Abstract
Background Exercise-triggered asthma (ETA) develops when physical activity triggers asthma symptoms during or directly after exercise. In patients prone to symptoms of supra-esophageal reflux, exercise may trigger gastroesophageal reflux (GER), resulting in such symptoms. Aims To determine the prevalence of abnormal pH in patients with ETA and to determine whether acid suppression improves symptoms in ETA patients. Methods We performed a randomized double-blind trial of rabeprazole versus placebo in the treatment of patients with ETA. Patients underwent treadmill protocol to determine their VO2max. Next, pH testing was initiated while undergoing a 30-min treadmill program exercising them at 65% of their VO2max. They were subsequently randomized to rabeprazole or placebo for 10 weeks. At the end of 10 weeks, exercise testing was repeated. Results A total of 31 patients completed the study (20 asthmatics, 11 non-asthmatics). Twenty-two out of 30 (73%) subjects had abnormal pH studies. For all subjects, rabeprazole improved symptoms more than placebo (P = 0.03). The association was stronger in the pH-positive group (P = 0.009). Conclusion Acid reflux is common in ETA patients. Many patients with exercise-related respiratory symptoms are misdiagnosed as chronic asthmatics. Exercise-related symptoms improve with the use of acid suppression. This study suggests that ETA patients may benefit from acid suppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Exercise-triggered asthma (ETA) is a common condition affecting up to 7% of active young adults [1]. ETA occurs when vigorous physical activity triggers respiratory symptoms during or directly after exercise with or without underlying chronic asthma. The classic presentation of ETA is coughing, excessive sputum production, wheezing, dyspnea, and/or chest tightness immediately following at least 6–8 min of strenuous exercise. However, many individuals will present with more subtle findings of chest discomfort, stomach ache, fatigue, feeling out of shape, inability to keep up with peers, or poorer performance than training would predict [1, 2].
ETA occurs in almost 90% of people who have chronic asthma [3]. ETA can also occur in otherwise healthy people without chronic asthma. In this group, exercise is the only stimulus for such bronchoreactive symptoms and may reflect a different pathophysiologic event than seen in chronic asthma.
To date, only one study has evaluated acid reflux and its potential relationship to ETA, and no association between the two was found [4]. However, this study had significant methodological limitations.
Gastroesophageal reflux disease (GERD) is present in 50–70% of chronic asthmatics [5, 6]. Some studies have demonstrated improvement of asthma symptoms with treatment of GERD. Exercise has been shown to induce gastroesophageal reflux events, likely due to decrease in the gastric–esophageal pressure gradient at the lower esophageal sphincter (LES) [7–11]. GER may reduce the threshold for bronchoconstriction and be the mechanism which produces respiratory symptoms in patients prone to exercise-induced asthma (EIA) [12, 13].
No study has examined distal esophageal acid exposure in patients with EIA. Additionally, there are no data to determine whether acid suppression decreases respiratory symptoms during exercise. We hypothesized that there is a high prevalence of GERD in ETA and further that acid suppression may improve symptoms of ETA.
Methods
Approval was obtained from the Institutional Review Board at the University of Utah. We performed a prospective, randomized, controlled trial of rabeprazole (QD and BID) versus placebo in the treatment of subjects with symptoms consistent with ETA. Participants were recruited from our supra-esophageal GERD clinic at the University of Utah Hospital from 2002 to 2005. To formally diagnose EIA, serial pulmonary function (PFT) tests for at least 20 min after intensive exercise are required [3]. Because we were unable to perform formal diagnostic testing with serial PFTs, we selected a population of “exercise-triggered” rather than “exercise-induced” asthma. All subjects complained of respiratory symptoms (cough, shortness of breath, chest tightness) which occurred during (within 15 min) or directly after (10–15 min) moderate exercise, typical of asthma-related symptoms [14]. Inclusion criteria consisted of the above-described exercise-induced respiratory symptoms with self-reported reflux symptoms less than twice weekly. This was intended to capture those subjects who would not normally present to physicians with reflux symptoms and thus be empirically treated with acid suppression. Subjects were excluded if they reported an average of greater than two episodes of heartburn weekly or concurrent use of acid suppression medication, were severe asthmatics with hospitalization within the preceding 6 months, suffered from pulmonary problems other than asthma, had a history of severe angina, cardiac arrhythmias, heart failure, myocardial infarction, or prior upper gastrointestinal surgery.
