Abstract
Reactive oxygen species (ROS) are increased in inflammatory bowel disease (IBD) and have been implicated as mediators of intestinal inflammation. We investigated the hypothesis that N-acetylcysteine (NAC) as a glutathione (GSH) precursor attenuates disease progression in a murine dextran sodium sulfate (DSS)-induced colitis model. A colitis model was induced by adding 5% DSS into the drinking water for 7 days. BALB/c mice were injiciatur enema with saline, 5-ASA, N-acetylcysteine, respectively, and free drinking water as control group. DSS-treated mice developed severe colitis as shown by bloody diarrhea, weight loss, and pathologic involvement. Colon lengths were significantly decreased in DSS-treated mice with decreased GSH activity too (P < 0.01). ROS in the colon, the level of interleukin 1β (IL-1β) in colonic mucosa, serum tumor necrosis factor a (TNF-α), MPO, and MDA were significantly increased in DSS-treated animals (P < 0.01), with decreased PON1 activity (P < 0.01). However, NAC significantly decreased colonic MPO activity, ROS, TNF-α and IL-1β levels and increased PON1 activity and GSH concentration. Moreover, NAC attenuated the macroscopic colonic damage and the histopathologic changes-induced by DSS while similar to 5-ASA group. These results suggest that NAC may be effective in the treatment of colitis through its up-regulating PON1 and scavenging oxygen-derived free radicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are the two major forms of inflammatory bowel disease (IBD) that comprise the conditions characterized by a tendency for chronic or relapsing immune activation and inflammation within the gastrointestinal tract. The mucosal immune system has evolved to balance the need to respond to pathogens while co-existing with commensal bacteria. In IBD, this hyporesponsiveness or tolerance breaks down, and inflammation supervenes driven by the intestinal microbial flora. Bacteria contain compounds and are recognized by a variety of receptors, including Toll-like receptors (TLRs) and NODs (a family of intracellular bacterial sensors) are potent stimuli of innate immune responses, which mediate the activation of nuclear factor-kappa B (NF-kB) and cytokines of the downstream link [1, 2]. Much evidence has shown that some cytokines such as IL-1 and tumor necrosis factor-alpha (TNF-α) are found elevated in both of these inflammatory bowel conditions [3, 4].
Recent studies suggest that TNF-α blocking agents and antibacterial may be effective in inducing remission in CD or UC. To determine whether anti-TNF-α and antibacterial therapy induce clinical response and remission in patients with CD or UC, some new meta-analysis studies have shown that anti-TNF-α is effective in inducing response and remission in patients with ulcerative colitis when administered in combination with corticosteroids, but not effective with CD; and suggest that adjunctive antibacterial therapy is effective for induction of clinical remission in UC and CD [5–8].
Besides the immune activation and inflammation, over the past decade there has been extensive focus on reactive oxygen species (ROS) as possible etiologic factors in the initiation or propagation of the inflammatory process. There are comparatively low tissue levels of endogenous antioxidants in the colonic mucosa, and oxidant stress therefore easily overwhelm the endogenous defenses that regulate ROS production. It has been suggested that the imbalance between prooxidant and antioxidant mechanisms in IBD may be controlled by antioxidant treatment. Several anti-oxidative drugs have been successfully used for the treatment of ulcerative colitis [9, 10]. On the other hand, the levels of the most important antioxidants have been found to be seriously impaired within the intestinal mucosa from IBD patients compared with normal mucosa [11].
Glutathione (GSH) is the most important intracellular defense against oxidative stress and is essential for both the functional and structural integrity of the gut, and GSH-deficient mice show severe degradation of the jejunum and colonic mucosa and diarrhea [12]. N-acetylcysteine (NAC) is known to act by raising intracellular concentrations of cysteine and hence of reduced glutathione (GSH) and by scavenging reactive oxygen species [13]. Recently, Fatemeh et al. stated that NAC has adequate potential for protection against DSS-induced colitis in mice by ameliorating colonic inflammation, oxidative and nitrosative stress of colonic damage, SOD and CAT activities, and colon TNF-α concentration [14].
Paraoxonase 1 (PON1) is a calcium-dependent esterase that is associated with HDL and recognized as an antioxidant enzyme because it hydrolyses lipid peroxides [15]. Baskol et al. revealed that serum PON1 activity was lower in UC patients [16]. Rothem et al. [17] demonstrated that PON mRNA and proteins are expressed in human biopsies from the gastrointestinal tract and in Caco-2 cells, and suggested that PONs may function in the intestine as detoxifiers or antioxidant mediators.
