserman J, Toomey TC, Hu YJ. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology. 1996;110:999–1007. doi:10.1053/gast.1996.v110.pm8613034.
Schutters SI, van Megen HJ, Westenberg HG. Efficacy of quetiapine in generalized social anxiety disorder: results from an open label-study. J Clin Psychiatry. 2005;66:540–542.
McIntyre A, Gendron A, McIntyre A. Quetiapine adjunct to selective serotonin reuptake inhibitors or venlafaxine in patients with major depression, comorbid anxiety, and residual depressive symptoms: a randomized, placebo-controlled pilot study. Depress Anxiety. 2007;24:487–494. doi:10.1002/da.20275.
Goldstein JM. Quetiapine fumarate (Seroquel): a new atypical antipsychotic. Drugs Today (Barc). 1999;35(3):193–210.
McConville B, Arvanitis LA, Thyrum PT, et al. Pharmacokinetics, tolerability, and clinical effectiveness of quetiapine fumarate: an open label trial in adolescents with psychotic disorders. J Clin Psychiatry. 2000;61:252–260.
Fass R, Fullerton S, Tung S, Mayer EA. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol. 2000;95:1195–1200. doi:10.1111/j.1572-0241.2000.02009.x.
Hamner MB, Deitsch SE, Brodrick PS, Ulmer HG, Lorberbaum JP. Quetiapine treatment in patients with post traumatic stress disorder: an open trial of adjuvant therapy. J Clin Psychopharmacol. 2003;23:15–20. doi:10.1097/00004714-200302000-00003.
Cohrs S, Rodenbeck A, Guan Z, et al. Sleep promoting properties of quetiapine in healthy subjects. Psychopharmacology (Berl). 2004;174:421–429.
Schreiber S, Getslev V, Backer MM. The atypical neuroleptics clozapine and olanzapine differ regarding their antipsychotic mechanisms and potency. Pharmacol Biochem Behav. 1999;64:75–80. doi:10.1016/S0091-3057(99)00107-0.
Shemo M, Shemo JPD, Anderson M. Beneficial effects of quetiapine treatment in patients with fibromyalgia. J Neuropsychiatry Clin Neurosci. 2003;15:282.
Passik SD, Lundberg J, Kirsh KL, et al. A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manage. 2002;23:526–532. doi:10.1016/S0885-3924(02)00391-3.
Hidalgo J, Rico-Villademoros F, Calandre EP. An open-label study of quetiapine in the treatment of fibromyalgia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:71–77. doi:10.1016/j.pnpbp.2006.06.023.
Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450–452. doi:10.1136/ard.2003.011502.
Hellewell JSE, Hands DC, McKellar J. Seroquel: an effective atypical antipsychotic with no greater EP’S than placebo across the full dose range. Schizophr Res. 1998;29:154–155.
Brooke NS, Wiersgalla M, Salzman C. Atypical uses of atypical antipsychotics. Harv Rev Psychiatry. 2005;13:317–339. doi:10.1080/10673220500433148.
Drossman DA, Chang L. Psychosocial factors in the care of patients with GI disorders. In: Yamada T, ed. Textbook of Gastroenterology, Chapter 29. Philadelphia: Lippincott; 2003:636–654.
Acknowledgments
Supported by the Gastrointestinal Biopsycho-social Research Center at UNC, NIH R24Dk067674.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grover, M., Dorn, S.D., Weinland, S.R. et al. Atypical Antipsychotic Quetiapine in the Management of Severe Refractory Functional Gastrointestinal Disorders. Dig Dis Sci 54, 1284–1291 (2009). https://doi.org/10.1007/s10620-009-0723-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-0723-6