Prior to enrollment, subjects were classified as “chronic asthmatic” or “non-chronic asthmatics” by a board-certified pulmonologist (W.M.S.) via clinical criteria. Chronic asthmatics had continual symptoms outside of exercise while non-chronic asthmatics appeared to have only an exercise trigger of their symptoms. To mimic real life, series of clinical and objective measures (symptoms, PFTs, inhaler use) were used confirm the diagnosis of asthma prior to enrollment. Diagnosis and confirmation of asthma was left to the discretion of a board-certified pulmonologist (W.M.S.). All outside medications were kept stable unless changed by their primary physician. At the request of the Institutional Review Board, subjects were allowed rescue medication (albuterol metered dose inhaler) use at any time during the study from randomization through follow-up.
Protocol
During the screening process, subjects were evaluated for entry criteria and categorized as asthmatics or non-asthmatics for future subgroup analysis. Upon enrollment, subjects underwent standardized exercise treadmill to determine their VO2max (aerobic capacity) and completed Mini-AQLQ (a quality of life assessment in asthma) and SF-36 questionnaire.
Next, after a 4-h fast, all subjects underwent esophageal manometry testing with localization of LES high pressure zone and subsequent placement of dual sensor pH monitor (antimony pH slimline probe; Medtronic, Shoreview, Minnesota). pH catheter was placed immediately prior to participating in the initial 30-min treadmill program designed to keep them exercising at 65% of their VO2max (chosen to provide moderate exercise for 30 min). Results of pH testing were considered positive if subjects demonstrated >4.2% time distal acid exposure or >0.9% time proximal acid exposure [15, 16]. BRAVO (Medtronic) testing was offered if the subjects felt anxious about a 24-h pH catheter or were unable to tolerate the slimline catheter.
Per IRB requirements, at any time during a treadmill test, subjects were allowed to utilize rescue inhalers.
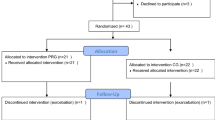
After completion of the exercise regimen and pH probe, subjects were randomized to rabeprazole 20 mg QAM and placebo QPM, rabeprazole 20 mg po BID, or placebo BID for 10–12 weeks. At the end of 10–12 weeks, 30-min exercise testing was repeated at 65% of the subjects’ pre-determined VO2max (Fig. 1). Before and after each exercise test at 65% VO2max, PFTs were performed. Our primary endpoint was a subjective determination by the subjects (yes/no) whether their exercise symptoms improved between each standardized 65% VO2max treadmill test. This outcome was utilized as prior studies in asthmatics have not demonstrated any validity of PFTs in reflecting asthmatic improvement.
Assignment
Subjects were randomized via a pre-determined computer generated randomization scheme to rabeprazole BID or QD or placebo. QD and BID dosing were chosen to determine whether high dose acid suppression was needed to result in an improvement. Subjects were analyzed in subgroups by asthma status (chronic or primarily exercise-triggered). The sample size (10 patients per arm) was determined based on a difference between rabeprazole (any dose) and placebo of 80% versus 30% response with alpha 0.05 and beta 20% (power 80%). Rationale for the sample size calculation was based upon our clinical experience within our asthma-GERD clinic where acid suppression has resulted in improvement in symptoms in daily symptoms in 80% of the patients. We anticipated an equal response to exercise-triggered symptoms—we have seen substantial exercise-triggered acid reflux in our population.
Masking
Both the subjects and the investigators were blinded to treatment allocation. Rabeprazole and placebo medications were supplied by the manufacturer of rabeprazole (PriCara, unit of Otho-McNeil, Inc. and Eisai, Inc.), so that both drugs were identical in appearance. The blinding was not broken for any subject enrolled. The randomization assignments remained concealed until all subjects completed the study.