Since abnormal oxidative metabolism is a central importance in IBD, increased mucosal production of ROS as well as lipid peroxidation by-products have been described in patients with IBD. The predominant purpose of this study was to investigate the protection of NAC as antioxidant by ameliorating the oxidative stress and improving pathologic inflammation factors release followed through up-regulating PON1 and GSH and down-regulating myeloperoxidase (MPO) activity in the colitis model induced by DSS.
Materials and Methods
Animals
Approximately 8-week-old male Balb/c mice were randomly allocated into five groups and housed in filter-top cages in a specific pathogen-free environment at the animal facilities under standard condition and fed standard rat chow and water ad libitum. The Ethical Committee of the Faculty of Veterinary Medicine approved all the experiments.
Drug
Drugs used were dextran sulfate sodium (DSS;MW 5000, Fluka); 5-ASA (Sigma); NAC (Sigma). All drugs were dissolved in saline and prepared immediately before use and administered injiciatur enema in a volume of 0.3 ml/20 g body weight.
Induction and Treatment of DSS Colitis
Mice were divided into five different groups with ten mice in each group. All animals except the normal group received 5% DSS for 7 days. In the whole process, they were separated into four groups as follows: (1) a colitis model group, which received no treatment; (2) a saline group, which received 0.3 ml NS injiciatur enema; (3) a 5-ASA group, which received 0.3 ml 5-ASA injiciatur enema; and (4) an NAC group, which received 0.3 ml NAC injiciatur enema. The mice were anaesthetized on the eighth day using pentobarbital sodium, then the abdomen was dissected, the colon (5 cm) was removed, and the blood was collected at the same time, which were all maintained at −80°C. To measure biochemical biomarkers, the colonic samples were minced on ice and homogenized in 10 ml of ice-cold 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% HETAB and 10 mM EDTA. The homogenates were then sonicated and centrifuged for 20 min at 12,000 x g and the supernatant was transformed into several microtubes for separate biochemical assays and frozen at −20°C until analysis.
Disease Activity Index (DAI) [18]
Disease activity index (DAI) was derived by scoring three major clinical signs, which were weight loss, diarrhea, and rectal bleeding, 7 days after DSS administration as described by Kanauchi et al. Loss of body weight was calculated as the difference between the initial and actual weight. Diarrhea was shown as mucus/fecal material adherent to anal fur. Diarrhea was defined by the absence of fecal pellet formation in the colon and the presence of continuous fluid fecal material in the colon. Rectal bleeding was defined as diarrhea containing visible blood and gross rectal bleeding and scored as described for diarrhea. Three major clinical signs were scored separately as shown in Table 1.
Histologic Scoring
Histologic scores were given based on the description by Kanauchi et al. [18], with light modifications (we added an extra 1.5 score representing the shortening and loss of the basal 1/2 of the crypt), see Table 2. The whole tissue present on the slides (Swiss rolls, in most cases prepared from around 90% of the colon) was blindly evaluated at high magnification (400×) under the light microscope. A score was given to each microscopic field. If in a field, more than one score was present, then they were multiplied by its estimated percentage in the field and added. At the end, average microscopic fields were recorded and analyzed statistically. The number of fields per section ranged between 16 and 44, with 30 fields being the average in both studies.
Determination of Myeloperoxidase Activity
Myeloperoxidase (MPO) activity was measured according to the modified method of Suzuki et al. [20]. Mice were killed 7 days after DSS treatment, the mucosa was scraped with glass slides, weighed, and homogenized in 50 mM phosphate buffer containing 0.5% hexadecyl-trimethylammonium bromide (HTAB; pH 6.0; Sigma). The homogenized samples were subjected to freezing and thawing three times and centrifuged at 2,000 rpm for 10 min at 4°C. MPO activity in the supernatant was determined by adding 100 μl of the supernatant to 1.9 ml of 10 mM phosphate buffer (pH 6.0) and 1 ml of 1.5 M o-dianisidine hydrochloride (Sigma) containing 0.0005% w/v hydrogen peroxide. The changes in absorbance at 450 nm of each sample were recorded on a Hitachi spectrophotometer (U-2000, Hitachi, lbaraki, Japan). Sample protein content was estimated by spectro-photometric assay (Peace protein assay kit, IL, USA), and the MPO activity was obtained from the slope of the reaction curve based on the following equation: specific activity (μmol H2O2/min/mg protein) = (OD/min)/OD/μmol H2O2 × mg protein.