Analysis
The primary endpoint was determined a priori to be a dichotomous value—whether the subjects’ exercise symptoms (during the treadmill) improved by the end of the trial (“yes” or “no”) with rabeprazole compared to placebo. This endpoint was chosen due to the lack of evidence that PFTs accurately reflect pulmonary status/improvement in asthmatics and due to a lack of validated questionnaires assessing pulmonary symptoms after exercise. The mini-AQLQ was not utilized as primary outcome as it did not assess exercise-related symptoms, only daily symptoms. Simple subjective reports of improvement would allow for future testing with objective measures such as pulmonary resistance and conductance measurements. Secondary endpoints include subgroup analysis between asthmatics and non-asthmatics, between +GER and −GER, and between rabeprazole QD to BID. Responses to the validated quality of life questionnaires (SF-36 and mini-AQLQ) were also compared between study groups before and after treatment with study medication. Fisher’s exact testing was used to evaluate the results as all results were proportional outcomes. All data was analyzed as intent-to-treat using Stat V8 (StataCorp LP, College Station, TX, USA). Mean changes in quality of life scores were assessed via independent samples t-test.
Results
Participant flow and follow-up
Thirty-seven total subjects were enrolled. Thirty-one of the 37 subjects completed the study (20 asthmatics, 11 non-asthmatics). Four dual channel pH probes were completed while the rest of the subjects exercised with BRAVO catheters. No participants reported difficulty exercising with either pH device. One pH probe malfunctioned but the subject completed the study leaving 30 subjects with adequate pH data. The drop-outs occurred prior to randomization due to intolerance of pH testing in six patients. No subjects dropped out after randomization to medication. Of the 31 subjects randomized, 8 received placebo and 23 received medication. Three patients in the rabeprazole group required rescue inhalers during the initial 30-min treadmill prior to starting medication. All three subjects took inhalers after the exercise regimen had been completed (prior to the spirometry). All subjects reported chest tightness with either cough or shortness of breath during the initial treadmill. No subjects in either treatment group required rescue inhalers during or after the final treadmill exam.
Patient characteristics are described in Table 1. All subjects in the asthma group complained of either cough or shortness of breath with exercise. Average distal pH value in the reflux group was 9.4 ± 7.8%; average proximal value was 2.3 ± 1.0%. Twenty-two of 30 patients had an abnormal 24-h pH reading along with reflux episodes detected during exercise. An additional two patients demonstrated reflux episodes during exercise without having abnormal 24-h acid exposure. Thus, 24 of 30 (80%) of subjects demonstrated acid reflux events during exercise (with an average of 10 reflux events during each 30-min period, range: 4–30).
In intent-to-treat analysis, rabeprazole therapy significantly improved symptoms compared to placebo [16/23 (70%) vs 2/8 (25%), respectively, P = 0.03] (Fig. 2). In subgroup analysis, the subjects with +GER also benefited from rabeprazole therapy [15/17 (88%) rabeprazole vs 1/5 (20%) placebo, P = 0.009] (Fig. 2). Looking at those who refluxed during exercise, 15/19 demonstrated improvement in the rabeprazole group while 1/5 improved in the placebo group (P = 0.03). There was no statistically significant improvement in symptoms when subgrouped into chronic asthmatics (P = 0.13, RRR = 0.42) or non-chronic asthmatics (P = 0.18). However, in chronic asthmatics with +GER, there was a significant improvement in symptoms in the rabeprazole group compared to placebo [10/11 (91%) vs 1/4 (25%), respectively, P = 0.03] (Fig. 3).
There was no significant difference in response between the rabeprazole QD and BID groups [(12/16 (75%) vs 4/7 (57%), respectively, P = 0.33] (Fig. 4). There was also no difference in PFTs (FEV1, FVC, FEV1/FVC) between the rabeprazole and placebo groups. By objective measures, rabeprazole did not provide any significant improvement in spirometry as compared to placebo (22% vs 50% respectively, P = 0.11). Quality of life scores were also not improved after treatment in the rabeprazole groups compared to placebo in either the SF-36 (P = 0.97, 95% CI: −1.05 to 0.99) or mini-AQLQ (P = 0.21, 95% CI: −0.53 to 2.27) (Table 2). There were no important adverse events in any treatment group.
Discussion
Our findings demonstrate that subjects with exercise-triggered respiratory symptoms subjectively improve with acid suppression therapy.
The exact relationship between gastroesophageal reflux and asthma remains controversial. Two recent randomized controlled studies found improvement in some but not all respiratory parameters measured in asthmatics treated with acid suppression. Littner et al. found decreased asthma exacerbations but no difference in quality of life between lansoprazole versus placebo [17]. In another randomized blinded study, Kiljander determined that asthma improved with PPI treatment only in asthmatics with reflux symptoms and nocturnal respiratory symptoms [18]. However, multiple previous studies have not been able to provide strong objective evidence of improvement in asthma with acid suppression by formal measures of respiratory function [19–21].
The relationship between ETA and GER is similarly controversial. In the only previous study that specifically examined ETA subjects, Weiner et al. attempted to study acid reflux in subjects with ETA. They found that there was little evidence of acid reflux during short bursts of exercise in ETA subjects [4]. However, this study was limited by the lack of complete 24-h pH monitoring, exceptionally stringent criteria for the diagnosis of a reflux episode, and small sample size. Thus, GER may have been under-diagnosed in this population.
Several recent studies have investigated acid reflux and its relationship to bronchoreactivity measured via methacholine and/or capsaicin-induced respiratory challenge [12, 13, 22–25]. Bagnato et al. found a lower threshold for bronchospasm during methacholine challenge testing in subjects with GERD [13]. Kiljander et al. determined that fundoplication resulted in improvement in bronchial hyper-responsiveness (BHR) in asthmatics studied [24]. Jiang et al. determined that treatment with acid suppression and promotility agents resulted in improvement in bronchial reactivity in asthmatics [25]. These data along with our own suggest that GER commonly occurs during exercise [26–28] and may result in BHR.
We found a high prevalence of acid reflux in our subjects, defined either by traditional 24-h pH monitoring (73%) or acid reflux events during exercise (80%). This is in agreement with the prior studies by Castell which demonstrated a high occurrence of reflux episodes during exercise [7, 8]. We intentionally chose subjects with less than two episodes of heartburn a week to study a population not already on acid suppression and, in fact, none of our subjects were currently taking acid suppression. This may have biased our study in favor of a negative result; instead we found the opposite. On the other hand, the medications used by our patients (albuterol, etc.) may have predisposed our subjects to developing acid reflux. These asthmatic medications have been found to be “refluxogenic” in prior studies [29, 30]. The improvement seen in our population (although subjective) suggests that, despite the etiology of the acid reflux, acid suppression relieved the exercise-related symptoms.
There are several limitations to our study. First, as EIA requires rigorous diagnostic testing to confirm its presence, we chose to evaluate persons who presented with the clinical symptoms of EIA without confirming the diagnosis via intensive exercise/pulmonary function testing. Thus, it is unclear how well our patient population may be representative of subjects with true exercise-induced asthma. Our population is better categorized as “exercise-triggered” asthma rather than EIA. We, however, feel confidant that our population represented a spectrum of asthma. We chose not to require objective measurements in the confirmation of the diagnosis of asthma due to their lack of specificity and their variability. Rather, we took a real-world approach and, through clinical manifestations as well as objective measures, allowed our subjects to be confirmed via pulmonology evaluation. Additionally, we did not subcategorize our patients into allergic versus non-allergic asthma, rather we categorized them into chronic (more than one symptom trigger other than exercise) versus non-chronic. However, 100% of our “chronic” asthmatics were triggered by known allergens.