Measurement of Serum MDA Concentration
Serum MDA levels were measured according to a method described elsewhere. The principle of the method is based on the spectrophotometric measurement of the color produced during the reaction of thiobarbituric acid with MDA. The concentration of thiobarbituric acid reactive substances was calculated by the absorbance coefficient of malondialdehyde–thiobarbituric acid complex and expressed in nmol/l. As a standard, MDA bis (dimethyl acetal)-TBA (thiobarbituricacid) complex was used.
Determination of Serum-Reduced Glutathione
Reduced glutathione was determined as previously described based on the reaction of 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) with the GSH present. The absorbance was measured at 412 nm in a Schimadzu double-beam spectrophotometer (UV 200S). The amount of glutathione present in the sample was calculated using a standard solution of GSH containing 1 mg of GSH/1 ml of 3% metaphosphoric acid. The increase in the extinction at 412 nm is proportional to the amount of GSH present.
Assay of Paraoxonase Activity
Serum PON1 activity was measured according to a method described elsewhere [19]. We measured the rate of hydrolysis of paraoxon by monitoring the increase in absorbance at 405 nm and at 25°C. The basal assay mixture contained 1.0 mM paraoxon and 1.0 mM CaCl2 in 0.05 M glycine buffer pH 10.5. One unit (IU) of PON1 activity is defined as 1 mmol of p-nitrophenol formed per minute, and activity was expressed as U/ml of serum.
Measurement of IL-1β and TNF-α Production
Mucosal level of these cytokine was assayed by using a commercially available IL-1β, level of TNF-α enzyme-linked immunosorbent assay kit (GENMED SCIENTIFICS INC, Shanghai, China), which also recognizes the mice form of IL-1β and TNF-α. The measurement of both TNF-α and IL-1β was performed step-by-step based on the protocol booklet of the ELISA kit. Briefly, the sample was incubated with mouse IgG monoclonal antibodies against IL-1β or TNF-α. Then, after incubation with a second biotinylated antibody, a streptavidin-biotinylated horseradish peroxidase complex was added. Orthophenylenediamine served as a substrate for the horseradish peroxidase complex and measured by a microplate reader (Biotek). The assay was carried out following the manufacturer’s instructions. The minimum of detectable concentration of mice TNF-α was <5 ng/l, and IL-1β was 3 ng/l.
Detection of Reactive Oxygen Species [19]
Isolated enterocytes and colonocytes (maintained at 37°C) from mice were divided into two treatment groups, which received either an oxidative challenge of 50 mM H2O2 for 5 min or no treatment (basal). This concentration of H2O2 was chosen as it was sufficient to create an oxidative stress without being toxic to the cells. Exposure time was necessarily limited due to the short-term viability of cells ex vivo. Samples were prepared in duplicate and incubated for 15 min with DCFDA, a fluorescence probe sensitive to such cellular oxidants as hydrogen peroxide (H2O2), hydroxyl radicals (OH−), and peroxyl radicals (OOH−). This probe passively diffuses into cells and upon oxidation by ROS forms a fluorescent adduct that remains trapped in the cell. Fluorescence was monitored on a Meridian Ultima confocal microscope (Meridian Instruments, Okemos, MI) with a 530-nm barrier filter and laser excitation at 488 nm, as previously described. Intensity of fluorescence is used as an indirect measure of prevalence of ROS.
Statistical Analyses
The statistical analyses were performed using SPSS for Windows computer software (SPSS Inc., Chicago, IL). Differences between groups were evaluated by one-way ANOVA, 95% confidence interval (if normally distributed) or by Kruskal–Wallis if not. Normal distribution was determined by homogeneity of variance (Levene) and Kolmogorov–Smirnov (skewness).
Results
Colitis Model and DAI Scoring
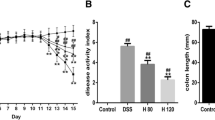
Mice administered with 5% DSS in drinking water for 7 days developed symptoms of colitis without mortality. Compared with controls, the model and saline groups induced loose stool or diarrhea, occult or gross rectal bleeding, significantly decreased weight (date not shown), and DAI scoring increased markedly (P < 0.05, Fig. 1), and caused a shortening of the colon (P < 0.05, Fig. 2). NAC or 5-ASA obviously improved the decreased weight or shortening of the colon and reduced DAI scoring (P < 0.05, Fig. 1 or 2).