Second, the majority of our study subjects were recruited from our supra-esophageal GERD clinic, although as stated previously we chose subjects with a low frequency of symptoms of GERD. Thus, our referral population may have had an increased prevalence of GER than that of the general ETA population. All subjects matched inclusion and exclusion criteria but there may have been underlying bias in our recruitment. In addition, validated GERD questionnaires were not used to assess heartburn. People were simply questioned as to self-reported regurgitation, heartburn, chest pain, dysphagia, acid reflux. As long as any of these symptoms were reported to occur less than twice per week, subjects were eligible for the study.
Third, due to our small sample size, block randomization would have provided a more equal distribution of therapy. We did not perform block randomization, accounting for our uneven numbers in each treatment group. To address concerns over power in our results, we performed post-hoc power calculations. Power suffered in our overall result (74%) but was preserved in our subgroup analyses (<85% in each). Thus, it appears that our results, based upon +GERD subgroups, are robust.
Fourth, a second pH probe was not performed to assess, compare and confirm efficacy of acid suppression in our different treatment arms. This would have allowed us to correlate improvement with acid suppression. An additional impedance/pH probe may have been beneficial in determining accurately whether acid or non-acid reflux existed during the second treadmill, contributing to symptoms. Thus, some non-responders and responders may have suffered from ongoing acid reflux despite PPI therapy. Mainie et al. demonstrated that 11% of patients with persistent reflux symptoms often had acid breakthrough on BID PPI therapy [31]. In contrary to Mainie, Charbel et al. have shown that <1% of subjects on PPI therapy will continue to have pathologic reflux [32]. Our population was different than these populations due to our subjects’ lack of symptomatic reflux. Additionally, it would have been highly unlikely for the therapy to have failed to suppress acid reflux at all and incomplete acid suppression may result in improvement in symptoms. The subjects were randomized and blinded to their therapy, making the difference between the therapy arms more valid.
Our primary endpoint was that of symptomatic improvement during exercise. We standardized our exercise to prevent subjects who felt better on acid suppression from exercising more or less vigorously on the second treadmill thus, interfering with our outcome measurements. However, symptomatic improvement did not correlate with respiratory improvement as measured by spirometry. This may be for a few reasons. First, per Institutional Review Board requirements, we allowed rescue inhaler use after exercise which a few subjects utilized after the first treadmill test. We controlled for this by having them describe their outcomes prior to use of the inhaler. Second, we did not standardize rest time between the exercise tests and the spirometry. Subjects simply performed spirometry when they felt ready. These facts may explain the trend (although non-significant) toward improvement in the spirometry in the placebo arm as compared to the rabeprazole arm (50% vs 22%, P = 0.11). Third, the lack of correlation between symptomatic and objective improvement by respiratory function testing is well recognized in the literature in this area. Our data are consistent with this observation.
Additionally, we chose not to use the results of the mini-AQLQ as our primary endpoint. This was due to a variety of reasons. Although, validated, the mini-AQLQ is only useful in determining changes in asthma-related quality of life. The instrument is not designed to detect primarily exercise-related improvement. And, as many of our patients had no symptoms unless they were exercising, the mini-AQLQ would not accurately reflect the outcomes we were striving to measure. Thus, we chose to use the mini-AQLQ as a secondary outcome, recognizing that it would not likely reflect exercise-related improvement.
Finally, the number of subjects in our study is relatively small increasing chance for type II error. We attempted to decrease this chance by comparing placebo to both medication arms combined. Further studies evaluating QD and BID therapy separately versus placebo are warranted.
Our blinded, randomized controlled trial study demonstrates that subjects who complain of exercise-triggered respiratory symptoms without excessive reflux symptoms improve symptomatically with acid suppression. Asthmatic subjects with underlying GER had an even higher response rate. Subjects with such exercise-related respiratory symptoms may be misdiagnosed as chronic asthmatics. Our data suggest acid reflux is a common finding in such subjects. Additionally, it appears that such exercise-related symptoms improve with the use of acid suppression. The results of this study suggest that such subjects may benefit from an empiric trial of acid suppression with PPI. Although, causality may only be hypothesized from this study.
Our results should be viewed as hypothesis-generating. Further studies are needed to determine whether this effect can be further corroborated by objective outcomes such as improvement in VO2max and/or exercise tolerance. Additionally, further studies evaluating both subjects with symptomatic GER (we tested those with very few symptoms) and studies evaluating the efficacy of “on-demand” therapy will be useful.
Study Highlights
What is current knowledge?
-
Gastroesophageal reflux occurs during exercise in people with and without GERD.
-
Triggered respiratory symptoms are common among active young adults.
-
The mechanism may be respiratory airway hyper-reactivity caused by acid reflux.
-
The relationship between reflux events and respiratory symptoms during exercise is unclear, and it is unknown if treatment of acid reflux improves symptoms during exercise.
What is new here?
-
The prevalence of abnormal 24-h pH studies is elevated in people with exercise-related respiratory symptoms.
-
Both asthmatic and non-asthmatics experience symptomatic improvement during exercise with acid suppression.
-
This symptomatic improvement with acid suppression is not paralleled by improvement in spirometry.
References
Storms WW (2005) Asthma associated with exercise. Immunol Allergy Clin North Am 25(1):31–43. doi:10.1016/j.iac.2004.09.007
Hermansen CL, Kirchner JT (2004) Identifying exercise-induced bronchospasm. Treatment hinges on distinguishing it from chronic asthma. Postgrad Med 115(6):15-6–21-5
Parsons JP, Mastroade JG (2005) Exercise-induced bronchoscontriction in athletes. Chest 128(6):3966–3974. doi:10.1378/chest.128.6.3966
Weiner P, Konson N, Sternberg A et al (1998) Is gastroesophageal reflux a factor in exercise induced asthma? Respir Med 92:1071–1075. doi:10.1016/S0954-6111(98)90357-2
Hogan WJ (1997) Spectrum of supraesophageal complications of gastroesophageal reflux disease. Am J Med 103(5A):77S–83S. doi:10.1016/S0002-9343(97)00329-x
Harding SM (2001) Gastroesophageal reflux, asthma, and mechanisms of interaction. Am J Med 111(8A):8S–12S. doi:10.1016/S0002-9343(01)00817-8
Kraus BB, Sinclair JW, Castell DO (1990) Gastroesophageal reflux in runners. Ann Intern Med 112:429–433
Clark CS, Kraus BB, Sinclair J et al (1989) Gastroesophageal reflux induced by exercise in healthy volunteers. JAMA 261(24):3599–3601. doi:10.1001/jama.261.24.3599
Banwait KS, Harnois DM, Tighe D et al (2004) The effect of exercise on the esophageal body and lower esophageal sphincter in normal and subjects with gastroesophageal reflux disease (GERD). Gastroenterology 126(4)Suppl 2:A-500–A-501:T1731
Maddison KJ, Shepherd KL, Hillman DR et al (2005) Function of the lower esophageal sphincter during and after high-intensity exercise. Med Sci Sports Exerc 37(10):1728–1733. doi:10.1249/01.mss.0000175051.47170.33
Sharma A, Levey J (2003) Gastroesophageal reflux disease in runners. AMAA J 16(1):7–11
Maddison K, Shepherd K, Hillman D et al (2005) Function of the lower esophageal sphincter during and after high-intensity exercise. Med Sci Sports Exerc 37:1728–1733. doi:10.1249/01.mss.0000175051.47170.33
Bagnato GF, Gulli S, Giacobbe O et al (2000) Bronchial hyperresponsiveness in subjects with gastroesophageal reflux. Respiration 67:507–509. doi:10.1159/000067464
National Asthma Education and Prevention Program (2007) Expert panel report III: guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute, Bethesda, MD, NIH publication no. 08-4051
Dobhan R, Castell DO (1993) Normal and abnormal proximal acid exposure; results of ambulatory dual probe pH monitoring. Am J Gastroenterol 88:25–30
Demeester J, Johnson D (1974) 24 hour pH monitoring of distal esophagus. Am J Gastroenterol 62:323–332