Effect of NAC on DAI in DSS-induced ulcerative colitis. DAI was calculated as described in Table 1. Data are expressed as the mean ± SEM (*P < 0.05 vs. control group, # P < 0.05 vs. model group; n = 10)
Microscopic Results and Histologic Scoring
When compared to the colon mucosa of control, the colonic sections of DSS-treated mice revealed a cryptic distortion with massive inflammatory infiltration and a loss of mucous membrane cells, leukocyte infiltration, and the bulk of glandular organ became unintegrated (Fig. 3a and b), increasing the histologic score (P < 0.05, Table 3). Treatment of NAC and 5-ASA (0.3 ml/20 g) remarkably attenuated the extent and severity of the histologic signs of cell damage, prevented the DSS-induced various pathologic changes of colitis (Fig. 3d and e), and decreased the histologic score significantly (P < 0.05, Table 3) compared to the model group (Fig. 4).
MPO Activity
Colitis was characterized by an increase in colonic MPO activity, compared to controls (P < 0.01). Injiciatur enema of NAC or 5-ASA significantly reduced colonic MPO activity (P < 0.05). The results were consistent with histologic findings.
Serum MDA Level, GSH, and PON1 Activity, ROS Content in Colon
Tables 4 and 5 demonstrate a significant elevation of MDA level and decrease the PON1 activity in serum, with remarkably increased the ROS content in the colitis colon, compared with control group, P < 0.01. NAC or 5-ASA evidently increase activity of PON1 and decrease the ROS and MDA concentration in serum or colon, compared with the model group (P < 0.05).
Interleukin-1β and TNF-α Concentration
Tables 4 and 5 show that the colonic inflammation induced by DSS significantly increased IL-1β level and TNF-α concentration as compared to the normal group (P < 0.01). Administration of NAC or 5-ASA resulted in a significant reduction in IL-1β and TNF-α concentration compared with the model group (P < 0.05).
Discussion
In the present study, we observed that NAC ameliorated all of the considered inflammatory symptoms in a DSS-induced colitis murine model, increased the activity of PON1, diminished the MPO activity, attenuated the enhanced ROS and MDA level, prevented the depletion of GSH, and decreased the elevation of IL-1β and TNF-α.
CD and UC are fairly common chronic inflammatory conditions of the gastrointestinal tract. The main pathologic feature of IBD is an infiltration of polymorphonuclear neutrophils and mononuclear cells into the intestinal tissues. Neutrophils and monocytes migration is in turn triggered by chemotactic bacterial cell-wall products and locally produced cytokines [20]. Measurement of MPO activity has been used as an indicator of neutrophil influx into inflamed gastrointestinal tissue. Our present study has shown that the MPO activity was remarkably increased in the intestinal tissue of DSS-treated mice compared to that of normal mice, and significantly decreased in mice administrated by NAC or 5-ASA, which was consistent with the histologic findings.
Oxidative stress is believed to play a key role in the pathogenesis of intestinal damage in IBD. As a matter of fact, intestinal mucosal damage in IBD, including CD and UC, is related to increased free-radical production and to a low concentration of endogenous antioxidant defense [21]. Similar studies reveal that the colons of IBD patients produce more ROS compared to those of control subjects [22]. Many studies indicate that super oxide (O2 −), hydrogen peroxide (H2O2), hypochlorous acid (HOCI), and hydroxyl radicals (OH−) have a role in mediating intestinal damage in IBD [23]. These compounds, which produce fatty acid radicals and lipid hydroperoxides, readily attack cell membranes that are known to be rich in polyunsaturated fatty acids. MDA is the abbreviation of malondialdehyde, it is an end product of lipid peroxidation induced by ROS. Our current data show the colon of colitis have significantly more ROS and creating more MDA content than normal group. However, the ROS and MDA content were markedly degraded by treated NAC or 5-ASA, which would suggest that lipid peroxidation could have an important role in the pathogenesis of UC.
It has been suggested that intestinal damage in IBD is related to a low concentration of endogenous antioxidant defenses, such as glutathione peroxidase (GSH-Px), superoxide dismutases (SOD) [24], etc., maybe including paraoxonase1 (PON1). PON1, an HDL-bound enzyme, is secreted by the liver and plays an important antioxidant role of hydrolyses lipid peroxides. Studies have indicated that the PON1 gene and protein are expressed in human intestinal biopsies and Caco-2 cells, and also showed that low PON1 activity in patients with ulcerative colitis. GSH is the most important intracellular defense against oxidative stress and is essential for both the functional and structural integrity of the gut [25]. PON1 protects LDL from oxidation induced by either copper-ion or free-radical generator. It possesses key point active sites: the antioxidant site, which is dependent on PON1’s free sulfhydryl (–SH) group on Cysteine-283. It was demonstrated that PON1’s free –SH group on Cys-283 is required for its ability to protect LDL against oxidation and PON1 inactivation is the result of an interaction between PON1’s free –SH group and specific oxidized lipids in Ox-LDL [26, 27]. It plays an essential role in cell biology and modulates cell response to redox changes associated with the ROS. Most of the beneficial effects of NAC are suggested as being a result of its ability to either reduce extracellular cystine to cysteine, or to be a source of –SH metabolites. In our previous study, we have found that NAC can up-regulate PON1 activity in vivo [28]. In this study, administration of NAC in the DSS-induced mice model increased PON1 and GSH activity, suggesting that up-regulated PON1 activity might play an important role in attenuating the chemically induced acute colitis. These data are consistent with other reports that NAC has adequate potential for protection against DSS-induced colitis in mice by ameliorating colonic inflammation, reducing the extent of colonic damage and colon TNF-α concentration, along with a decrease in MPO activity, preventing the depletion of colonic GSH [14, 29].
It is proposed that TNF-α production of ROS in turn activates nuclear factor-kappa B (NF-kB), which then enhances further TNF-α production and cytokines, such as IL-1β, IL-6 etc., propagating a vicious cycle [30, 31]. Kumon et al. reported that cytokines, IL-1β, and TNF-α downregulated serum PON 1 activity [32]. Macrophages produce certain cytokines, such as TNF-α and IL-1β, the levels of which are often increased in both animal models and patients with UC [33, 34]. In addition, IL-1β appears to be a primary stimulator of diarrhea, the major symptom of intestinal inflammation. TNF-α is involved directly in tissue damage and cell death in the intestinal mucosa in inflammatory bowel diseases. Our results showed an elevation in the levels of IL-1β in the colon and TNF-α in serum of mice induced by DSS, but was reduced by NAC or 5-ASA, which decreased the ROS content.
In conclusion, our results demonstrate that NAC is protective in acute DSS experimental colitis. The antioxidant activity of NAC may be partly responsible for the observed protection in colitis. In our work, we tried to elevate the defensive factors such as PON1 and GSH so as to reduce excessive ROS production and IL-1β or TNF-α followed in colitis. Further studies should be trialed in mice colitis made by other stimulus, administer NAC in various ways, and search for other possible related mechanisms.
References
Strober W, Murray PJ, Kitani A, et al. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi:10.1038/nri1747.
Netea MG, Kullberg BJ, de Jong DJ, et al. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn’s disease. Eur J Immunol. 2004;34:2052–2059. doi:10.1002/eji.200425229.
Mawdsley JE, Jenkins DG, Macey MG, et al. The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am J Gastroenterol. 2008;103(6):1460–1469. doi:10.1111/j.1572-0241.2008.01845.x.
Lee HS, Han SY, Bae EA, et al. Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. Int Immunopharmacol. 2008;8(4):574–580. doi:10.1016/j.intimp.2008.01.009.
Rahimi R, Nikfar S, Abdollahi M. Meta-analysis technique confirms the effectiveness of anti-TNF-alpha in the management of active ulcerative colitis when administered in combination with corticosteroids. Med Sci Monit. 2007;13(7):PI13–PI18.
Rahimi R, Nikfar S, Abdollahi Mohammad. Do anti-tumor necrosis factors induce response and remission in patients with acute refractory Crohn’s disease? A systematic meta-analysis of controlled clinical trials. Biomed Pharmacother. 2007;61(1):75–80. doi:10.1016/j.biopha.2006.06.022.
Rahimi R, Nikfar S, Rezaie A, et al. A meta-analysis of broad spectrum antibiotic therapy in patients with active Crohn’s disease. Clin Ther. 2006;28:1983–1988. doi:10.1016/j.clinthera.2006.12.012.
Rahimi R, Nikfar S, Rezaie A, et al. A meta-analysis of antibiotic therapy for active ulcerative colitis. Dig Dis Sci. 2007;52(11):2920–2925. doi:10.1007/s10620-007-9760-1.
Helieh Oza TS, Theresa Chenb S, Craig McClain J, et al. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297–304. doi:10.1016/j.jnutbio.2004.09.007.