Littner MR, Leung FW, Ballard ED et al (2005) Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life and pulmonary function in adult asthmatic subjects with acid reflux symptoms. Chest 128:1128–1135. doi:10.1378/chest.128.3.1128
Kilijander TO, Harding SM, Field SK et al (2006) Effects of esomeprazole 40 mg twice daily on asthma. Am J Respir Crit Care Med 173:191–1097
Kilijander TO, Salomaa ER, Hietanen EK et al (1999) Gastroesophageal reflux in asthmatics: a double-blind, placebo-controlled crossover study with omeprazole. Chest 116(5):1257–1264. doi:10.1378/chest.116.5.1257
Boeree MJ, Peters FTM, Postrna DS et al (1998) No effects of high dose omeprazole in subjects with severe airway hyper-responsiveness and (a)symptomatic gastroesophageal reflux. Eur Respir J 11:1070–1074. doi:10.1183/09031936.98.11051070
Harding SM, Richter JE (1997) The role of gastroesophageal reflux in chronic cough and asthma. Chest 111:1389–1402. doi:10.1378/chest.111.5.1389
Vincent D, Cohen-Jonathan AM, Leport J et al (1997) Gastro-esophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 10:2255–2259. doi:10.1183/09031936.97.10102255
Field SK, Evans JA, Price LM (1998) The effects of acid perfusion of the esophagus on ventilation and respiratory sensation. Am J Respir Crit Care Med 157:1058–1062
Kiljander TO, Salomaa ER, Hietanen Ek et al (2002) Gastroesophageal reflux and bronchial hyperresponsiveness: correlation and the effect of fundoplication. Respiration 69:434–439. doi:10.1159/000064021
Jiang SP, Liang RY, Zeng ZY et al (2003) Effects of antireflux treatment on bronchial hyperresponsiveness and lung function in asthmatic subjects with gastroesophageal reflux disease. World J Gastroenterol 9:1123–1125
Soffer EE, Merchant RK, Duethman G et al (1993) Effect of graded exercise on esophageal motility and gastroesophageal reflux in trained athletes. Dig Dis Sci 38:220–224. doi:10.1007/BF01307538
Soffer EE, Wilson J, Duethman G et al (1994) Effect of graded exercise on esophageal motility and gastroesophageal reflux in nontrained subjects. Dig Dis Sci 39:193–198. doi:10.1007/BF02090082
Van Nieuwenhoven MA, Brummer RJM, Brouns F (2000) Gastrointestinal function during exercise: comparison of water, sports drink, and sports drink with caffeine. J Appl Physiol 89:1079–1085
Corley DA, Levin TR, Habel LA, Buffler PA (2006) Barrett’s esophagus and medications that relax the lower esophageal sphincter. Am J Gastroenterol 101:937–944. doi:10.1111/j.1572-0241.2006.00539.x
Lagergren J, Bergstrom R, Adami H, Nyren O (2000) Association between medications that relax the LES and esophageal adenocarcinoma. Ann Intern Med 133:165–175
Mainie I, Tatuian R et al (2006) Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy; a multi-centre study using combined ambulatory impedance monitoring. Gut 55:1398–1402. doi:10.1136/gut.2005.087668
Charbel S, Khandwala F, Vaezi M (2005) The role of esophageal pH monitoring in symptomatic Subjects on PPI therapy. Am J Gastroenterol 100:283–289. doi:10.1111/j.1572-0241.2005.41210.x
Acknowledgments
Declaration of personal interests:
Dr. Kathryn Peterson received research support from PriCara, unit of Ortho-McNeil, Inc. and the AGA Foundation. No other personal interests exist.
Declaration of Funding Interests
This study was funded in part by PriCara, unit of Ortho-McNeil, Inc. and the AGA Foundation for the 2000 Fellowship to Faculty Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peterson, K.A., Samuelson, W.M., Ryujin, D.T. et al. The Role of Gastroesophageal Reflux in Exercise-Triggered Asthma: A Randomized Controlled Trial. Dig Dis Sci 54, 564–571 (2009). https://doi.org/10.1007/s10620-008-0396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0396-6