Isozaki Y, Yoshida N, Kuroda M, et al. Effect of a novel water-soluble vitamin E derivative as a cure for TNBS-induced colitis in rats. Int J Mol Med. 2006;17(3):497–502.
Kruidenier L, Kuiper I, Lamers CB, et al. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201(1):28–36. doi:10.1002/path.1409.
Mrtensson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci USA. 1990;87:1715–1719. doi:10.1073/pnas.87.5.1715.
Soltan-Sharifi MS, Mojtahedzadeh M, Najafi A, et al. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Hum Exp Toxicol. 2007;26(9):697–703. doi:10.1177/0960327107083452.
Ebrahimi F, Esmaily H, Baeeri M, et al. Molecular evidences on the benefit of N-acetylcysteine in experimental colitis. Cent Eur J Biol 2008;3(2):135–142. doi:10.2478/s11535-008-0005-x.
Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–480.
Baskol G, Baskol M, Yurci A, et al. Serum paraoxonase 1 activity and malondialdehyde levels in patients with ulcerative colitis. Cell Biochem Funct. 2006;24(3):283–286. doi:10.1002/cbf.1224.
Rothem L, Hartman C, Dahan A, et al. Paraoxonases are associated with intestinal inflammatory diseases and intracellularly localized to the endoplasmic reticulum. Free Radic Biol Med. 2007;43:730–739. doi:10.1016/j.freeradbiomed.2007.05.003.
Kanauchi O, Nakamura T, Agata K, et al. Effects of germinated barley food stuff on dextran sulfate sodium-induced colitis in rats. J Gastroenterol. 1998;33:179–188. doi:10.1007/s005350050067.
Sanders LM, Henderson CE, Hong MY, et al. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett. 2004;208:155–161. doi:10.1016/j.canlet.2003.12.007.
Suzuki K, Sugimura K, Hasegawa K, et al. Activated platelets in ulcerative colitis enhance the production of reactive oxygen species by polymorphonuclear leukocytes. Scand J Gastroenterol. 2001;36:1301–1306. doi:10.1080/003655201317097164.
Qualls JE, Kaplan AM, van Rooijen N, et al. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80(4):802–815. doi:10.1189/jlb.1205734.
Rezaie A, Parker RD, Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig Dis Sci. 2007;52(9):2015–2021. doi:10.1007/s10620-006-9622-2.
Banan A, Choudhary S, Zhang Y, et al. Oxidant-induced intestinal barrier disruption and its prevention by growth factors in a human colonic cell line: role of the microtubule cytoskeleton. Free Radic Biol Med. 2000;28(5):727–738. doi:10.1016/S0891-5849(00)00160-X.
Kruidenier L, Kuiper I, Van Duijn W, et al. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol. 2003;201(1):17–27. doi:10.1002/path.1408.
Aw TY. Intestinal glutathione: determinant of mucosal peroxide transport, metabolism, and oxidative susceptibility. Toxicol Appl Pharmacol. 2005;204(3):320–328. doi:10.1016/j.taap.2004.11.016.
Aviram M, Billecke S, Sorenson R, et al. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different than that required for its arylesterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler Thromb Vasc Biol. 1998;18:1617–1624.
Aviram M, Rosenblat M, Billecke S, et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999;26:892–904.
Liu Y-H, Liu L-Y, Wu J-X, et al. Comparison of Captopril and Enalapril to study the role of the sulfhydryl-group in improvement of endothelial dysfunction with ACE inhibitors in high dieted methionine mice. J Cardiovasc Pharmacol. 2006;47(1):82–88.
Nosal’ova V, Cerna S, Bauer V. Effect of N-acetylcysteine on colitis induced by acetic acid in rats. Gen.Pharmaco. 2000;35(2):77–81.
Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14.
Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071.
Kumon Y, Nakauchi Y, Suehiro T, et al. Proinflammatory cytokines but not acute phase serum amyloid A or C-reactive protein, downregulate paraoxonase 1 (PON1) expression by Hep G2. Amyloid. 2002;9:160–164.
Chromik AM, Müller AM, Albrecht M, et al. Oral administration of taurolidine ameliorates chronic DSS colitis in mice. J Invest Surg. 2007;20(5):273–282.
Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007;13(4):416–423.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, Y., Fu, JJ., Meng, J. et al. Effect of N-acetylcysteine on the Murine Model of Colitis Induced by Dextran Sodium Sulfate Through Up-Regulating PON1 Activity. Dig Dis Sci 54, 1643–1650 (2009). https://doi.org/10.1007/s10620-008-0563-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0563-9